 Found 672 hits with Last Name = 'koeberle' and Initial = 'a'
Found 672 hits with Last Name = 'koeberle' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50532250
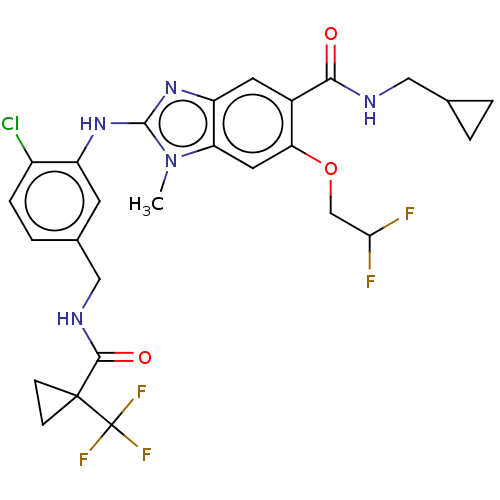
(CHEMBL4444875)Show SMILES Cn1c(Nc2cc(CNC(=O)C3(CC3)C(F)(F)F)ccc2Cl)nc2cc(C(=O)NCC3CC3)c(OCC(F)F)cc12 Show InChI InChI=1S/C27H27ClF5N5O3/c1-38-20-10-21(41-13-22(29)30)16(23(39)34-11-14-2-3-14)9-19(20)37-25(38)36-18-8-15(4-5-17(18)28)12-35-24(40)26(6-7-26)27(31,32)33/h4-5,8-10,14,22H,2-3,6-7,11-13H2,1H3,(H,34,39)(H,35,40)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES-1 expressed in CHO-K1 cells using PGH2 as substrate assessed as PGE2 formation preincubated for 20 mins followed by additio... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50532250

(CHEMBL4444875)Show SMILES Cn1c(Nc2cc(CNC(=O)C3(CC3)C(F)(F)F)ccc2Cl)nc2cc(C(=O)NCC3CC3)c(OCC(F)F)cc12 Show InChI InChI=1S/C27H27ClF5N5O3/c1-38-20-10-21(41-13-22(29)30)16(23(39)34-11-14-2-3-14)9-19(20)37-25(38)36-18-8-15(4-5-17(18)28)12-35-24(40)26(6-7-26)27(31,32)33/h4-5,8-10,14,22H,2-3,6-7,11-13H2,1H3,(H,34,39)(H,35,40)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES-1 expressed in CHO-K1 cells using PGH2 as substrate assessed as PGE2 formation preincubated for 20 mins followed by additio... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302429
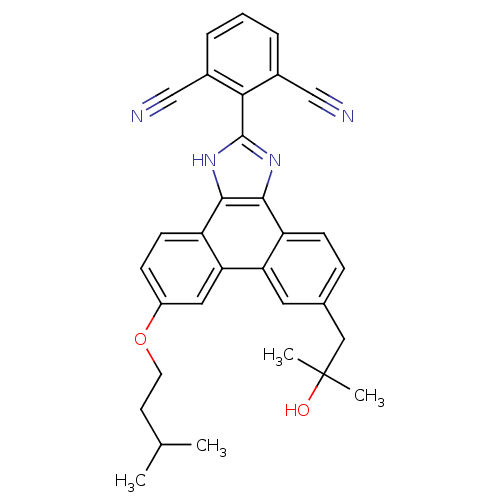
(2-(9-(2-hydroxy-2-methylpropyl)-6-(isopentyloxy)-1...)Show SMILES CC(C)CCOc1ccc2c3[nH]c(nc3c3ccc(CC(C)(C)O)cc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C32H30N4O2/c1-19(2)12-13-38-23-9-11-25-27(15-23)26-14-20(16-32(3,4)37)8-10-24(26)29-30(25)36-31(35-29)28-21(17-33)6-5-7-22(28)18-34/h5-11,14-15,19,37H,12-13,16H2,1-4H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50109832

(CHEMBL1289402)Show SMILES Fc1cccc(Cl)c1-c1nc(c(Br)[nH]1)-c1ccc(nc1)C#Cc1ccccc1 Show InChI InChI=1S/C22H12BrClFN3/c23-21-20(27-22(28-21)19-17(24)7-4-8-18(19)25)15-10-12-16(26-13-15)11-9-14-5-2-1-3-6-14/h1-8,10,12-13H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50337654
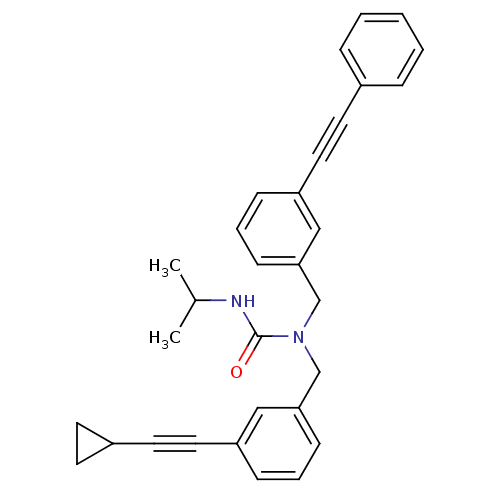
(1-(3-(cyclopropylethynyl)benzyl)-3-isopropyl-1-(3-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#CC1CC1)Cc1cccc(c1)C#Cc1ccccc1 Show InChI InChI=1S/C31H30N2O/c1-24(2)32-31(34)33(23-30-13-7-11-28(21-30)19-17-26-14-15-26)22-29-12-6-10-27(20-29)18-16-25-8-4-3-5-9-25/h3-13,20-21,24,26H,14-15,22-23H2,1-2H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50109831
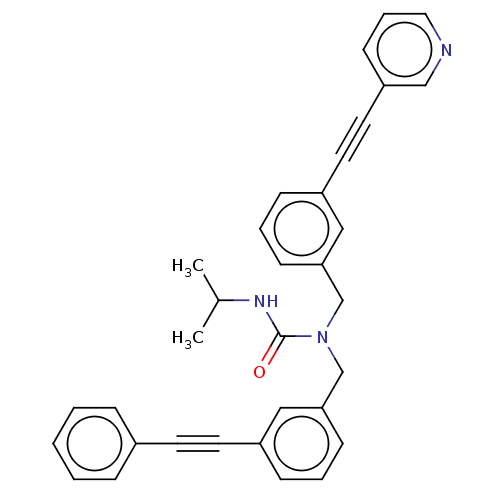
(CHEMBL3604189)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)C#Cc1ccccc1)Cc1cccc(c1)C#Cc1cccnc1 Show InChI InChI=1S/C33H29N3O/c1-26(2)35-33(37)36(24-31-13-6-11-28(21-31)17-16-27-9-4-3-5-10-27)25-32-14-7-12-29(22-32)18-19-30-15-8-20-34-23-30/h3-15,20-23,26H,24-25H2,1-2H3,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50302421
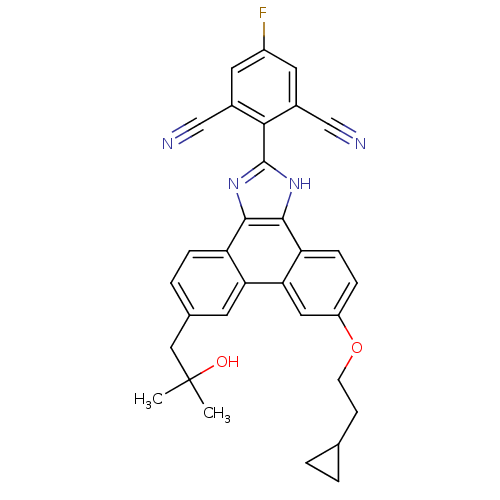
(2-(6-(2-cyclopropylethoxy)-9-(2-hydroxy-2-methylpr...)Show SMILES CC(C)(O)Cc1ccc2c3nc([nH]c3c3ccc(OCCC4CC4)cc3c2c1)-c1c(cc(F)cc1C#N)C#N Show InChI InChI=1S/C32H27FN4O2/c1-32(2,38)15-19-5-7-24-26(11-19)27-14-23(39-10-9-18-3-4-18)6-8-25(27)30-29(24)36-31(37-30)28-20(16-34)12-22(33)13-21(28)17-35/h5-8,11-14,18,38H,3-4,9-10,15H2,1-2H3,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50532250

(CHEMBL4444875)Show SMILES Cn1c(Nc2cc(CNC(=O)C3(CC3)C(F)(F)F)ccc2Cl)nc2cc(C(=O)NCC3CC3)c(OCC(F)F)cc12 Show InChI InChI=1S/C27H27ClF5N5O3/c1-38-20-10-21(41-13-22(29)30)16(23(39)34-11-14-2-3-14)9-19(20)37-25(38)36-18-8-15(4-5-17(18)28)12-35-24(40)26(6-7-26)27(31,32)33/h4-5,8-10,14,22H,2-3,6-7,11-13H2,1H3,(H,34,39)(H,35,40)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells assessed as PGE2-alpha formation preincubated for 60 mins prior to IL-beta stimulation measured after 24 hr... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50532250

(CHEMBL4444875)Show SMILES Cn1c(Nc2cc(CNC(=O)C3(CC3)C(F)(F)F)ccc2Cl)nc2cc(C(=O)NCC3CC3)c(OCC(F)F)cc12 Show InChI InChI=1S/C27H27ClF5N5O3/c1-38-20-10-21(41-13-22(29)30)16(23(39)34-11-14-2-3-14)9-19(20)37-25(38)36-18-8-15(4-5-17(18)28)12-35-24(40)26(6-7-26)27(31,32)33/h4-5,8-10,14,22H,2-3,6-7,11-13H2,1H3,(H,34,39)(H,35,40)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells assessed as PGE2-alpha formation preincubated for 60 mins prior to IL-beta stimulation measured after 24 hr... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAP in human PMNC assessed as inhibition of A23187-induced LTB4 biosynthesis pre-incubated for 2 mins followed by addition of... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University Jena
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAP in human PMNC assessed as inhibition of A23187-induced LTB4 biosynthesis pre-incubated for 2 mins followed by addition of... |
J Med Chem 59: 5970-86 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01750
BindingDB Entry DOI: 10.7270/Q2902793 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50109830

(CHEMBL1289055)Show SMILES Fc1cccc(Cl)c1-c1nc(c[nH]1)-c1ccc(nc1)C#Cc1cccc(Cl)c1 Show InChI InChI=1S/C22H12Cl2FN3/c23-16-4-1-3-14(11-16)7-9-17-10-8-15(12-26-17)20-13-27-22(28-20)21-18(24)5-2-6-19(21)25/h1-6,8,10-13H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in IL-1beta-stimulated human A549 cell microsomal membranes assessed as reduction in PGE2 formation incubated for 15 mins using... |
Bioorg Med Chem 23: 4839-45 (2015)
Article DOI: 10.1016/j.bmc.2015.05.045
BindingDB Entry DOI: 10.7270/Q2M90BF8 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Mus musculus) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in mouse RAW264.7 cells assessed as reduction in LPS-induced PGE2 production after 24 hrs |
Bioorg Med Chem Lett 26: 5193-5197 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.070
BindingDB Entry DOI: 10.7270/Q2W097WN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273442
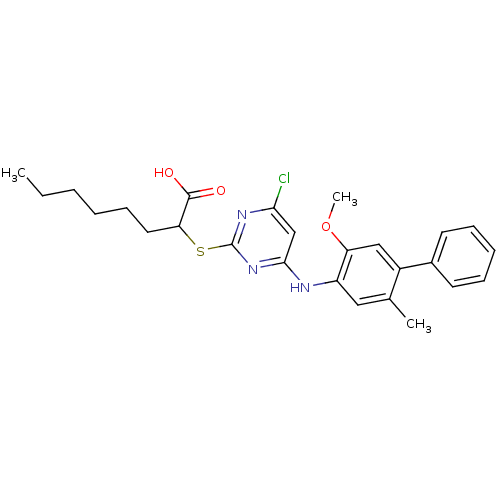
(2-(4-Chloro-6-(5-methoxy-2-methylbiphenyl-4-ylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cc(C)c(cc2OC)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30ClN3O3S/c1-4-5-6-10-13-22(25(31)32)34-26-29-23(27)16-24(30-26)28-20-14-17(2)19(15-21(20)33-3)18-11-8-7-9-12-18/h7-9,11-12,14-16,22H,4-6,10,13H2,1-3H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273441
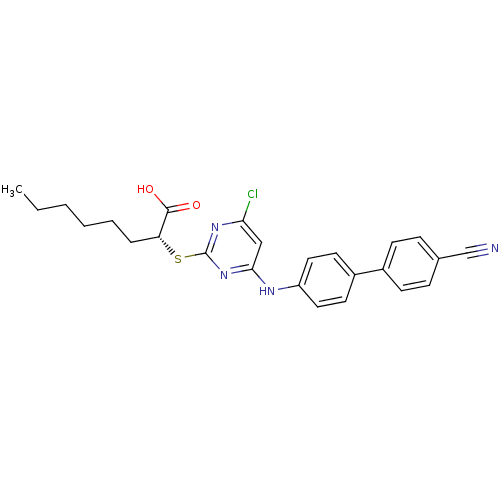
((R)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273390
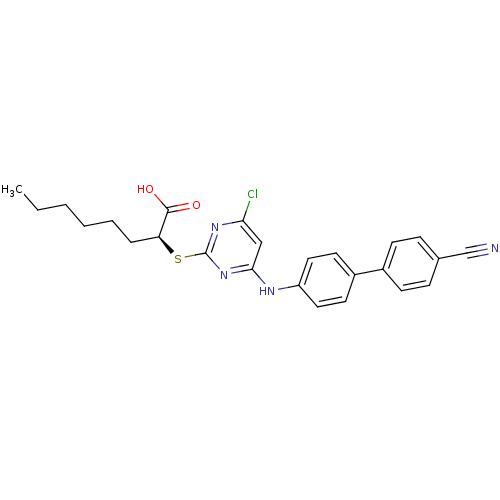
((S)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273389
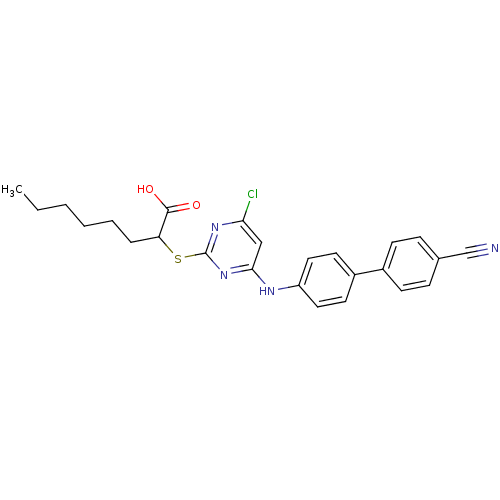
(2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273388
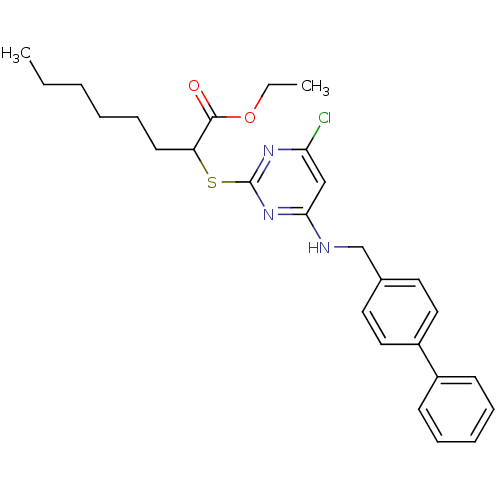
(CHEMBL456575 | Ethyl 2-(4-(biphenyl-4-ylmethylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C27H32ClN3O2S/c1-3-5-6-10-13-23(26(32)33-4-2)34-27-30-24(28)18-25(31-27)29-19-20-14-16-22(17-15-20)21-11-8-7-9-12-21/h7-9,11-12,14-18,23H,3-6,10,13,19H2,1-2H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273710
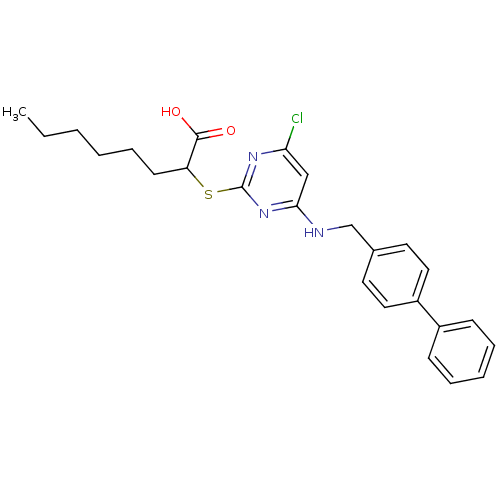
(2-(4-(Biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C25H28ClN3O2S/c1-2-3-4-8-11-21(24(30)31)32-25-28-22(26)16-23(29-25)27-17-18-12-14-20(15-13-18)19-9-6-5-7-10-19/h5-7,9-10,12-16,21H,2-4,8,11,17H2,1H3,(H,30,31)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273709
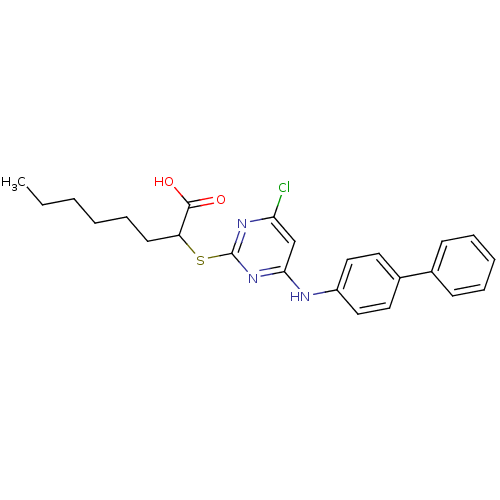
(2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C24H26ClN3O2S/c1-2-3-4-8-11-20(23(29)30)31-24-27-21(25)16-22(28-24)26-19-14-12-18(13-15-19)17-9-6-5-7-10-17/h5-7,9-10,12-16,20H,2-4,8,11H2,1H3,(H,29,30)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273708
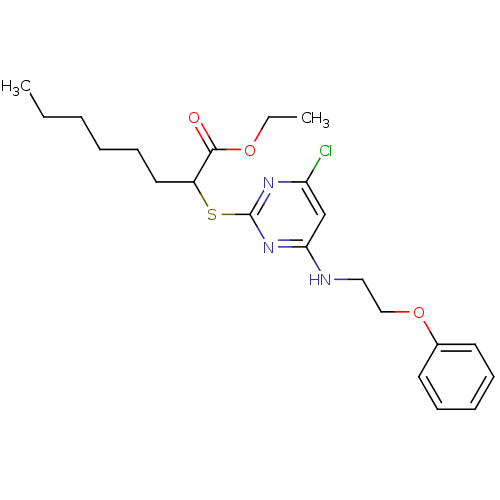
(CHEMBL460615 | Ethyl 2-(4-chloro-6-(2-phenoxyethyl...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCCOc2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C22H30ClN3O3S/c1-3-5-6-10-13-18(21(27)28-4-2)30-22-25-19(23)16-20(26-22)24-14-15-29-17-11-8-7-9-12-17/h7-9,11-12,16,18H,3-6,10,13-15H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273707
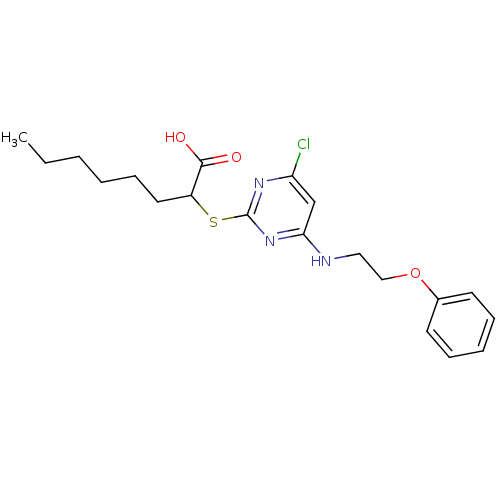
(2-(4-Chloro-6-(2-phenoxyethylamino)pyrimidin-2-ylt...)Show InChI InChI=1S/C20H26ClN3O3S/c1-2-3-4-8-11-16(19(25)26)28-20-23-17(21)14-18(24-20)22-12-13-27-15-9-6-5-7-10-15/h5-7,9-10,14,16H,2-4,8,11-13H2,1H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273685
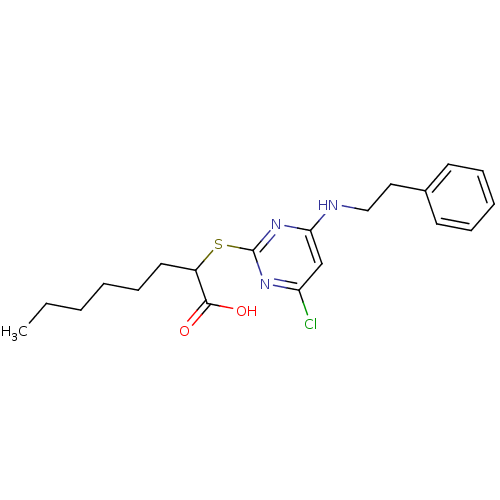
(2-(4-Chloro-6-(phenethylamino)pyrimidin-2-ylthio)o...)Show InChI InChI=1S/C20H26ClN3O2S/c1-2-3-4-8-11-16(19(25)26)27-20-23-17(21)14-18(24-20)22-13-12-15-9-6-5-7-10-15/h5-7,9-10,14,16H,2-4,8,11-13H2,1H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273684
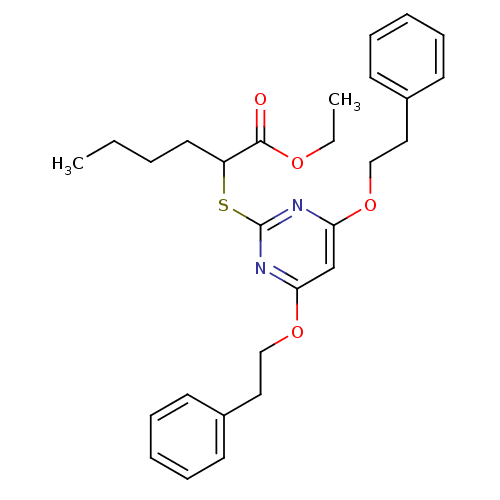
(CHEMBL460184 | Ethyl 2-(4,6-diphenethoxypyrimidin-...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C28H34N2O4S/c1-3-5-16-24(27(31)32-4-2)35-28-29-25(33-19-17-22-12-8-6-9-13-22)21-26(30-28)34-20-18-23-14-10-7-11-15-23/h6-15,21,24H,3-5,16-20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273683
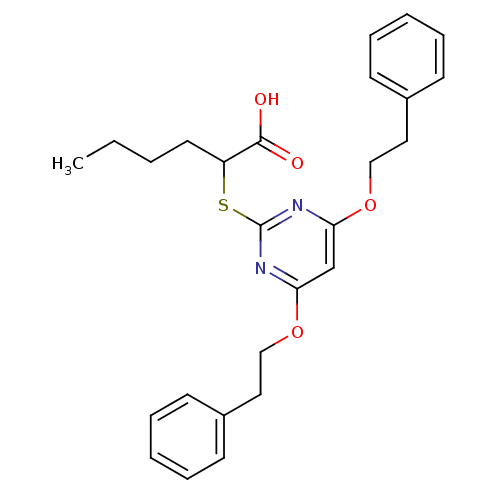
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273682
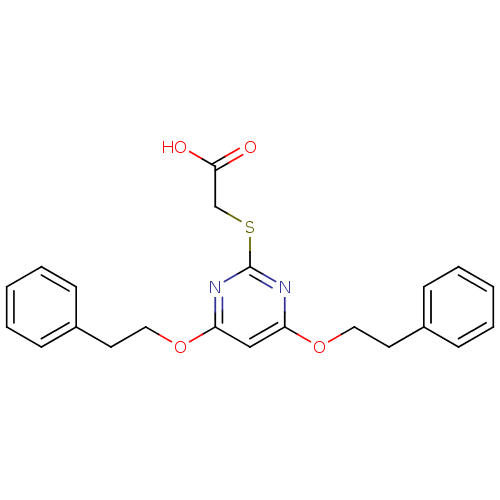
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)acetic acid ...)Show InChI InChI=1S/C22H22N2O4S/c25-21(26)16-29-22-23-19(27-13-11-17-7-3-1-4-8-17)15-20(24-22)28-14-12-18-9-5-2-6-10-18/h1-10,15H,11-14,16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24565
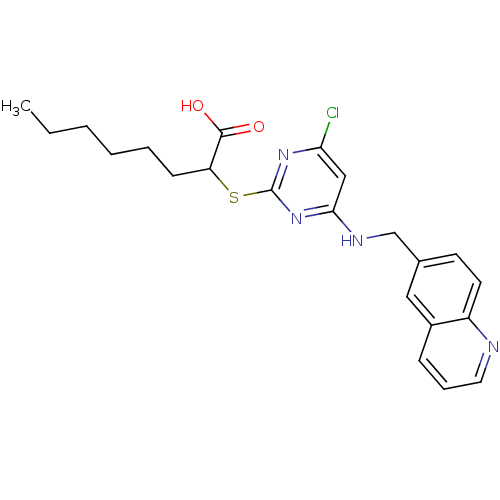
(2-({4-chloro-6-[(quinolin-6-ylmethyl)amino]pyrimid...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S/c1-2-3-4-5-8-18(21(28)29)30-22-26-19(23)13-20(27-22)25-14-15-9-10-17-16(12-15)7-6-11-24-17/h6-7,9-13,18H,2-5,8,14H2,1H3,(H,28,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253880
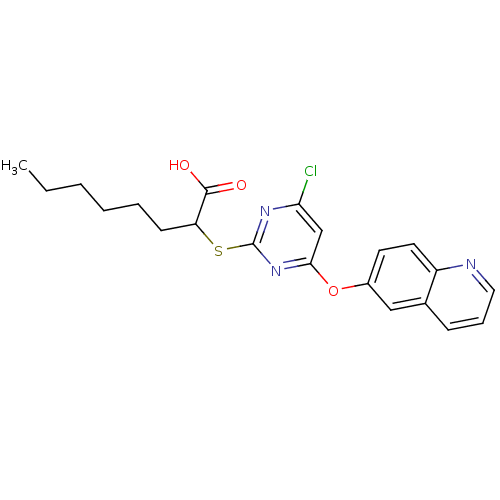
(2-(4-chloro-6-(quinolin-6-yloxy)pyrimidin-2-ylthio...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Oc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H22ClN3O3S/c1-2-3-4-5-8-17(20(26)27)29-21-24-18(22)13-19(25-21)28-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24561
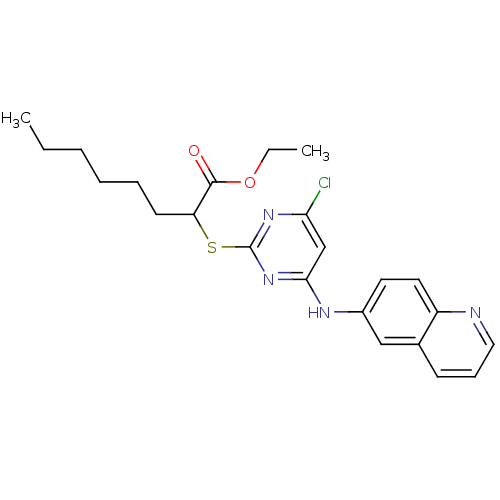
(CHEMBL459722 | Pirinixic acid-based compound, 6d |...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(=O)OCC Show InChI InChI=1S/C23H27ClN4O2S/c1-3-5-6-7-10-19(22(29)30-4-2)31-23-27-20(24)15-21(28-23)26-17-11-12-18-16(14-17)9-8-13-25-18/h8-9,11-15,19H,3-7,10H2,1-2H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24564
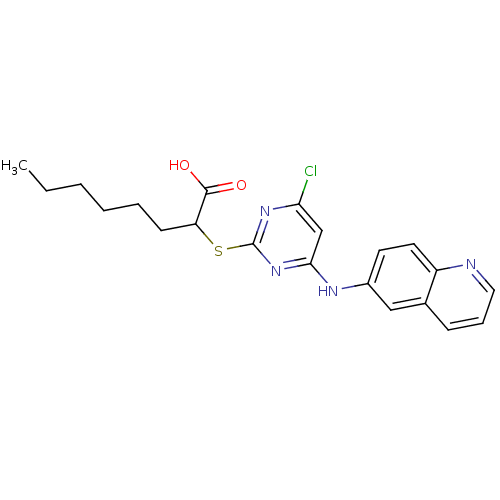
(2-{[4-chloro-6-(quinolin-6-ylamino)pyrimidin-2-yl]...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S/c1-2-3-4-5-8-17(20(27)28)29-21-25-18(22)13-19(26-21)24-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253803
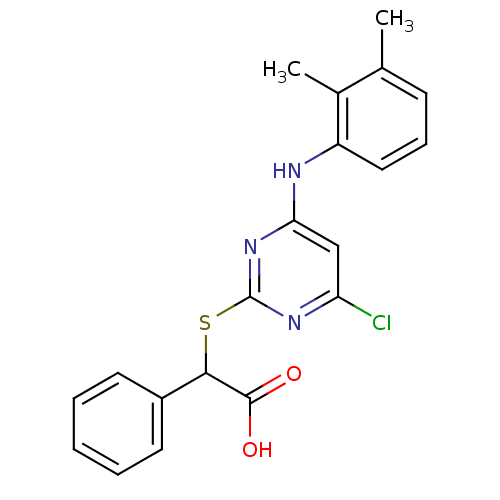
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES Cc1cccc(Nc2cc(Cl)nc(SC(C(O)=O)c3ccccc3)n2)c1C Show InChI InChI=1S/C20H18ClN3O2S/c1-12-7-6-10-15(13(12)2)22-17-11-16(21)23-20(24-17)27-18(19(25)26)14-8-4-3-5-9-14/h3-11,18H,1-2H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273385

(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES CCCCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C22H30ClN3O2S/c1-4-5-6-7-8-9-13-18(21(27)28)29-22-25-19(23)14-20(26-22)24-17-12-10-11-15(2)16(17)3/h10-12,14,18H,4-9,13H2,1-3H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253879
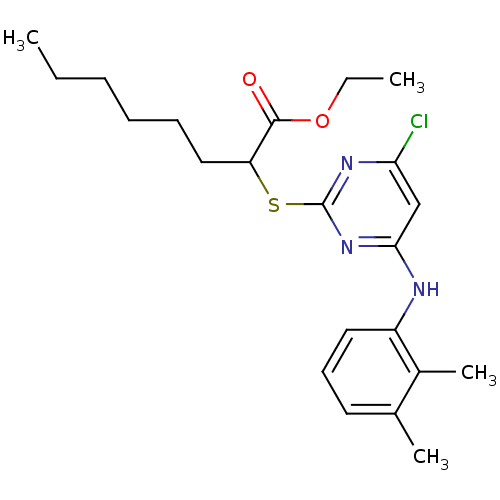
(CHEMBL459720 | ethyl 2-(4-chloro-6-(2,3-dimethylph...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(=O)OCC Show InChI InChI=1S/C22H30ClN3O2S/c1-5-7-8-9-13-18(21(27)28-6-2)29-22-25-19(23)14-20(26-22)24-17-12-10-11-15(3)16(17)4/h10-12,14,18H,5-9,13H2,1-4H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24560
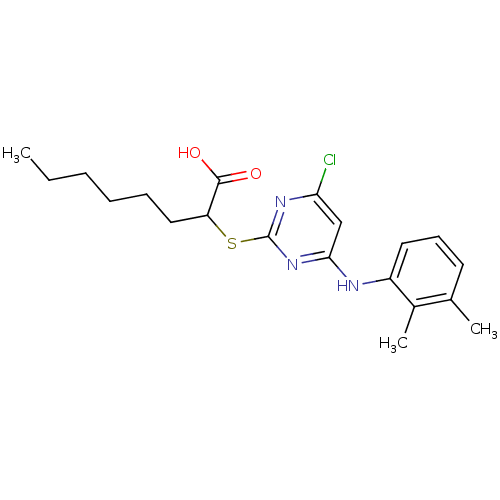
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C20H26ClN3O2S/c1-4-5-6-7-11-16(19(25)26)27-20-23-17(21)12-18(24-20)22-15-10-8-9-13(2)14(15)3/h8-10,12,16H,4-7,11H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273681
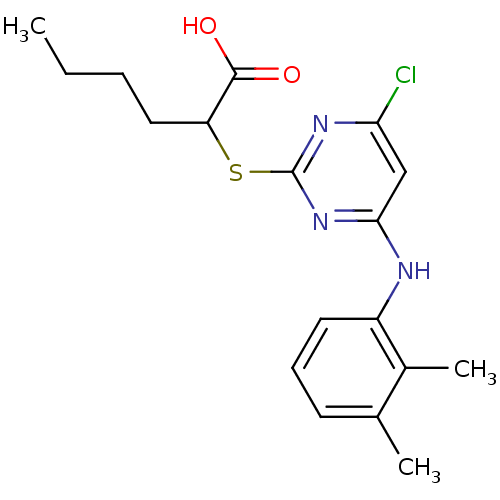
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C18H22ClN3O2S/c1-4-5-9-14(17(23)24)25-18-21-15(19)10-16(22-18)20-13-8-6-7-11(2)12(13)3/h6-8,10,14H,4-5,9H2,1-3H3,(H,23,24)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273680
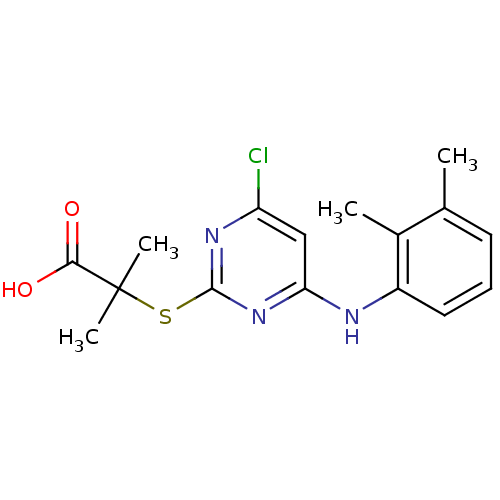
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C16H18ClN3O2S/c1-9-6-5-7-11(10(9)2)18-13-8-12(17)19-15(20-13)23-16(3,4)14(21)22/h5-8H,1-4H3,(H,21,22)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253802
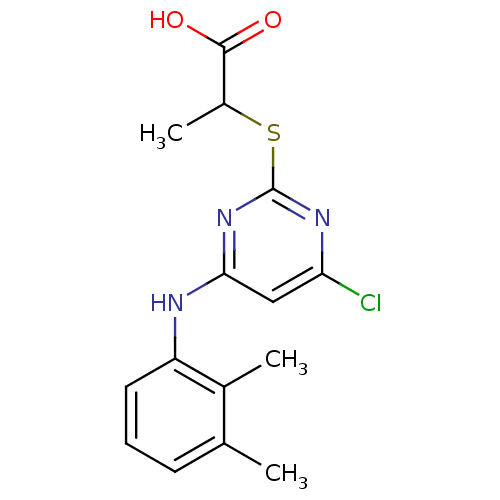
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C15H16ClN3O2S/c1-8-5-4-6-11(9(8)2)17-13-7-12(16)18-15(19-13)22-10(3)14(20)21/h4-7,10H,1-3H3,(H,20,21)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM24566
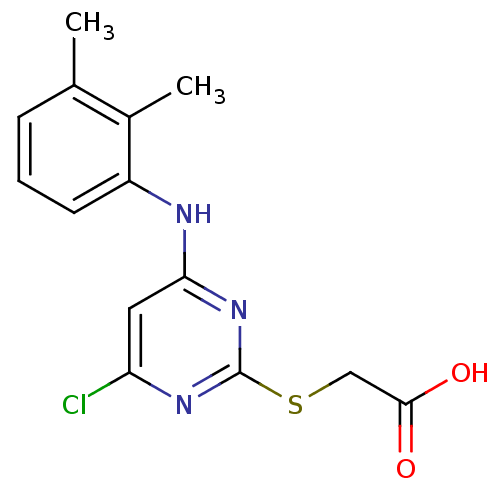
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24566

(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273442

(2-(4-Chloro-6-(5-methoxy-2-methylbiphenyl-4-ylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cc(C)c(cc2OC)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30ClN3O3S/c1-4-5-6-10-13-22(25(31)32)34-26-29-23(27)16-24(30-26)28-20-14-17(2)19(15-21(20)33-3)18-11-8-7-9-12-18/h7-9,11-12,14-16,22H,4-6,10,13H2,1-3H3,(H,31,32)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273441

((R)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273390

((S)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273389

(2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273388

(CHEMBL456575 | Ethyl 2-(4-(biphenyl-4-ylmethylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C27H32ClN3O2S/c1-3-5-6-10-13-23(26(32)33-4-2)34-27-30-24(28)18-25(31-27)29-19-20-14-16-22(17-15-20)21-11-8-7-9-12-21/h7-9,11-12,14-18,23H,3-6,10,13,19H2,1-2H3,(H,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50273710

(2-(4-(Biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C25H28ClN3O2S/c1-2-3-4-8-11-21(24(30)31)32-25-28-22(26)16-23(29-25)27-17-18-12-14-20(15-13-18)19-9-6-5-7-10-19/h5-7,9-10,12-16,21H,2-4,8,11,17H2,1H3,(H,30,31)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data