 Found 366 hits with Last Name = 'nishimura' and Initial = 'a'
Found 366 hits with Last Name = 'nishimura' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
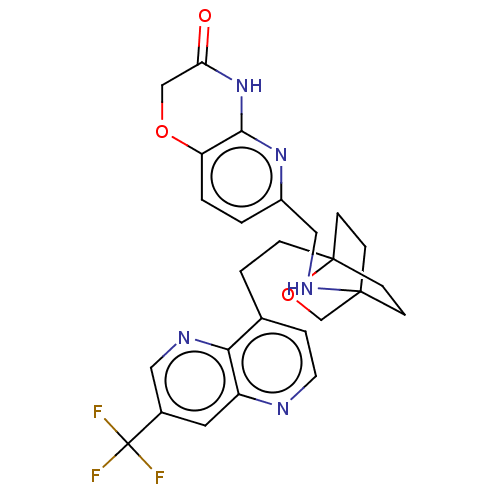
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426652
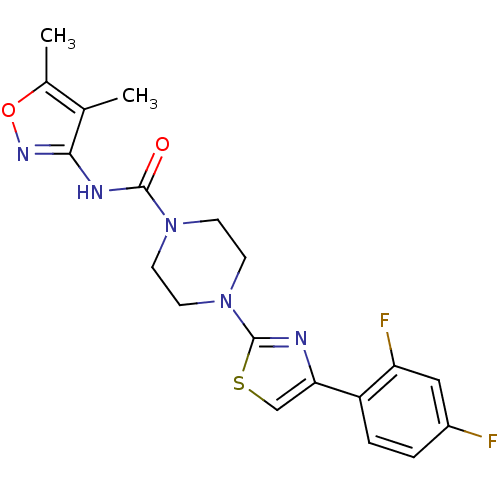
(CHEMBL2326178)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccc(F)cc2F)c1C Show InChI InChI=1S/C19H19F2N5O2S/c1-11-12(2)28-24-17(11)23-18(27)25-5-7-26(8-6-25)19-22-16(10-29-19)14-4-3-13(20)9-15(14)21/h3-4,9-10H,5-8H2,1-2H3,(H,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426652

(CHEMBL2326178)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccc(F)cc2F)c1C Show InChI InChI=1S/C19H19F2N5O2S/c1-11-12(2)28-24-17(11)23-18(27)25-5-7-26(8-6-25)19-22-16(10-29-19)14-4-3-13(20)9-15(14)21/h3-4,9-10H,5-8H2,1-2H3,(H,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426651
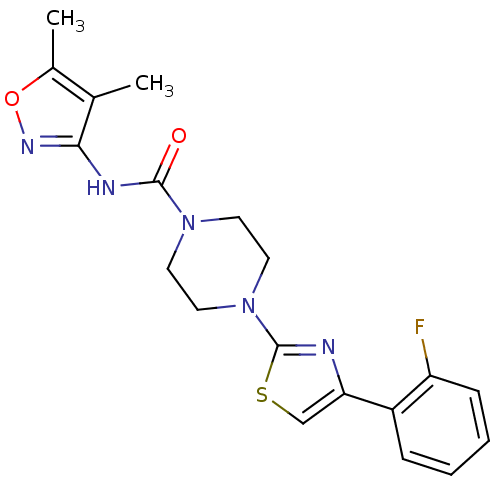
(CHEMBL2326194)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccccc2F)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-7-9-25(10-8-24)19-21-16(11-28-19)14-5-3-4-6-15(14)20/h3-6,11H,7-10H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426650
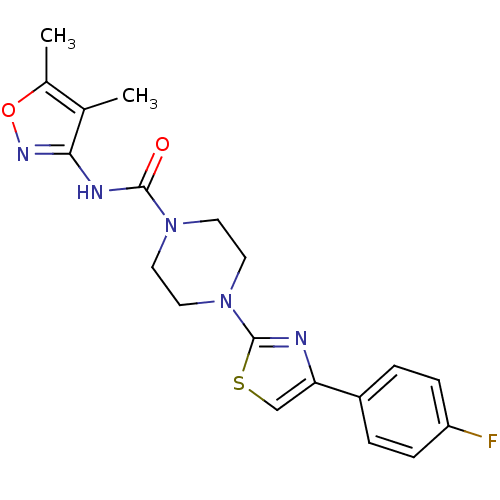
(CHEMBL2326177)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-7-9-25(10-8-24)19-21-16(11-28-19)14-3-5-15(20)6-4-14/h3-6,11H,7-10H2,1-2H3,(H,22,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
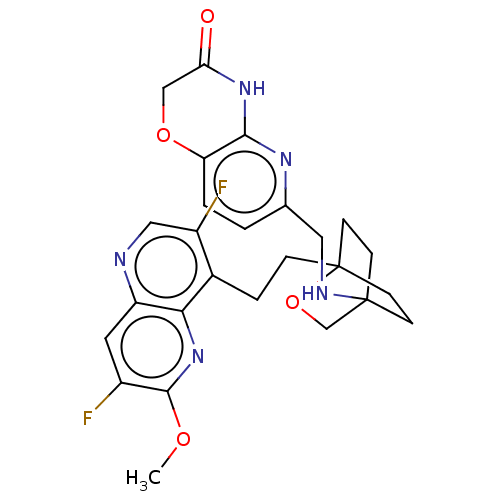
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067612
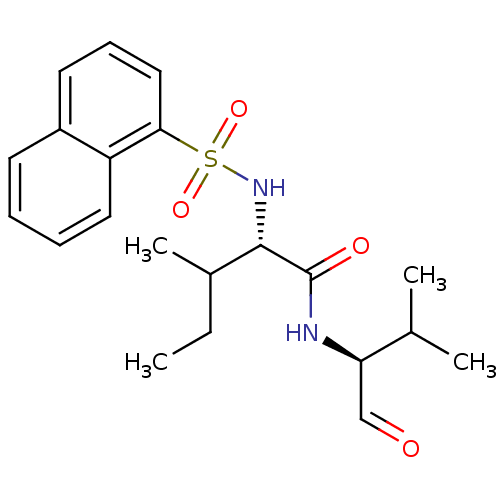
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](C=O)C(C)C Show InChI InChI=1S/C21H28N2O4S/c1-5-15(4)20(21(25)22-18(13-24)14(2)3)23-28(26,27)19-12-8-10-16-9-6-7-11-17(16)19/h6-15,18,20,23H,5H2,1-4H3,(H,22,25)/t15?,18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426651

(CHEMBL2326194)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccccc2F)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-7-9-25(10-8-24)19-21-16(11-28-19)14-5-3-4-6-15(14)20/h3-6,11H,7-10H2,1-2H3,(H,22,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426648
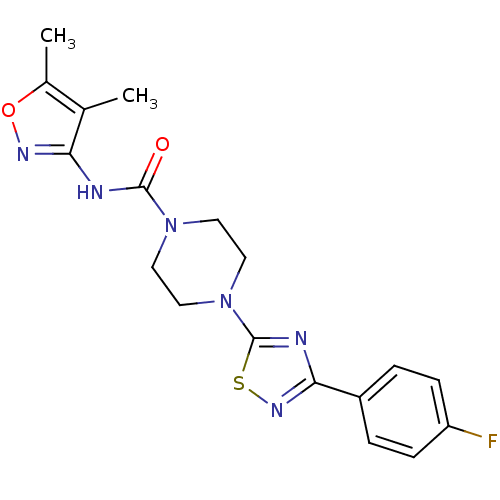
(CHEMBL2326197)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C18H19FN6O2S/c1-11-12(2)27-22-15(11)20-17(26)24-7-9-25(10-8-24)18-21-16(23-28-18)13-3-5-14(19)6-4-13/h3-6H,7-10H2,1-2H3,(H,20,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426650

(CHEMBL2326177)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-7-9-25(10-8-24)19-21-16(11-28-19)14-3-5-15(20)6-4-14/h3-6,11H,7-10H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
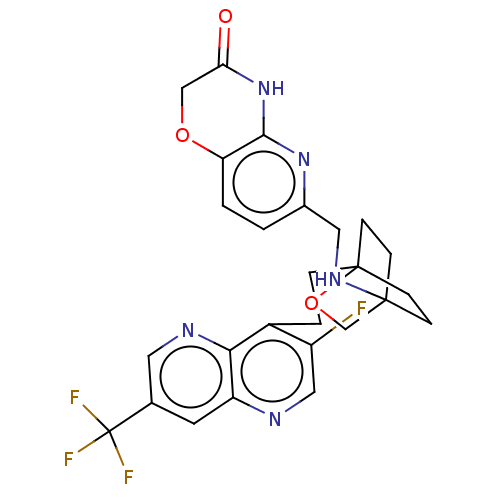
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426649
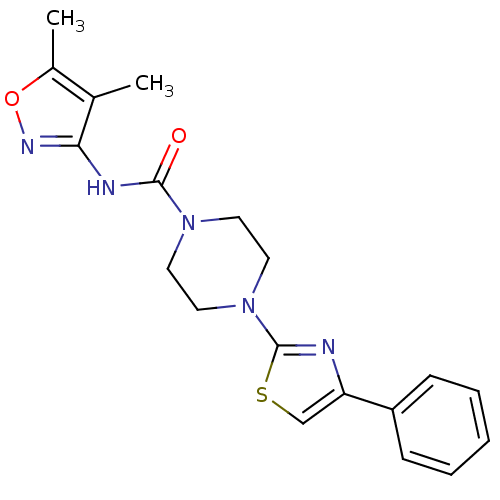
(CHEMBL2326192)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccccc2)c1C Show InChI InChI=1S/C19H21N5O2S/c1-13-14(2)26-22-17(13)21-18(25)23-8-10-24(11-9-23)19-20-16(12-27-19)15-6-4-3-5-7-15/h3-7,12H,8-11H2,1-2H3,(H,21,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067606
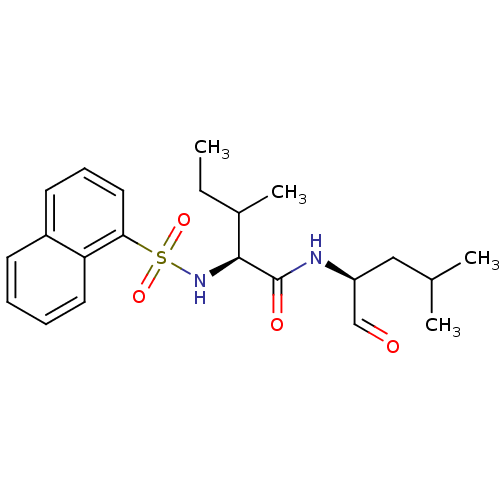
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](CC(C)C)C=O Show InChI InChI=1S/C22H30N2O4S/c1-5-16(4)21(22(26)23-18(14-25)13-15(2)3)24-29(27,28)20-12-8-10-17-9-6-7-11-19(17)20/h6-12,14-16,18,21,24H,5,13H2,1-4H3,(H,23,26)/t16?,18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067608
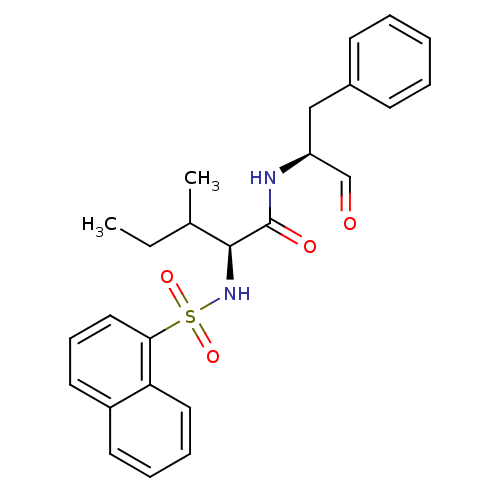
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C25H28N2O4S/c1-3-18(2)24(25(29)26-21(17-28)16-19-10-5-4-6-11-19)27-32(30,31)23-15-9-13-20-12-7-8-14-22(20)23/h4-15,17-18,21,24,27H,3,16H2,1-2H3,(H,26,29)/t18?,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426649

(CHEMBL2326192)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2ccccc2)c1C Show InChI InChI=1S/C19H21N5O2S/c1-13-14(2)26-22-17(13)21-18(25)23-8-10-24(11-9-23)19-20-16(12-27-19)15-6-4-3-5-7-15/h3-7,12H,8-11H2,1-2H3,(H,21,22,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067604

((S)-N-[(R)-1-Formyl-2-(1H-indol-3-yl)-ethyl]-3-met...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C26H27N3O4S/c1-17(2)25(29-34(32,33)24-13-7-9-18-8-3-4-11-22(18)24)26(31)28-20(16-30)14-19-15-27-23-12-6-5-10-21(19)23/h3-13,15-17,20,25,27,29H,14H2,1-2H3,(H,28,31)/t20-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082440

(CHEMBL3422977)Show SMILES FC(F)(F)c1ccc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,.93,;-4.02,1.54,;-4.02,2.78,;-5.09,2.16,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)20-4-2-18-22(33-20)16(6-12-30-18)5-7-25-10-8-24(9-11-25,15-37-25)31-13-17-1-3-19-23(32-17)34-21(35)14-36-19/h1-4,6,12,31H,5,7-11,13-15H2,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426647
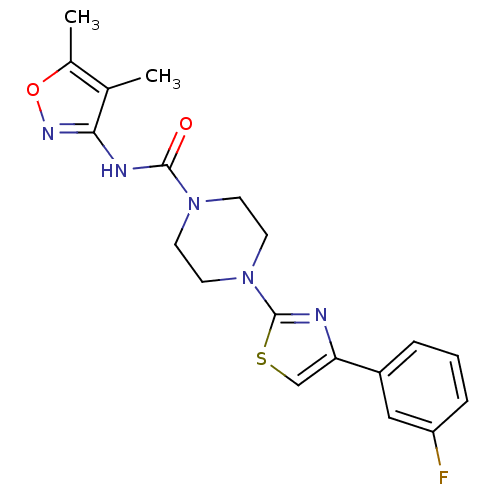
(CHEMBL2326196)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2cccc(F)c2)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-6-8-25(9-7-24)19-21-16(11-28-19)14-4-3-5-15(20)10-14/h3-5,10-11H,6-9H2,1-2H3,(H,22,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082382
(CHEMBL3422954)Show SMILES CCOc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,3.71,;-4.02,3.09,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-3-36-23-12-17(2)24-25(34-23)19(20(29)14-30-24)6-7-28-10-8-27(9-11-28,16-38-28)31-13-18-4-5-21-26(32-18)33-22(35)15-37-21/h4-5,12,14,31H,3,6-11,13,15-16H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082435
(CHEMBL3422976)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)25-19(28)13-20-23(33-25)17(6-12-29-20)5-7-27-10-8-26(9-11-27,16-37-27)30-14-18-3-4-21-24(31-18)32-22(35)15-36-21/h3-4,6,12-13,30H,5,7-11,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426645
(CHEMBL2326193)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccccc2F)c1C Show InChI InChI=1S/C18H19FN6O2S/c1-11-12(2)27-22-15(11)20-17(26)24-7-9-25(10-8-24)18-21-16(23-28-18)13-5-3-4-6-14(13)19/h3-6H,7-10H2,1-2H3,(H,20,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426648

(CHEMBL2326197)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C18H19FN6O2S/c1-11-12(2)27-22-15(11)20-17(26)24-7-9-25(10-8-24)18-21-16(23-28-18)13-3-5-14(19)6-4-13/h3-6H,7-10H2,1-2H3,(H,20,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426646
(CHEMBL2326189)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccccc2)c1C Show InChI InChI=1S/C18H20N6O2S/c1-12-13(2)26-21-15(12)19-17(25)23-8-10-24(11-9-23)18-20-16(22-27-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,19,21,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082390
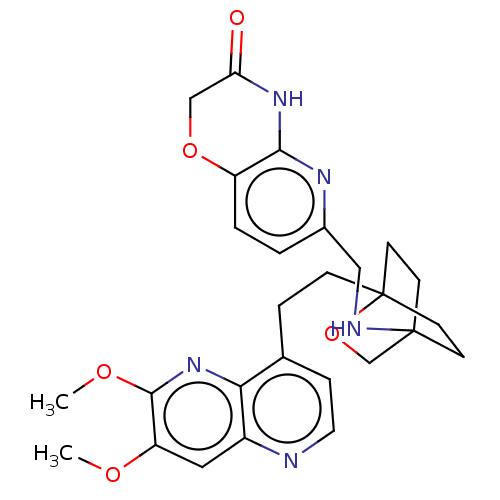
(CHEMBL3422964)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1OC |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5/c1-34-21-13-19-23(32-25(21)35-2)17(6-12-28-19)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-20-24(30-18)31-22(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426647

(CHEMBL2326196)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(cs2)-c2cccc(F)c2)c1C Show InChI InChI=1S/C19H20FN5O2S/c1-12-13(2)27-23-17(12)22-18(26)24-6-8-25(9-7-24)19-21-16(11-28-19)14-4-3-5-15(20)10-14/h3-5,10-11H,6-9H2,1-2H3,(H,22,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067613
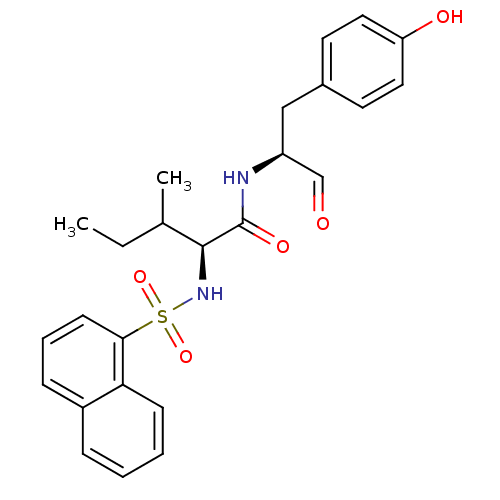
((S)-3-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CCC(C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C=O Show InChI InChI=1S/C25H28N2O5S/c1-3-17(2)24(25(30)26-20(16-28)15-18-11-13-21(29)14-12-18)27-33(31,32)23-10-6-8-19-7-4-5-9-22(19)23/h4-14,16-17,20,24,27,29H,3,15H2,1-2H3,(H,26,30)/t17?,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082394

(CHEMBL3422966)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-25-18(13-28)12-20-23(33-25)17(5-11-29-20)4-6-27-9-7-26(8-10-27,16-37-27)30-14-19-2-3-21-24(31-19)32-22(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082383

(CHEMBL3422957)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C27H27F4N5O4/c1-38-24-17(27(29,30)31)10-19-22(36-24)16(18(28)12-32-19)4-5-26-8-6-25(7-9-26,14-40-26)33-11-15-2-3-20-23(34-15)35-21(37)13-39-20/h2-3,10,12,33H,4-9,11,13-14H2,1H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426646

(CHEMBL2326189)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccccc2)c1C Show InChI InChI=1S/C18H20N6O2S/c1-12-13(2)26-21-15(12)19-17(25)23-8-10-24(11-9-23)18-20-16(22-27-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,19,21,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082388
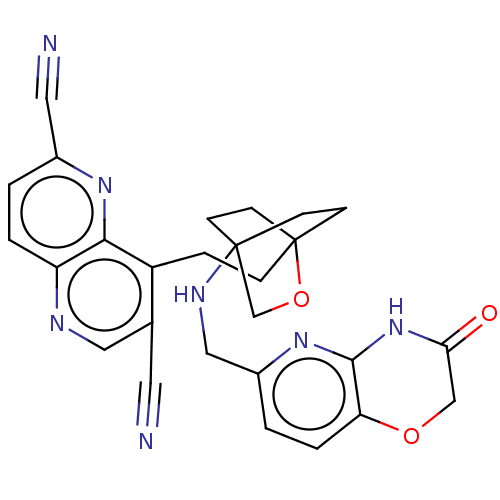
(CHEMBL3422962)Show SMILES O=C1COc2ccc(CNC34CCC(CCc5c(cnc6ccc(nc56)C#N)C#N)(CC3)OC4)nc2N1 |(10.96,15.37,;9.76,15.09,;8.71,16.22,;7.21,15.87,;6.76,14.39,;5.26,14.04,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.09,2.16,;4,1.54,;5.07,2.16,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,)| Show InChI InChI=1S/C27H25N7O3/c28-11-17-13-30-21-3-1-18(12-29)32-24(21)20(17)5-6-27-9-7-26(8-10-27,16-37-27)31-14-19-2-4-22-25(33-19)34-23(35)15-36-22/h1-4,13,31H,5-10,14-16H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082427
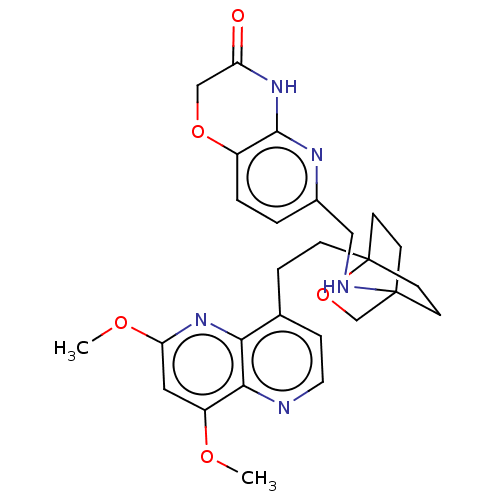
(CHEMBL3422968)Show SMILES COc1cc(OC)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-3.08,;-2.39,-3.71,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-20-13-22(35-2)32-23-17(6-12-28-24(20)23)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-19-25(30-18)31-21(33)15-36-19/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369397
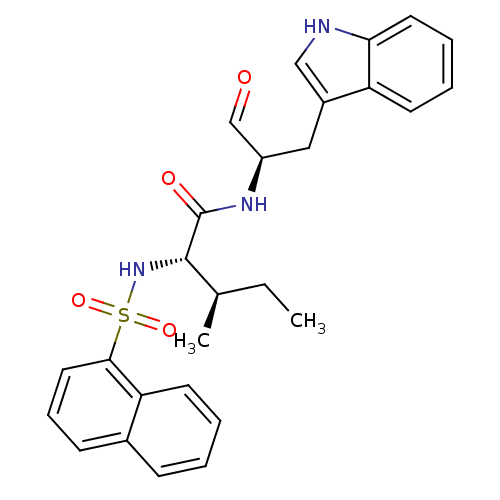
(CHEMBL1790993)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-10-19-9-4-5-12-23(19)25)27(32)29-21(17-31)15-20-16-28-24-13-7-6-11-22(20)24/h4-14,16-18,21,26,28,30H,3,15H2,1-2H3,(H,29,32)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082430
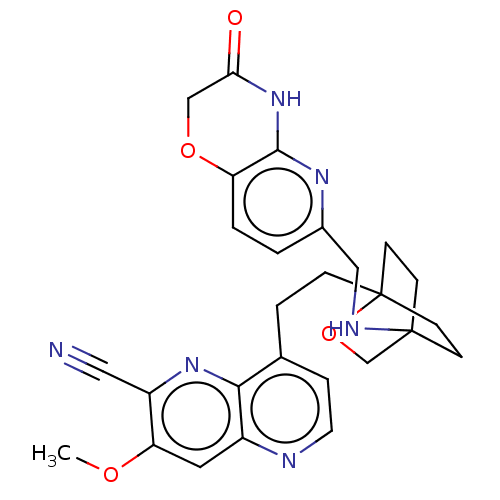
(CHEMBL3422971)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1C#N |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-22-12-19-24(32-20(22)13-28)17(5-11-29-19)4-6-27-9-7-26(8-10-27,16-37-27)30-14-18-2-3-21-25(31-18)33-23(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1 [30-579]
(Rattus norvegicus (rat)) | BDBM50426644
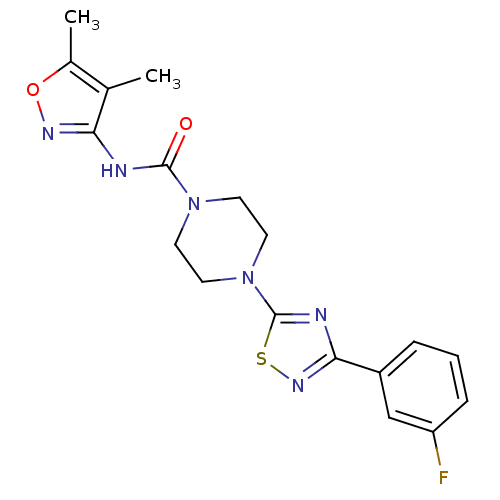
(CHEMBL2326195)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2cccc(F)c2)c1C Show InChI InChI=1S/C18H19FN6O2S/c1-11-12(2)27-22-15(11)20-17(26)24-6-8-25(9-7-24)18-21-16(23-28-18)13-4-3-5-14(19)10-13/h3-5,10H,6-9H2,1-2H3,(H,20,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of rat FAAH after 30 mins |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369397

(CHEMBL1790993)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-3-18(2)26(30-35(33,34)25-14-8-10-19-9-4-5-12-23(19)25)27(32)29-21(17-31)15-20-16-28-24-13-7-6-11-22(20)24/h4-14,16-18,21,26,28,30H,3,15H2,1-2H3,(H,29,32)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was measured for inhibition of collagenolytic of human Cathepsin L |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369410

(CHEMBL1790989)Show SMILES CC[C@@H](C)[C@H](NC(=O)Nc1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C28H30N4O3/c1-3-18(2)26(32-28(35)31-25-14-8-10-19-9-4-5-11-22(19)25)27(34)30-21(17-33)15-20-16-29-24-13-7-6-12-23(20)24/h4-14,16-18,21,26,29H,3,15H2,1-2H3,(H,30,34)(H2,31,32,35)/t18-,21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369405

(CHEMBL1790991)Show SMILES CC[C@@H](C)[C@H](NC(=O)Nc1ccccc1C(F)(F)F)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H27F3N4O3/c1-3-15(2)22(32-24(35)31-21-11-7-5-9-19(21)25(26,27)28)23(34)30-17(14-33)12-16-13-29-20-10-6-4-8-18(16)20/h4-11,13-15,17,22,29H,3,12H2,1-2H3,(H,30,34)(H2,31,32,35)/t15-,17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50426645

(CHEMBL2326193)Show SMILES Cc1onc(NC(=O)N2CCN(CC2)c2nc(ns2)-c2ccccc2F)c1C Show InChI InChI=1S/C18H19FN6O2S/c1-11-12(2)27-22-15(11)20-17(26)24-7-9-25(10-8-24)18-21-16(23-28-18)13-5-3-4-6-14(13)19/h3-6H,7-10H2,1-2H3,(H,20,22,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082432
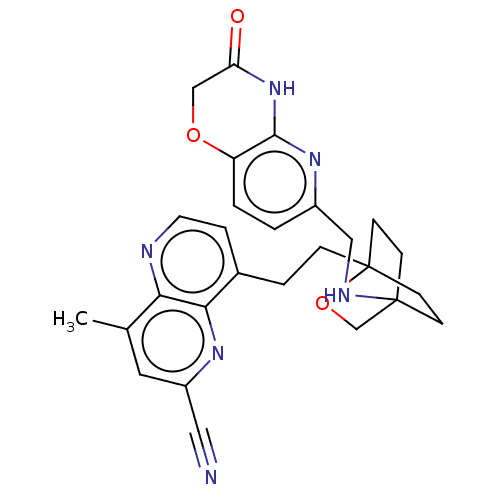
(CHEMBL3422973)Show SMILES Cc1cc(nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12)C#N |(-1.33,-2.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-12-20(13-28)31-24-18(5-11-29-23(17)24)4-6-27-9-7-26(8-10-27,16-36-27)30-14-19-2-3-21-25(32-19)33-22(34)15-35-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369402

(CHEMBL1790996)Show SMILES CC[C@@H](C)[C@H](NC(=O)c1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H27N3O3/c1-3-16(2)22(27-23(29)17-9-5-4-6-10-17)24(30)26-19(15-28)13-18-14-25-21-12-8-7-11-20(18)21/h4-12,14-16,19,22,25H,3,13H2,1-2H3,(H,26,30)(H,27,29)/t16-,19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50067597
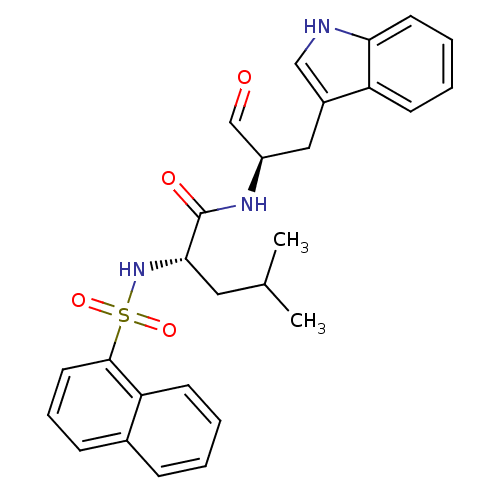
((S)-4-Methyl-2-(naphthalene-1-sulfonylamino)-penta...)Show SMILES CC(C)C[C@H](NS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C27H29N3O4S/c1-18(2)14-25(30-35(33,34)26-13-7-9-19-8-3-4-11-23(19)26)27(32)29-21(17-31)15-20-16-28-24-12-6-5-10-22(20)24/h3-13,16-18,21,25,28,30H,14-15H2,1-2H3,(H,29,32)/t21-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
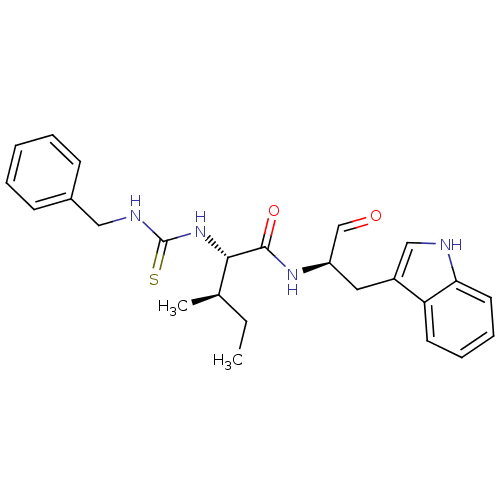
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369403
(CHEMBL1790995)Show SMILES CC[C@@H](C)[C@H](NC(=S)NCc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C25H30N4O2S/c1-3-17(2)23(29-25(32)27-14-18-9-5-4-6-10-18)24(31)28-20(16-30)13-19-15-26-22-12-8-7-11-21(19)22/h4-12,15-17,20,23,26H,3,13-14H2,1-2H3,(H,28,31)(H2,27,29,32)/t17-,20-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369400
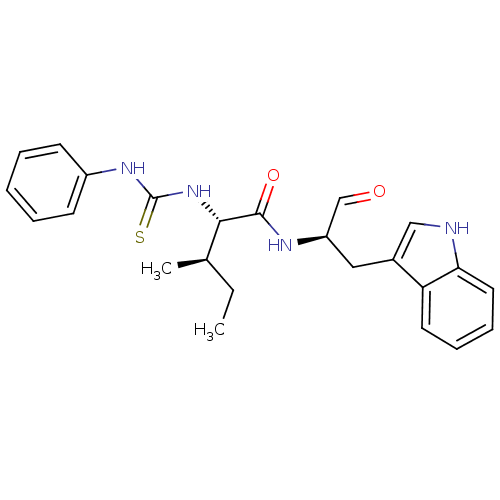
(CHEMBL1791000)Show SMILES CC[C@@H](C)[C@H](NC(=S)Nc1ccccc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H28N4O2S/c1-3-16(2)22(28-24(31)27-18-9-5-4-6-10-18)23(30)26-19(15-29)13-17-14-25-21-12-8-7-11-20(17)21/h4-12,14-16,19,22,25H,3,13H2,1-2H3,(H,26,30)(H2,27,28,31)/t16-,19-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50369398
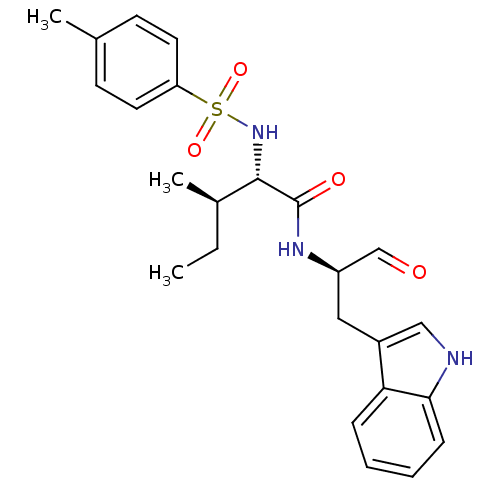
(CHEMBL1790999)Show SMILES CC[C@@H](C)[C@H](NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C=O Show InChI InChI=1S/C24H29N3O4S/c1-4-17(3)23(27-32(30,31)20-11-9-16(2)10-12-20)24(29)26-19(15-28)13-18-14-25-22-8-6-5-7-21(18)22/h5-12,14-15,17,19,23,25,27H,4,13H2,1-3H3,(H,26,29)/t17-,19-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L. |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082434

(CHEMBL3422975)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C28H31F3N6O3/c1-37(2)25-19(28(29,30)31)13-20-23(36-25)17(6-12-32-20)5-7-27-10-8-26(9-11-27,16-40-27)33-14-18-3-4-21-24(34-18)35-22(38)15-39-21/h3-4,6,12-13,33H,5,7-11,14-16H2,1-2H3,(H,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50244993
(4-(3-phenyl-1,2,4-thiadiazol-5-yl)-N-(pyridin-3-yl...)Show InChI InChI=1S/C18H18N6OS/c25-17(20-15-7-4-8-19-13-15)23-9-11-24(12-10-23)18-21-16(22-26-18)14-5-2-1-3-6-14/h1-8,13H,9-12H2,(H,20,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Apparent inhibition of human FAAH expressed in CHO-K1 cells using ethanolamine 1-3[H] as substrate after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 28-41 (2012)
Article DOI: 10.1016/j.bmc.2012.11.006
BindingDB Entry DOI: 10.7270/Q2FN17H6 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50286441

((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C20H38N6O5/c1-11(2)9-15(24-13(5)27)17(28)26-16(10-12(3)4)18(29)25-14(19(30)31)7-6-8-23-20(21)22/h11-12,14-16H,6-10H2,1-5H3,(H,24,27)(H,25,29)(H,26,28)(H,30,31)(H4,21,22,23)/t14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Compound was measured for inhibition of collagenolytic of human Cathepsin L |
J Med Chem 41: 4301-8 (1998)
Article DOI: 10.1021/jm9803065
BindingDB Entry DOI: 10.7270/Q2VQ33CX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data