 Found 223 hits with Last Name = 'naik' and Initial = 'a'
Found 223 hits with Last Name = 'naik' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324297

(4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...)Show SMILES C1CN(CCN1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C22H26N6O/c1-6-25-21(26-18-3-11-29-12-4-18)14-16(1)20-13-17-15-24-5-2-19(17)22(27-20)28-9-7-23-8-10-28/h1-2,5-6,13-15,18,23H,3-4,7-12H2,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324315

(CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc2cnccc2c(n1)N1CCNCC1 Show InChI InChI=1S/C23H28N6/c1-2-4-19(5-3-1)27-22-15-17(6-9-26-22)21-14-18-16-25-8-7-20(18)23(28-21)29-12-10-24-11-13-29/h6-9,14-16,19,24H,1-5,10-13H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559322
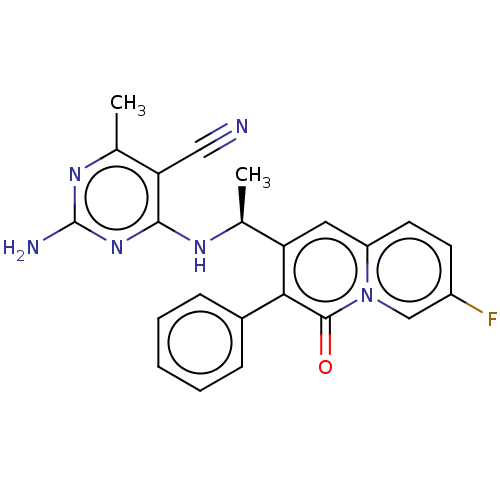
(CHEMBL4787382)Show SMILES C[C@H](Nc1nc(N)nc(C)c1C#N)c1cc2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472
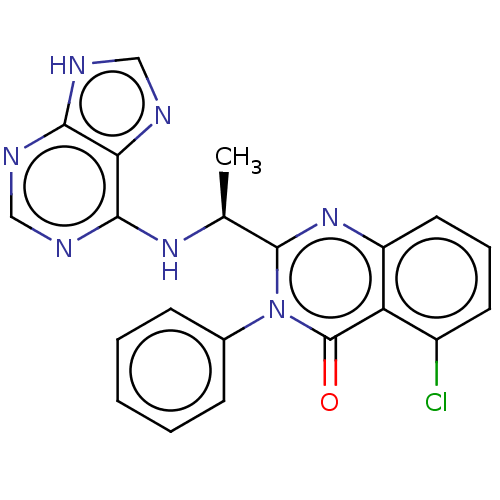
(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate in presence of ATP measured after 45 mins by HTRF assay relative to control |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324305

(CHEMBL1214711 | N-(2-(pyrrolidin-1-yl)ethyl)-1-(3-...)Show SMILES O=C(NC1CCNCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C30H39N7O/c38-30(35-25-7-12-31-13-8-25)21-10-16-37(17-11-21)29-26-9-14-32-20-23(26)18-27(36-29)22-6-15-33-28(19-22)34-24-4-2-1-3-5-24/h6,9,14-15,18-21,24-25,31H,1-5,7-8,10-13,16-17H2,(H,33,34)(H,35,38) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324296
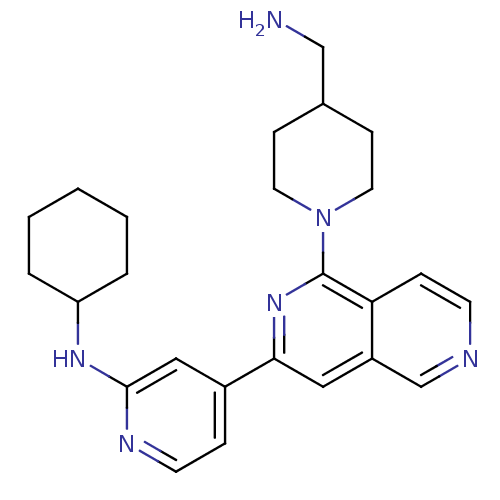
(1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...)Show SMILES NCC1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C25H32N6/c26-16-18-8-12-31(13-9-18)25-22-7-10-27-17-20(22)14-23(30-25)19-6-11-28-24(15-19)29-21-4-2-1-3-5-21/h6-7,10-11,14-15,17-18,21H,1-5,8-9,12-13,16,26H2,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324314
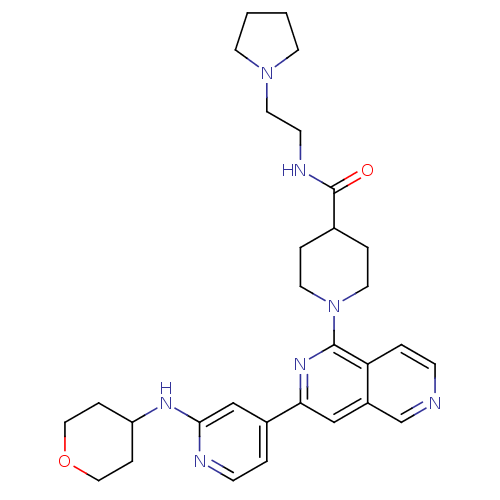
(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES O=C(NCCN1CCCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C30H39N7O2/c38-30(33-11-16-36-12-1-2-13-36)22-5-14-37(15-6-22)29-26-4-9-31-21-24(26)19-27(35-29)23-3-10-32-28(20-23)34-25-7-17-39-18-8-25/h3-4,9-10,19-22,25H,1-2,5-8,11-18H2,(H,32,34)(H,33,38) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324298
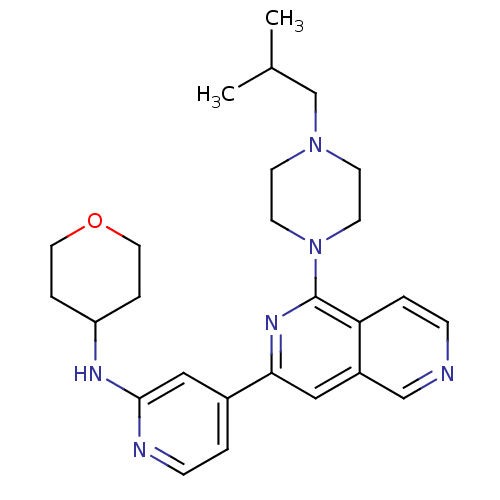
(CHEMBL1215712 | {4-[1-(4-Isobutylpiperazin-1-yl)[2...)Show SMILES CC(C)CN1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C26H34N6O/c1-19(2)18-31-9-11-32(12-10-31)26-23-4-7-27-17-21(23)15-24(30-26)20-3-8-28-25(16-20)29-22-5-13-33-14-6-22/h3-4,7-8,15-17,19,22H,5-6,9-14,18H2,1-2H3,(H,28,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324306
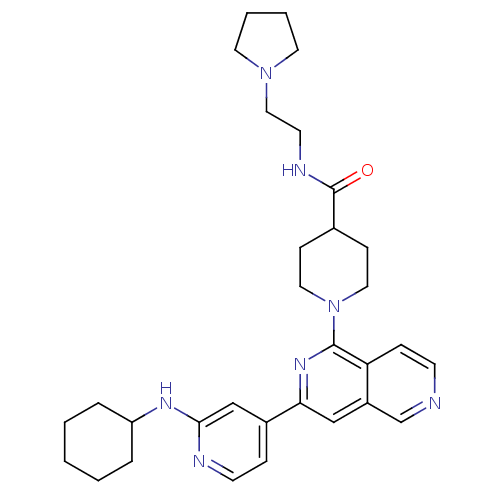
(1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...)Show SMILES O=C(NCCN1CCCC1)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C31H41N7O/c39-31(34-14-19-37-15-4-5-16-37)23-10-17-38(18-11-23)30-27-9-12-32-22-25(27)20-28(36-30)24-8-13-33-29(21-24)35-26-6-2-1-3-7-26/h8-9,12-13,20-23,26H,1-7,10-11,14-19H2,(H,33,35)(H,34,39) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469565
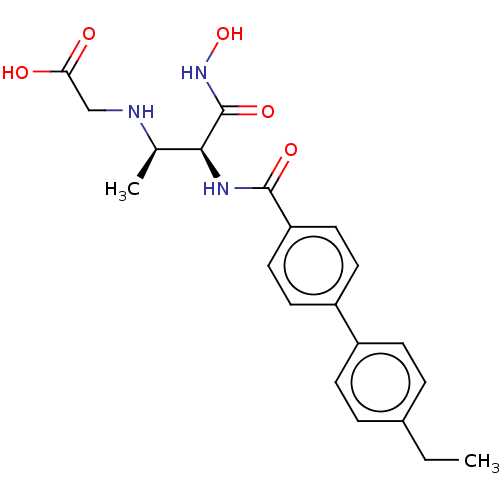
(CHEMBL4082918)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)NCC(O)=O)C(=O)NO |r| Show InChI InChI=1S/C21H25N3O5/c1-3-14-4-6-15(7-5-14)16-8-10-17(11-9-16)20(27)23-19(21(28)24-29)13(2)22-12-18(25)26/h4-11,13,19,22,29H,3,12H2,1-2H3,(H,23,27)(H,24,28)(H,25,26)/t13-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50168472

(CHEMBL3805348 | US9765060, Compound X)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C21H16ClN7O/c1-12(27-19-17-18(24-10-23-17)25-11-26-19)20-28-15-9-5-8-14(22)16(15)21(30)29(20)13-6-3-2-4-7-13/h2-12H,1H3,(H2,23,24,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559321
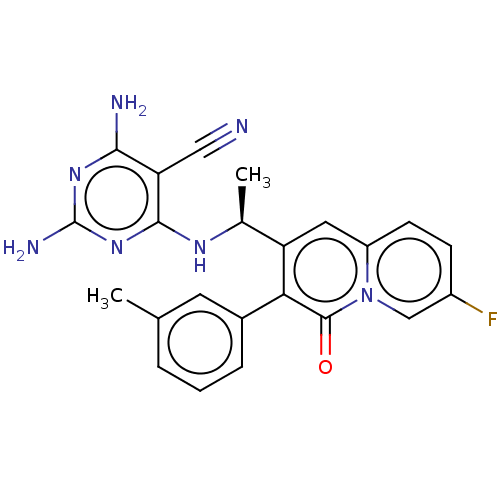
(CHEMBL4754291)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1cc2ccc(F)cn2c(=O)c1-c1cccc(C)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469558
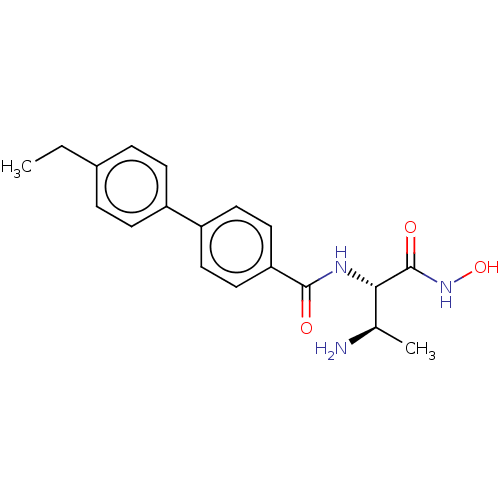
(CHEMBL4061041)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)N)C(=O)NO |r| Show InChI InChI=1S/C19H23N3O3/c1-3-13-4-6-14(7-5-13)15-8-10-16(11-9-15)18(23)21-17(12(2)20)19(24)22-25/h4-12,17,25H,3,20H2,1-2H3,(H,21,23)(H,22,24)/t12-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559323
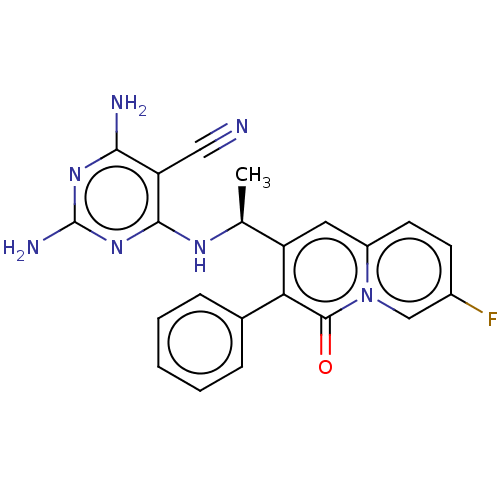
(CHEMBL4794328)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1cc2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559327

(CHEMBL4787659)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2cccc(C)n2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324312

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES CC(C)CNC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C28H36N6O2/c1-19(2)17-31-28(35)20-5-11-34(12-6-20)27-24-4-9-29-18-22(24)15-25(33-27)21-3-10-30-26(16-21)32-23-7-13-36-14-8-23/h3-4,9-10,15-16,18-20,23H,5-8,11-14,17H2,1-2H3,(H,30,32)(H,31,35) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324292

(CHEMBL1215641 | Cyclohexyl-{4-[1-(4-methylpiperazi...)Show SMILES CN1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C24H30N6/c1-29-11-13-30(14-12-29)24-21-8-9-25-17-19(21)15-22(28-24)18-7-10-26-23(16-18)27-20-5-3-2-4-6-20/h7-10,15-17,20H,2-6,11-14H2,1H3,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324294

(CHEMBL1215643 | Cyclohexyl-{4-[1-(4-cyclopropylmet...)Show SMILES C(C1CC1)N1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C27H34N6/c1-2-4-23(5-3-1)30-26-17-21(8-11-29-26)25-16-22-18-28-10-9-24(22)27(31-25)33-14-12-32(13-15-33)19-20-6-7-20/h8-11,16-18,20,23H,1-7,12-15,19H2,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324309

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES NC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C24H28N6O2/c25-23(31)16-3-9-30(10-4-16)24-20-2-7-26-15-18(20)13-21(29-24)17-1-8-27-22(14-17)28-19-5-11-32-12-6-19/h1-2,7-8,13-16,19H,3-6,9-12H2,(H2,25,31)(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324313

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES OCCNC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C26H32N6O3/c33-12-9-29-26(34)18-3-10-32(11-4-18)25-22-2-7-27-17-20(22)15-23(31-25)19-1-8-28-24(16-19)30-21-5-13-35-14-6-21/h1-2,7-8,15-18,21,33H,3-6,9-14H2,(H,28,30)(H,29,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559341
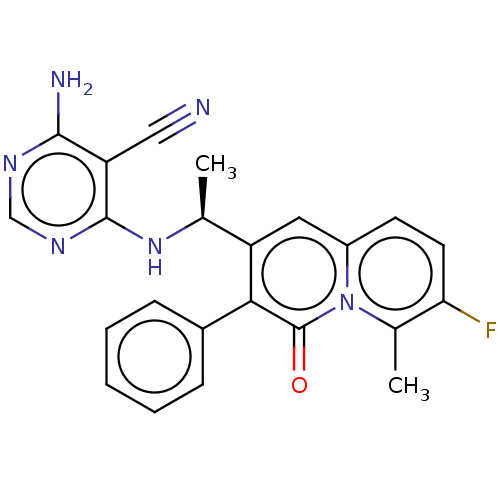
(CHEMBL4783353)Show SMILES C[C@H](Nc1ncnc(N)c1C#N)c1cc2ccc(F)c(C)n2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 using rhodamine as substrate after 1 hrs by fluorescence assay |
Bioorg Med Chem 19: 4626-34 (2011)
Article DOI: 10.1016/j.bmc.2011.06.030
BindingDB Entry DOI: 10.7270/Q2319W8N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324311
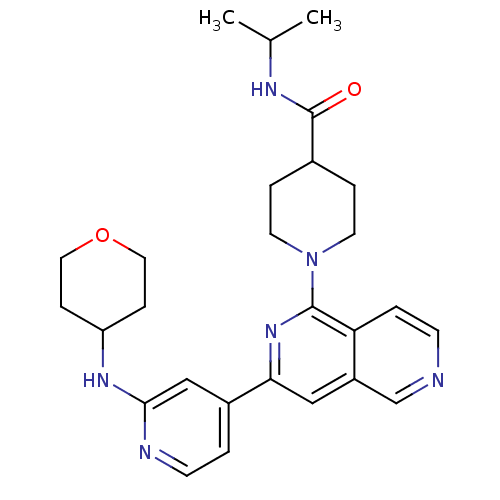
(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES CC(C)NC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C27H34N6O2/c1-18(2)30-27(34)19-5-11-33(12-6-19)26-23-4-9-28-17-21(23)15-24(32-26)20-3-10-29-25(16-20)31-22-7-13-35-14-8-22/h3-4,9-10,15-19,22H,5-8,11-14H2,1-2H3,(H,29,31)(H,30,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324304

(1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...)Show SMILES CNC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C26H32N6O/c1-27-26(33)18-9-13-32(14-10-18)25-22-8-11-28-17-20(22)15-23(31-25)19-7-12-29-24(16-19)30-21-5-3-2-4-6-21/h7-8,11-12,15-18,21H,2-6,9-10,13-14H2,1H3,(H,27,33)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324310

(1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...)Show SMILES CCNC(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C26H32N6O2/c1-2-28-26(33)18-5-11-32(12-6-18)25-22-4-9-27-17-20(22)15-23(31-25)19-3-10-29-24(16-19)30-21-7-13-34-14-8-21/h3-4,9-10,15-18,21H,2,5-8,11-14H2,1H3,(H,28,33)(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324291

(CHEMBL1215640 | Cyclohexyl-{4-[1-((cis-3,5-dimethy...)Show SMILES C[C@H]1CN(C[C@@H](C)N1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 |r| Show InChI InChI=1S/C25H32N6/c1-17-15-31(16-18(2)28-17)25-22-9-10-26-14-20(22)12-23(30-25)19-8-11-27-24(13-19)29-21-6-4-3-5-7-21/h8-14,17-18,21,28H,3-7,15-16H2,1-2H3,(H,27,29)/t17-,18+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50249583

(CHEMBL4097399)Show SMILES CC#CCOc1ccc(cc1)C(=O)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C16H21N3O4/c1-4-5-10-23-12-8-6-11(7-9-12)14(20)18-13(15(21)19-22)16(2,3)17/h6-9,13,22H,10,17H2,1-3H3,(H,18,20)(H,19,21)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559325
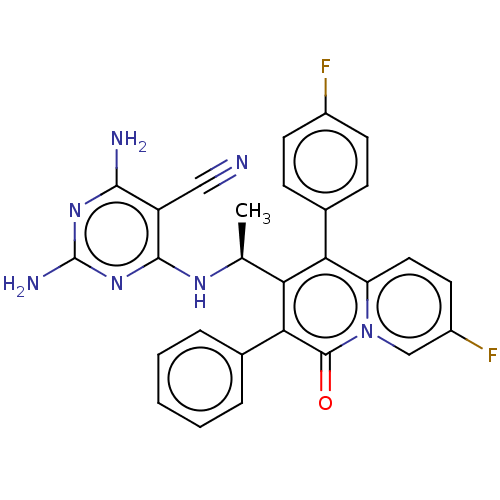
(CHEMBL4756563)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(-c2ccc(F)cc2)c2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate in presence of ATP measured after 45 mins by HTRF assay relative to control |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559330
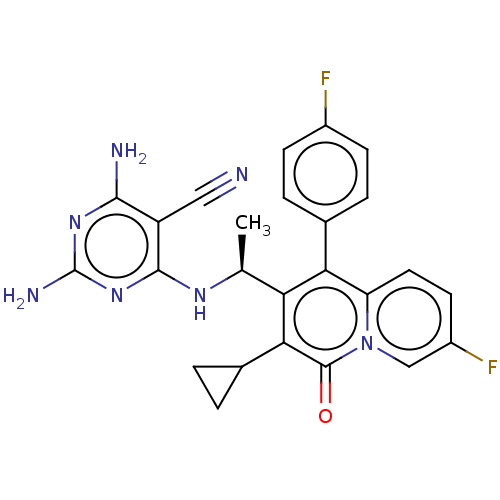
(CHEMBL4778687)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(-c2ccc(F)cc2)c2ccc(F)cn2c(=O)c1C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559338
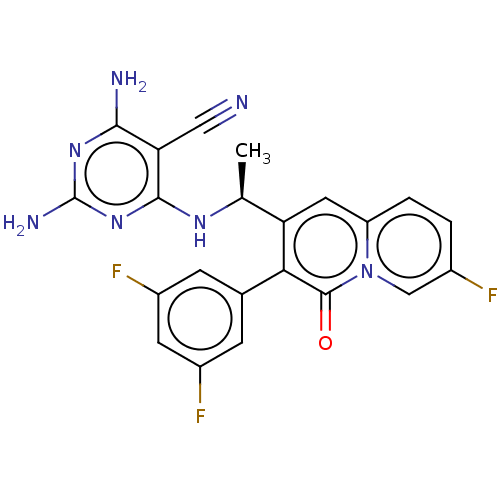
(CHEMBL4752055)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1cc2ccc(F)cn2c(=O)c1-c1cc(F)cc(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469562
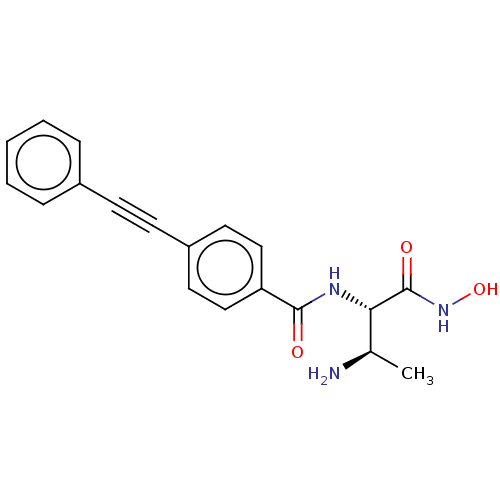
(CHEMBL4069725)Show SMILES C[C@@H](N)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H19N3O3/c1-13(20)17(19(24)22-25)21-18(23)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-13,17,25H,20H2,1H3,(H,21,23)(H,22,24)/t13-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559324
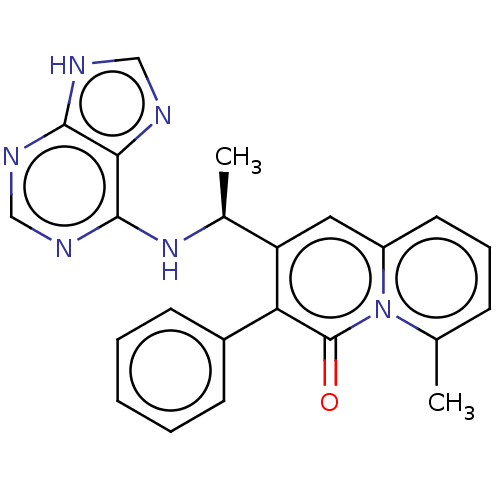
(CHEMBL4782337)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1cc2cccc(C)n2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3033
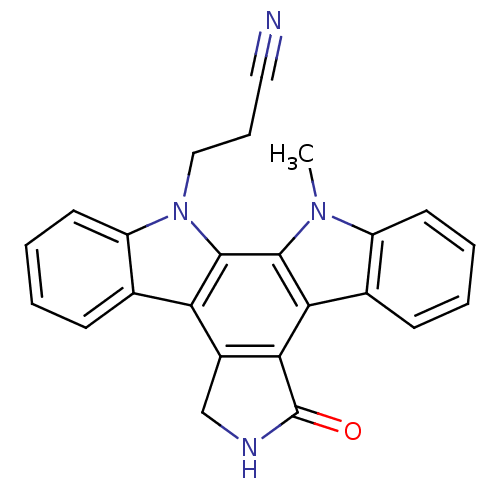
(3-{23-methyl-14-oxo-3,13,23-triazahexacyclo[14.7.0...)Show SMILES Cn1c2ccccc2c2c3C(=O)NCc3c3c4ccccc4n(CCC#N)c3c12 Show InChI InChI=1S/C24H18N4O/c1-27-17-9-4-2-7-14(17)20-21-16(13-26-24(21)29)19-15-8-3-5-10-18(15)28(12-6-11-25)23(19)22(20)27/h2-5,7-10H,6,12-13H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 using rhodamine as substrate after 1 hrs by fluorescence assay |
Bioorg Med Chem 19: 4626-34 (2011)
Article DOI: 10.1016/j.bmc.2011.06.030
BindingDB Entry DOI: 10.7270/Q2319W8N |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324307
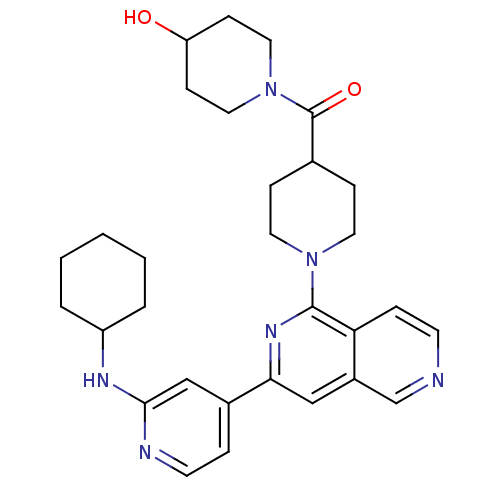
(CHEMBL1214786 | {1-[3-(2-Cyclohexylaminopyridin-4-...)Show SMILES OC1CCN(CC1)C(=O)C1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C30H38N6O2/c37-25-10-16-36(17-11-25)30(38)21-8-14-35(15-9-21)29-26-7-12-31-20-23(26)18-27(34-29)22-6-13-32-28(19-22)33-24-4-2-1-3-5-24/h6-7,12-13,18-21,24-25,37H,1-5,8-11,14-17H2,(H,32,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469559
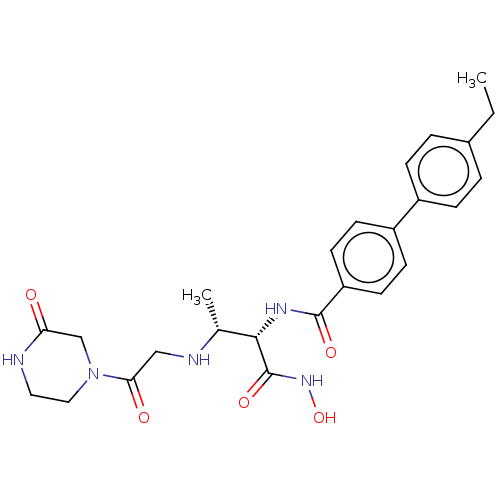
(CHEMBL4063087)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)NCC(=O)N1CCNC(=O)C1)C(=O)NO |r| Show InChI InChI=1S/C25H31N5O5/c1-3-17-4-6-18(7-5-17)19-8-10-20(11-9-19)24(33)28-23(25(34)29-35)16(2)27-14-22(32)30-13-12-26-21(31)15-30/h4-11,16,23,27,35H,3,12-15H2,1-2H3,(H,26,31)(H,28,33)(H,29,34)/t16-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469555

(CHEMBL4090716)Show SMILES C[C@@H](NCC(=O)N1CCNC(=O)C1)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N5O5/c1-17(27-15-22(32)30-14-13-26-21(31)16-30)23(25(34)29-35)28-24(33)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,23,27,35H,13-16H2,1H3,(H,26,31)(H,28,33)(H,29,34)/t17-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324308
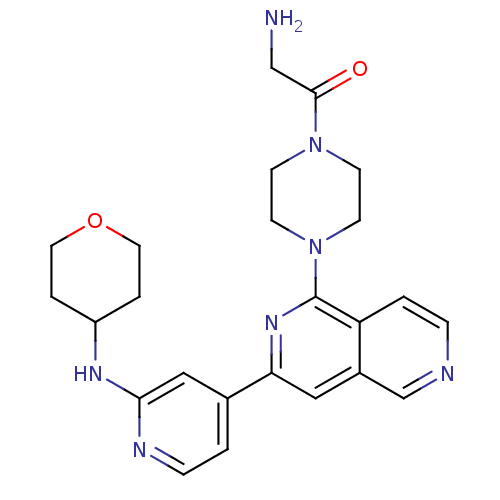
(2-Amino-1-(4-{3-[2-(tetrahydropyran-4-ylamino)pyri...)Show SMILES NCC(=O)N1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C24H29N7O2/c25-15-23(32)30-7-9-31(10-8-30)24-20-2-5-26-16-18(20)13-21(29-24)17-1-6-27-22(14-17)28-19-3-11-33-12-4-19/h1-2,5-6,13-14,16,19H,3-4,7-12,15,25H2,(H,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559339
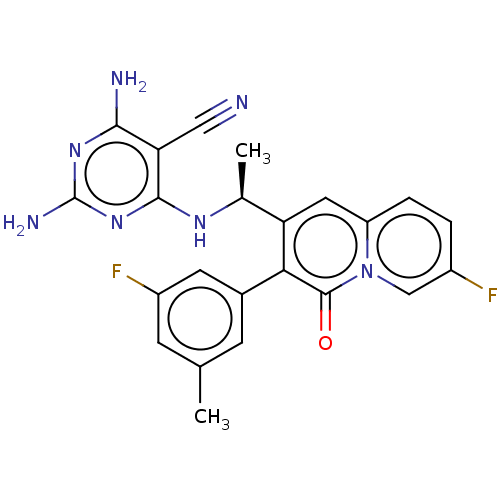
(CHEMBL4750109)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1cc2ccc(F)cn2c(=O)c1-c1cc(C)cc(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559319
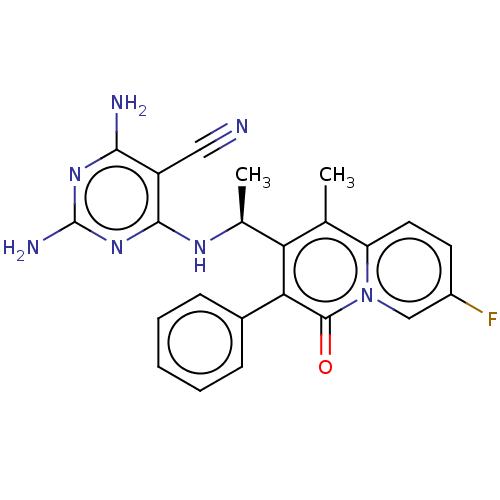
(CHEMBL4791717)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(C)c2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559318
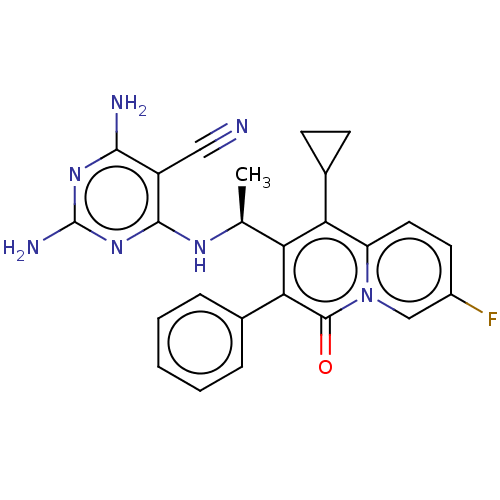
(CHEMBL4751779)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(C2CC2)c2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324293

(CHEMBL1215642 | Cyclohexyl-{4-[1-(4-isobutylpipera...)Show SMILES CC(C)CN1CCN(CC1)c1nc(cc2cnccc12)-c1ccnc(NC2CCCCC2)c1 Show InChI InChI=1S/C27H36N6/c1-20(2)19-32-12-14-33(15-13-32)27-24-9-10-28-18-22(24)16-25(31-27)21-8-11-29-26(17-21)30-23-6-4-3-5-7-23/h8-11,16-18,20,23H,3-7,12-15,19H2,1-2H3,(H,29,30) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559340
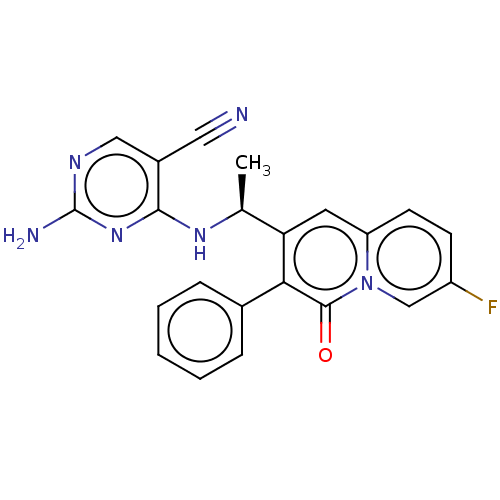
(CHEMBL4742973)Show SMILES C[C@H](Nc1nc(N)ncc1C#N)c1cc2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559316
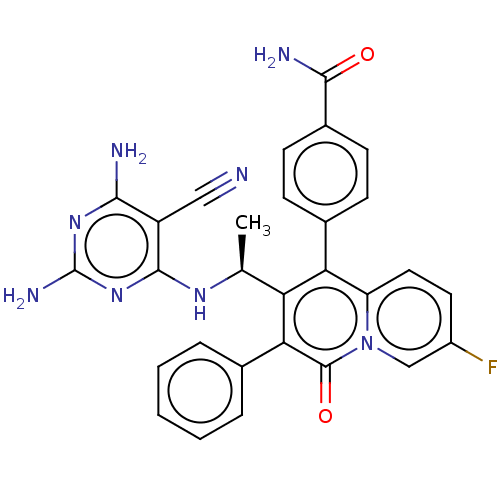
(CHEMBL4750646)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(-c2ccc(cc2)C(N)=O)c2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559333
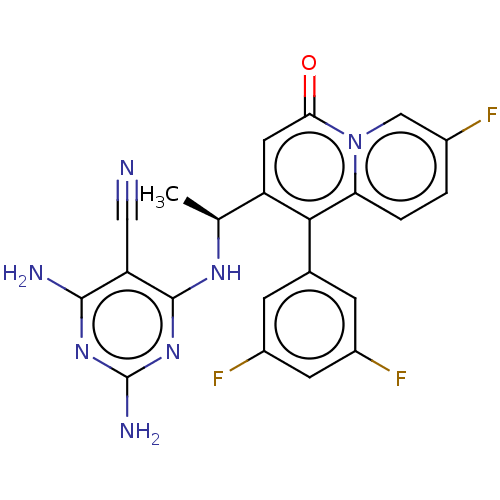
(CHEMBL4755417)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1cc(=O)n2cc(F)ccc2c1-c1cc(F)cc(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559325

(CHEMBL4756563)Show SMILES C[C@H](Nc1nc(N)nc(N)c1C#N)c1c(-c2ccc(F)cc2)c2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50559326
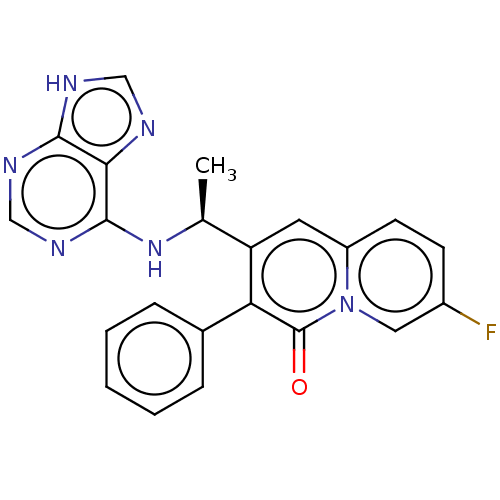
(CHEMBL4749877)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1cc2ccc(F)cn2c(=O)c1-c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3Kdelta in human Raji cells assessed as reduction in phosphorylation of AKT at 473 measured after 2 hr by alpha screen assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01264
BindingDB Entry DOI: 10.7270/Q2RN3CJV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324297

(4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...)Show SMILES C1CN(CCN1)c1nc(cc2cnccc12)-c1ccnc(NC2CCOCC2)c1 Show InChI InChI=1S/C22H26N6O/c1-6-25-21(26-18-3-11-29-12-4-18)14-16(1)20-13-17-15-24-5-2-19(17)22(27-20)28-9-7-23-8-10-28/h1-2,5-6,13-15,18,23H,3-4,7-12H2,(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 assessed as HDAC5 neuclear export |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324286

(1-(1-Piperazinyl)-3-(2-cyclohexylaminopyrid-4-yl)i...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc2ccccc2c(n1)N1CCNCC1 Show InChI InChI=1S/C24H29N5/c1-2-7-20(8-3-1)27-23-17-19(10-11-26-23)22-16-18-6-4-5-9-21(18)24(28-22)29-14-12-25-13-15-29/h4-6,9-11,16-17,20,25H,1-3,7-8,12-15H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5400-21 (2010)
Article DOI: 10.1021/jm100075z
BindingDB Entry DOI: 10.7270/Q2T153T9 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50469560
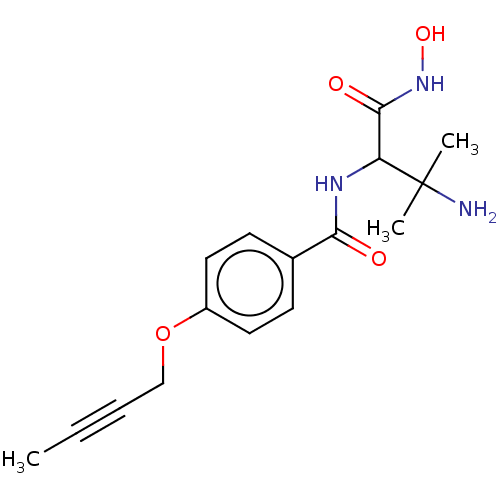
(CHEMBL4083624)Show InChI InChI=1S/C16H21N3O4/c1-4-5-10-23-12-8-6-11(7-9-12)14(20)18-13(15(21)19-22)16(2,3)17/h6-9,13,22H,10,17H2,1-3H3,(H,18,20)(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... |
J Med Chem 60: 5002-5014 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00377
BindingDB Entry DOI: 10.7270/Q2FN18MZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data