

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4696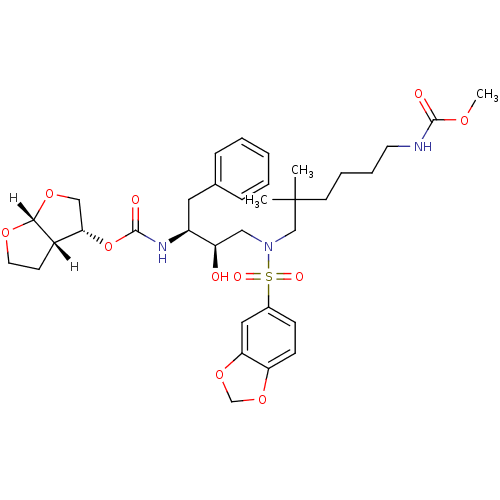 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000140 | -80.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4699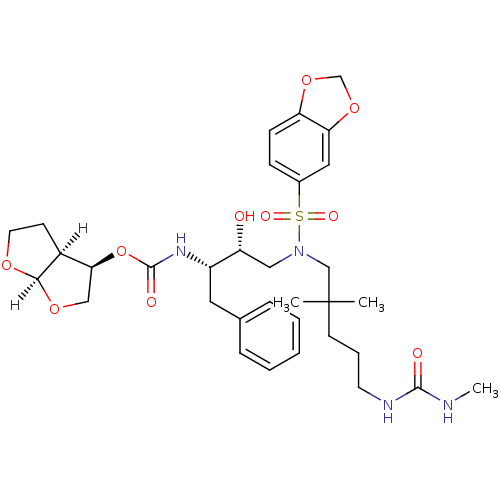 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000480 | -77.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4698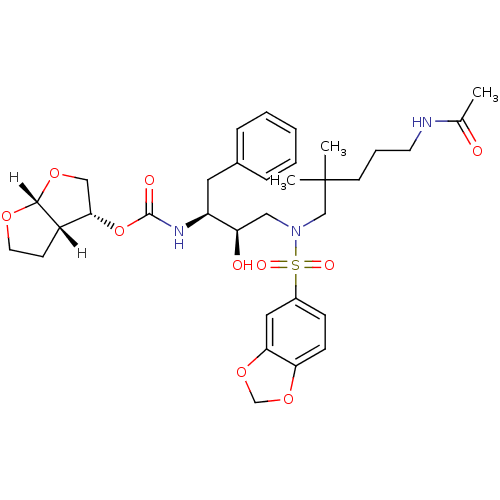 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000540 | -77.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4700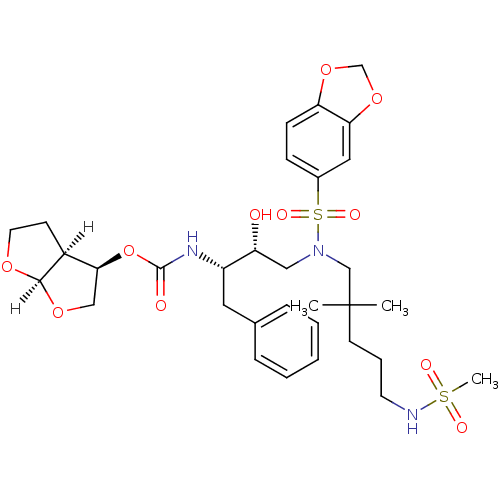 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000580 | -76.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4691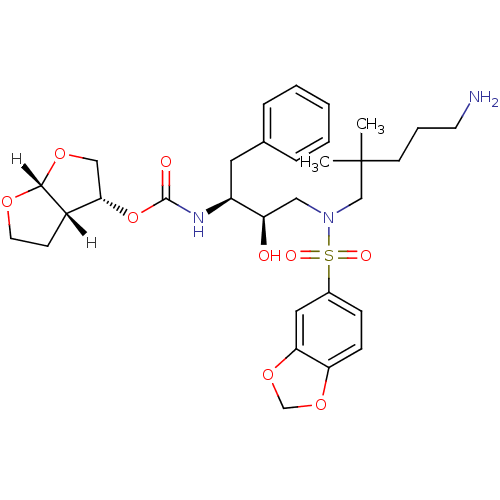 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0000610 | -76.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4697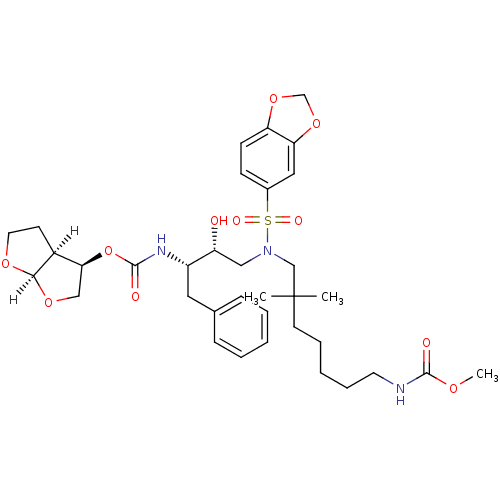 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000120 | -75.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4695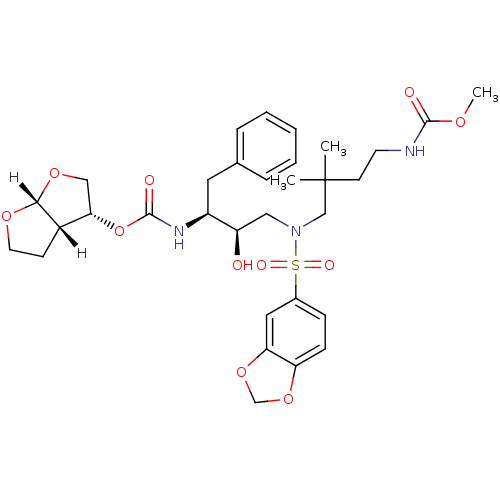 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000160 | -74.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4693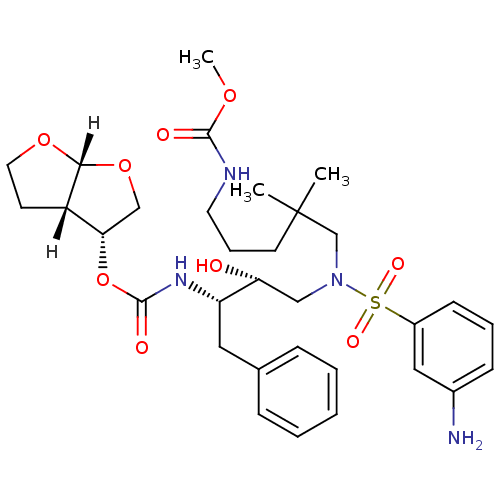 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000210 | -73.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4692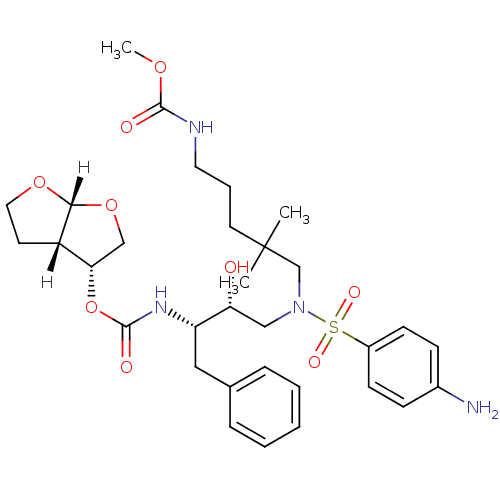 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000260 | -73.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4694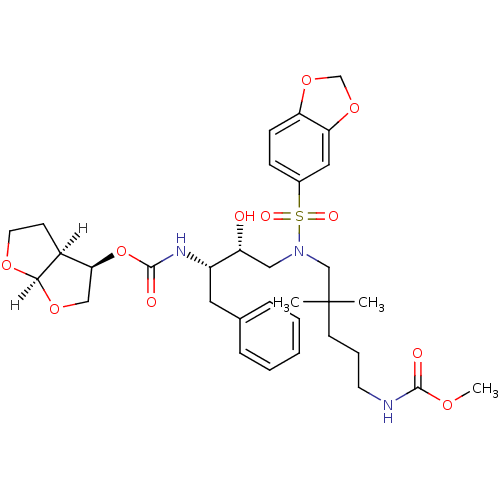 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000520 | -71.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50545673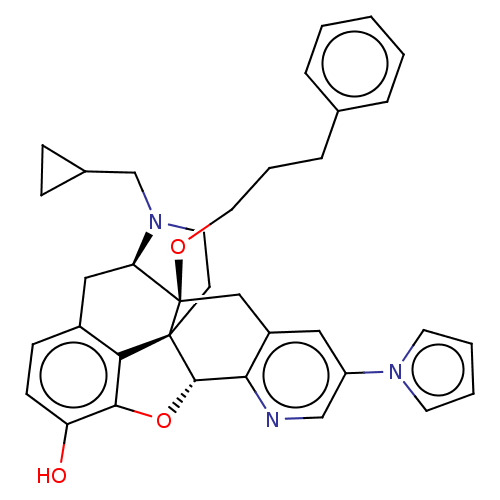 (CHEMBL4634079) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35226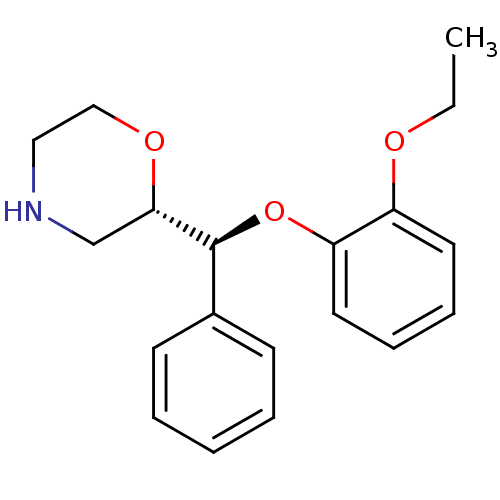 ((S,S)-reboxetine | Reboxetine | Vestra) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103072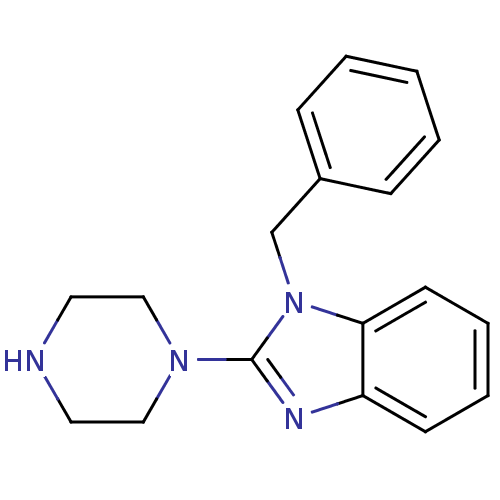 (1-Benzyl-2-piperazin-1-yl-1H-benzoimidazole | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50187673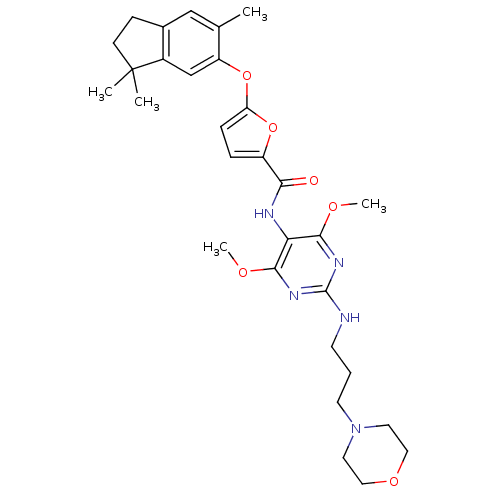 (5-(3,3,6-trimethyl-indan-5-yloxy)-furan-2-carboxyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to rat pituitary GnRH receptor | J Med Chem 49: 3362-7 (2006) Article DOI: 10.1021/jm060012g BindingDB Entry DOI: 10.7270/Q2F76C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50187673 (5-(3,3,6-trimethyl-indan-5-yloxy)-furan-2-carboxyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Binding affinity to human recombinant GnRH receptor | J Med Chem 49: 3362-7 (2006) Article DOI: 10.1021/jm060012g BindingDB Entry DOI: 10.7270/Q2F76C54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368883 (CHEMBL1159458) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103070 (1-(4-Methoxy-benzyl)-2-piperazin-1-yl-1H-benzoimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Sus scrofa) | BDBM50201438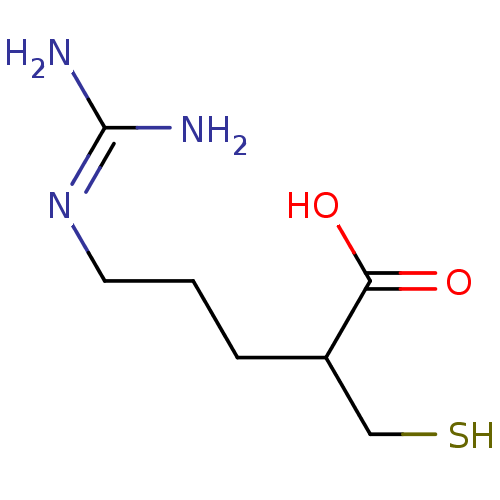 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of porcine pancreatic carboxypeptidase B | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50533716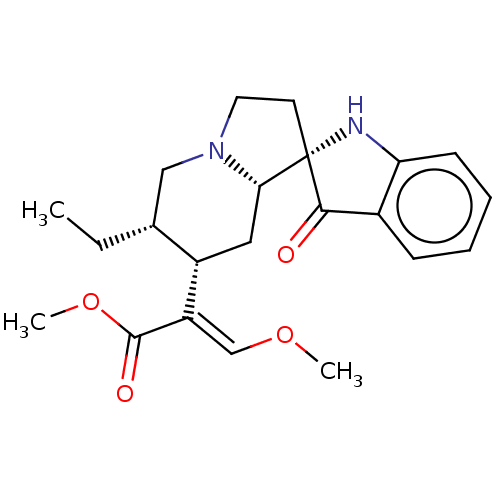 (CHEMBL4453504) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35255 (2-Chlor-11-(2-dimethylaminoaethoxy)-dibenzo(b,f)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] ketanserin binding to membranes from CHO cells, stably transfected with the human 5-HT2A receptor. Da... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50533717 (CHEMBL4449284) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50545677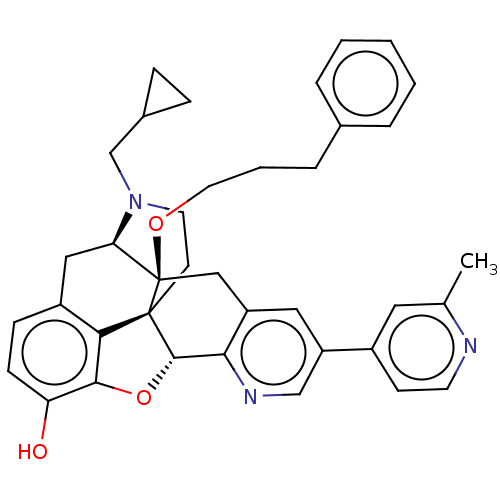 (CHEMBL4645508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50133231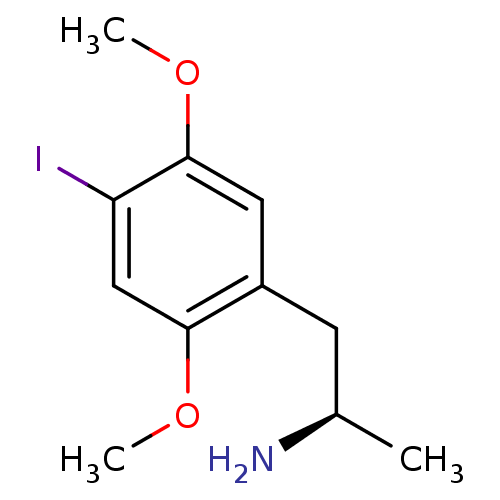 ((R)-1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2A receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50474150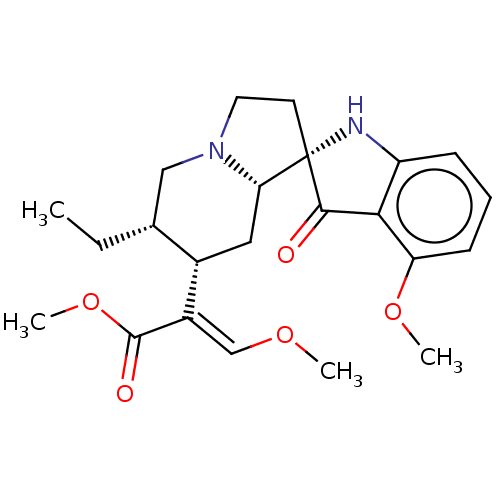 (CHEMBL58362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50528969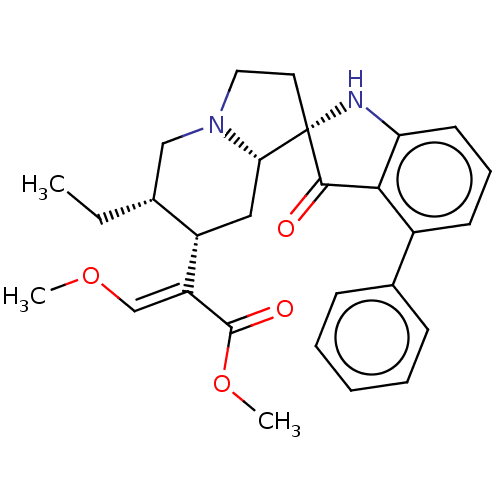 (CHEMBL4458337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50528969 (CHEMBL4458337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50533719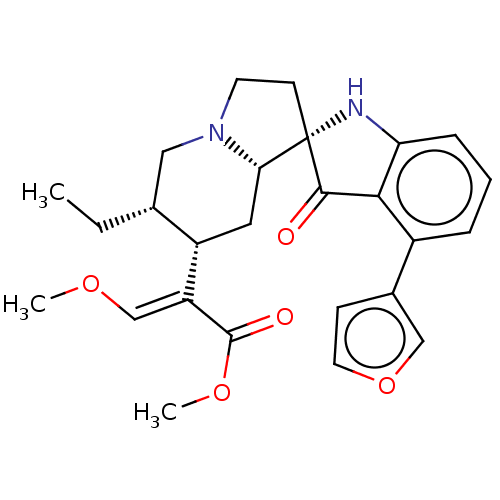 (CHEMBL4441457) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50180258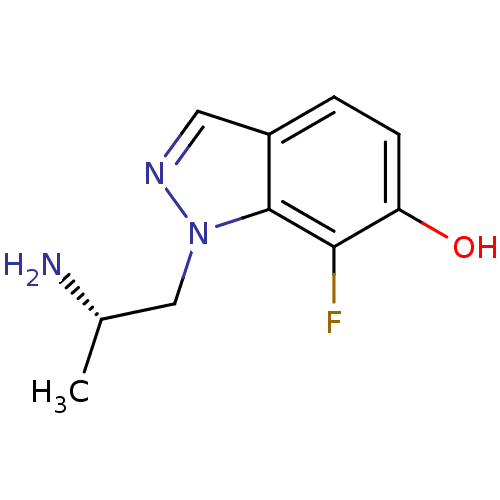 (1-((S)-2-aminopropyl)-7-fluoro-1H-indazol-6-ol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2C receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039276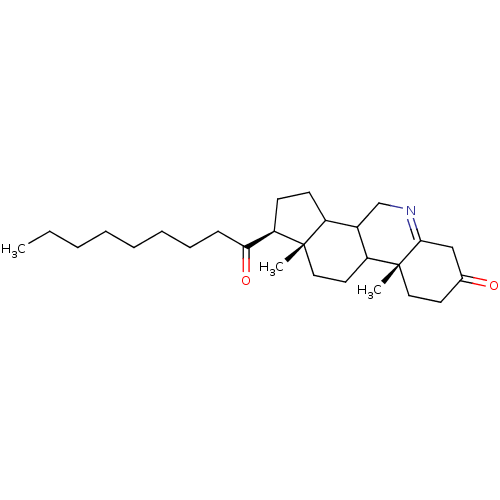 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-nonanoyl-1,2,3,3a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039277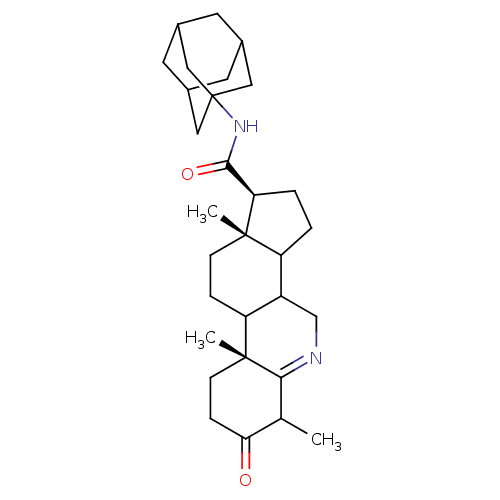 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50545677 (CHEMBL4645508) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bindin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50180255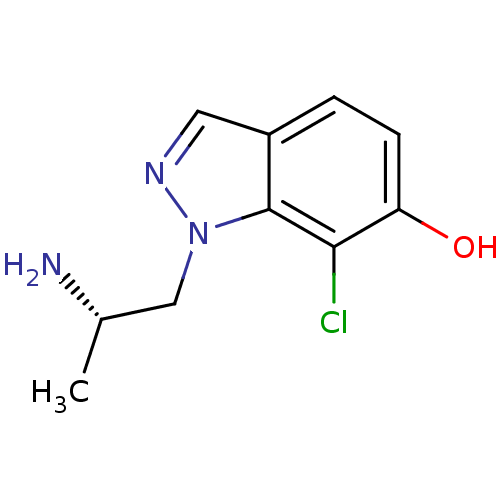 (1-((S)-2-aminopropyl)-7-chloro-1H-indazol-6-ol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2C receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103076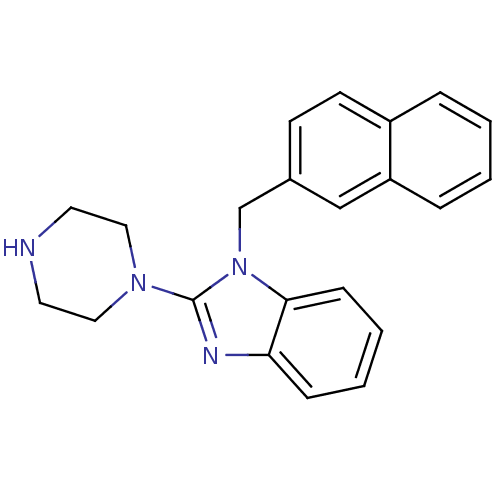 (1-Naphthalen-2-ylmethyl-2-piperazin-1-yl-1H-benzoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50533718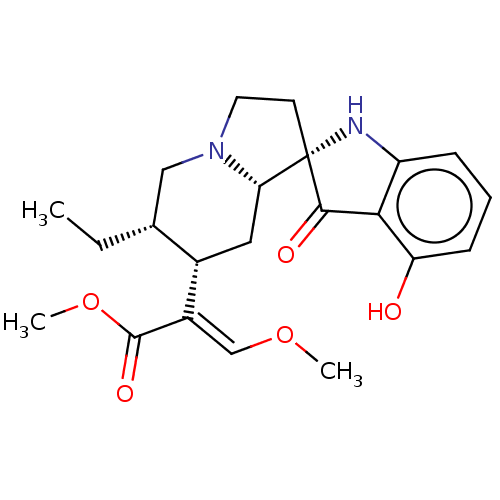 (CHEMBL4530421) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50180252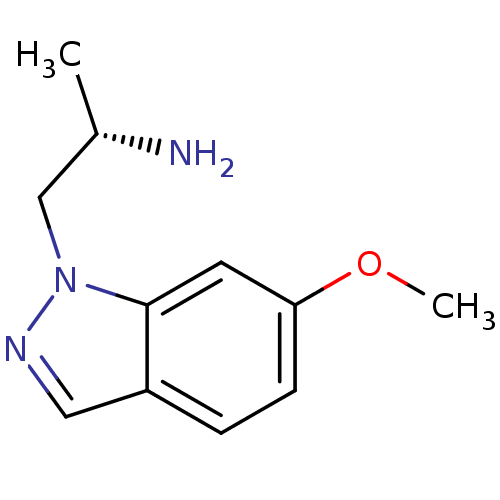 ((2S)-1-(6-methoxy-1H-indazol-1-yl)propan-2-amine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2C receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50533719 (CHEMBL4441457) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50121472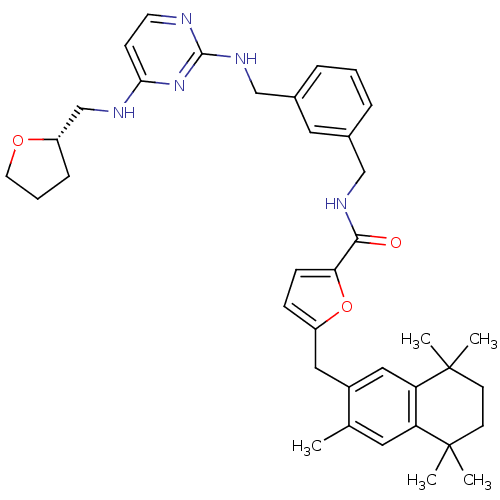 (5-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-La Jolla/Agouron Pharmaceuticals Curated by ChEMBL | Assay Description In vitro binding affinity towards rat gonadotropin-releasing hormone receptor | Bioorg Med Chem Lett 12: 3635-9 (2002) BindingDB Entry DOI: 10.7270/Q2XK8DXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50180258 (1-((S)-2-aminopropyl)-7-fluoro-1H-indazol-6-ol | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2B receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50103075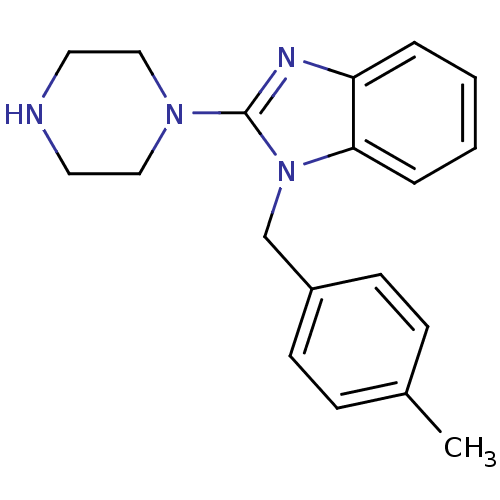 (1-(4-Methyl-benzyl)-2-piperazin-1-yl-1H-benzoimida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Louisiana at Monroe Curated by ChEMBL | Assay Description Binding affinity of the compound against human 5-hydroxytryptamine 3A receptor | Bioorg Med Chem Lett 11: 2133-6 (2001) BindingDB Entry DOI: 10.7270/Q20G3JGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50180253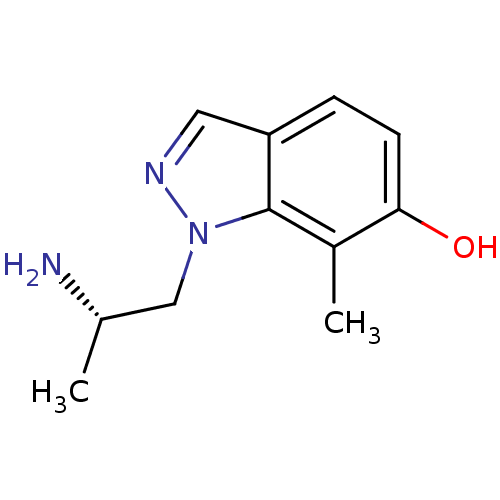 (1-((S)-2-aminopropyl)-7-methyl-1H-indazol-6-ol | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alcon Research, Ltd. Curated by ChEMBL | Assay Description Displacement of [125I]DOI from cloned human 5HT2A receptor expressed in CHO cells | J Med Chem 49: 318-28 (2006) Article DOI: 10.1021/jm050663x BindingDB Entry DOI: 10.7270/Q23B5ZQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase N catalytic chain (Homo sapiens (Human)) | BDBM50201438 ((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human plasma carboxypeptidase N | J Med Chem 50: 6095-103 (2007) Article DOI: 10.1021/jm0702433 BindingDB Entry DOI: 10.7270/Q2T153CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in MDCK-Net6 cells | J Med Chem 53: 4511-21 (2010) Article DOI: 10.1021/jm100053t BindingDB Entry DOI: 10.7270/Q2PC32JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in MDCK-Net6 cells | J Med Chem 54: 6824-31 (2011) Article DOI: 10.1021/jm200733r BindingDB Entry DOI: 10.7270/Q2XD1227 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human norepinephrine transporter expressed in MDCK-Net6 cells | J Med Chem 51: 4038-49 (2008) Article DOI: 10.1021/jm8002262 BindingDB Entry DOI: 10.7270/Q2TB16PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50398760 (CHEMBL2179652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50545658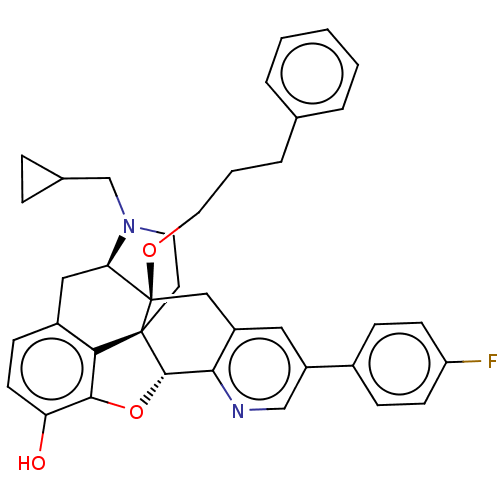 (CHEMBL4644543) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by competition radioligand bindin... | J Med Chem 63: 7663-7694 (2020) Article DOI: 10.1021/acs.jmedchem.0c00503 BindingDB Entry DOI: 10.7270/Q2B56P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50533717 (CHEMBL4449284) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse delta opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 7 incubated for 1 hr prior to testing measured for 10 to 100 secs by phenol red-based stopped-flow... | J Med Chem 63: 321-333 (2020) Article DOI: 10.1021/acs.jmedchem.9b01669 BindingDB Entry DOI: 10.7270/Q2TX3JR0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50533720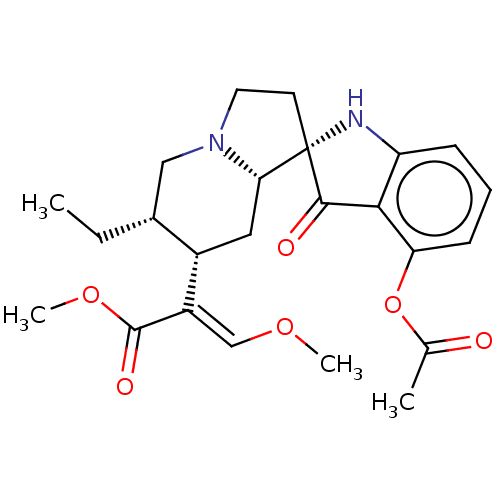 (CHEMBL4557905) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan Kettering Cancer Center Curated by ChEMBL | Assay Description Displacement of [125I]-IBNtxA from mouse mu opioid receptor-1 expressed in CHO cell membranes incubated for 90 mins | J Med Chem 59: 8381-97 (2016) Article DOI: 10.1021/acs.jmedchem.6b00748 BindingDB Entry DOI: 10.7270/Q27H1P2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5507 total ) | Next | Last >> |