

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM24722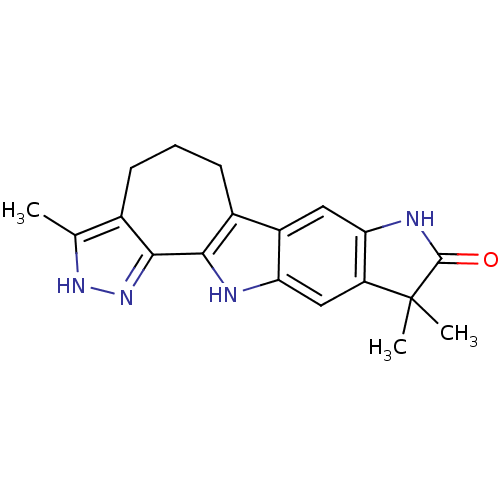 (7,7,16-trimethyl-5,11,14,15-tetraazapentacyclo[10....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 51: 4465-75 (2008) Article DOI: 10.1021/jm800052b BindingDB Entry DOI: 10.7270/Q2H70D4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM24722 (7,7,16-trimethyl-5,11,14,15-tetraazapentacyclo[10....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 51: 4465-75 (2008) Article DOI: 10.1021/jm800052b BindingDB Entry DOI: 10.7270/Q2H70D4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195608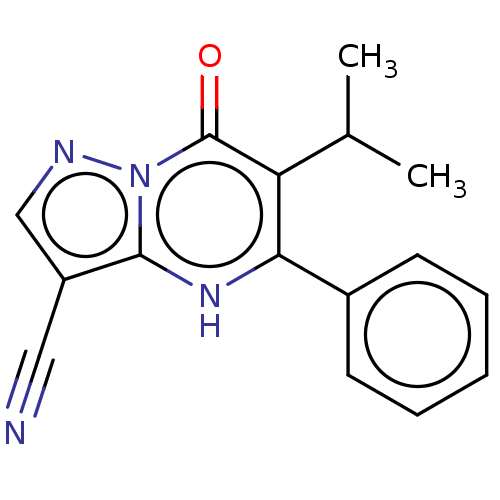 (CPI-455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genentech Inc | Assay Description Time-resolved fluorescence resonance energy transfer (TR-FRET) assays were carried out using full-length KDM5 enzymes in 384-well black ProxiPlates (... | Nat Chem Biol 12: 531-8 (2016) Article DOI: 10.1038/nchembio.2085 BindingDB Entry DOI: 10.7270/Q2CF9NXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31837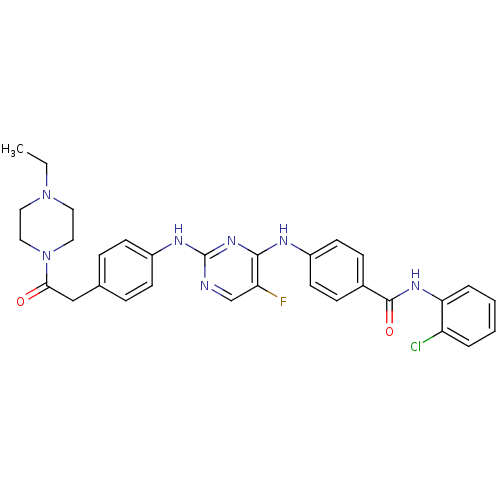 (2,4-Bisanilinopyrimidine, 10 | Aurora Inhibitor, 3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503407 (CHEMBL4440829) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM24721 (5-ethyl-7,7,16-trimethyl-5,11,14,15-tetraazapentac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 51: 4465-75 (2008) Article DOI: 10.1021/jm800052b BindingDB Entry DOI: 10.7270/Q2H70D4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31836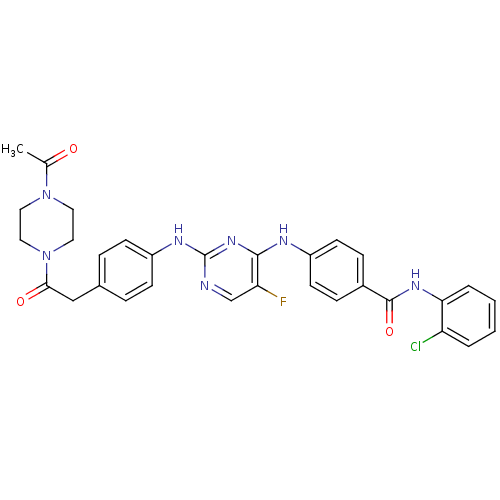 (2,4-Bisanilinopyrimidine, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM24721 (5-ethyl-7,7,16-trimethyl-5,11,14,15-tetraazapentac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 51: 4465-75 (2008) Article DOI: 10.1021/jm800052b BindingDB Entry DOI: 10.7270/Q2H70D4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321424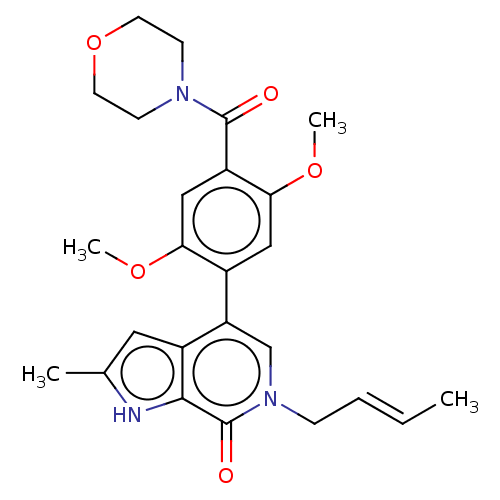 (6-[(E)-but-2-enyl]-4-[2,5-dimethoxy-4-(morpholine-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321411 (6-[(E)-but-2-enyl]-4-[3-methoxy-4-(morpholine-4-ca...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321463 (6-(but-3-en-1-yl)-4-(3-(difluoromethoxy)-5-(morpho...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31833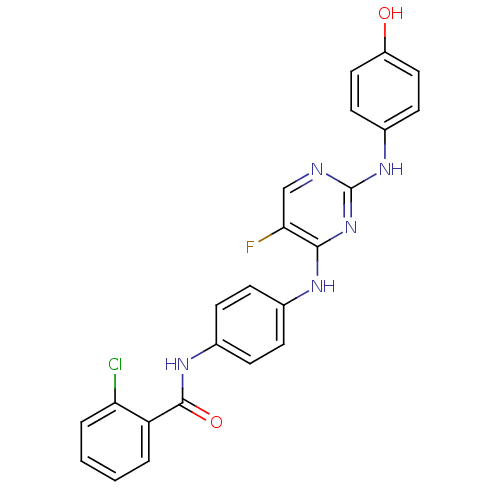 (2,4-Bisanilinopyrimidine, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503399 (CHEMBL4441257) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 51: 4465-75 (2008) Article DOI: 10.1021/jm800052b BindingDB Entry DOI: 10.7270/Q2H70D4F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321467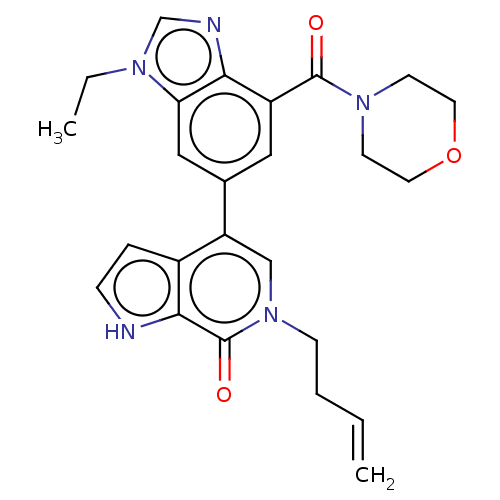 (6-but-3-enyl-4-[3-ethyl-7-(morpholine-4-carbonyl)b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503413 (CHEMBL4566599) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321412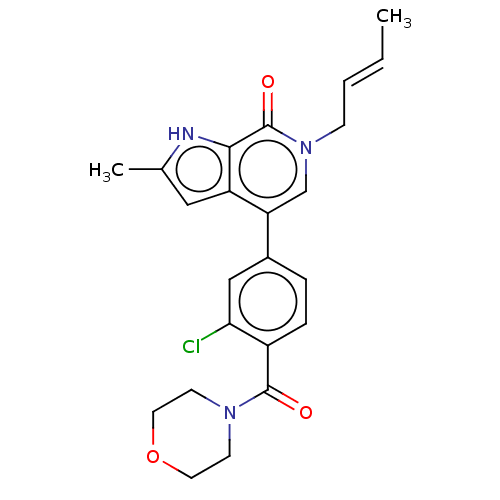 (6-[(E)-but-2-enyl]-4-[3-chloro-4-(morpholine-4-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321437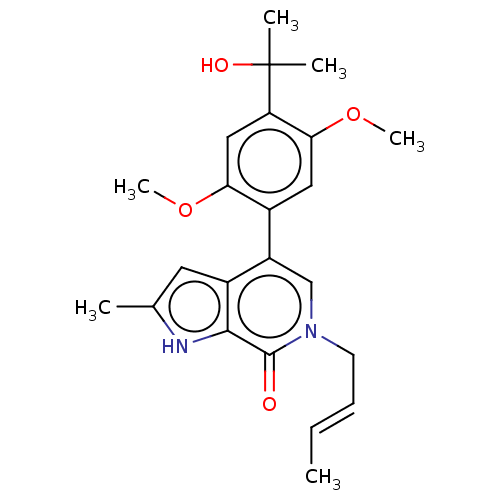 ((E)-6-(but-2-en-1-yl)-4-(4-(2-hydroxypropan-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31858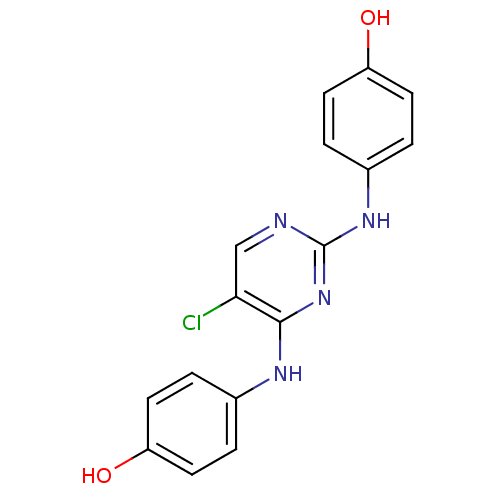 (2,4-Bisanilinopyrimidine, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321422 (5-[6-[(E)-but-2-enyl]-2-methyl-7-oxo-1H-pyrrolo[2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein polybromo-1 (Homo sapiens (Human)) | BDBM394583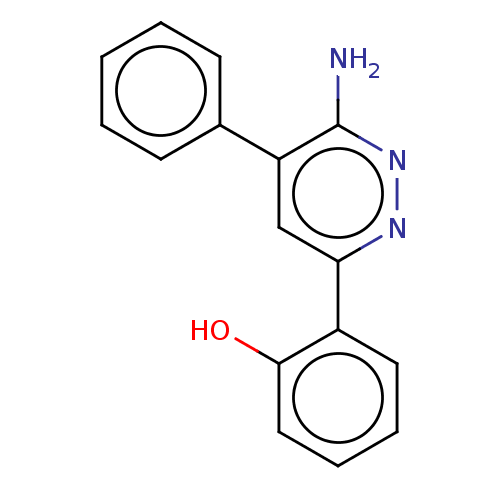 (2-(6-amino-5-phenylpyridazin-3-yl)phenol | US10308...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00662 BindingDB Entry DOI: 10.7270/Q2TB1BX4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321415 (6-[(E)-but-2-enyl]-2-methyl-4-[3-methyl-4-(morphol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31842 (2,4-Bisanilinopyrimidine, 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503406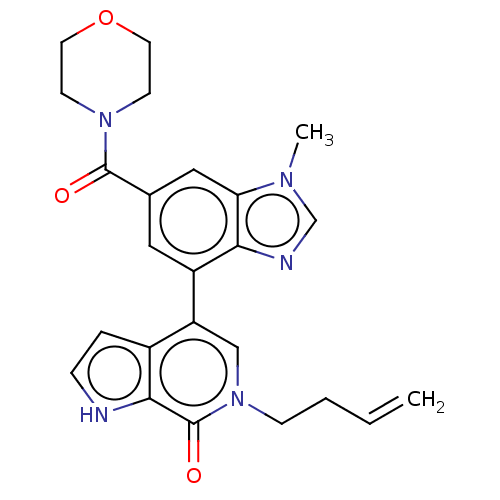 (CHEMBL4463538) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321462 (6-but-3-enyl-4-[4-fluoro-3-(morpholine-4-carbonyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM195608 (CPI-455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genentech Inc | Assay Description Time-resolved fluorescence resonance energy transfer (TR-FRET) assays were carried out using full-length KDM5 enzymes in 384-well black ProxiPlates (... | Nat Chem Biol 12: 531-8 (2016) Article DOI: 10.1038/nchembio.2085 BindingDB Entry DOI: 10.7270/Q2CF9NXW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31834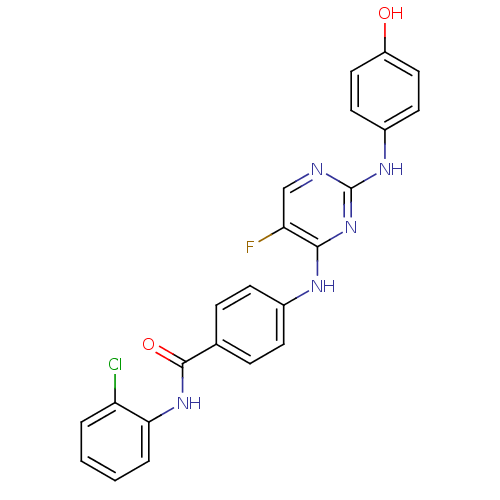 (2,4-Bisanilinopyrimidine, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein/Histone acetyltransferase p300 (Homo sapiens (Human)) | BDBM50200375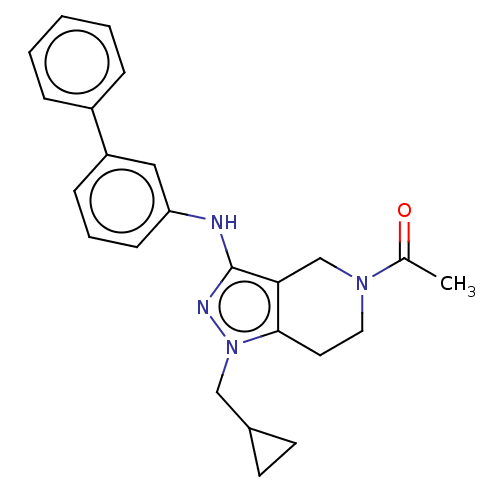 (CHEMBL3902514) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of biotinylated ligand from recombinant His-tagged CBP/EP300 (unknown origin) measured after 10 mins by TR-FRET assay | J Med Chem 59: 10549-10563 (2016) Article DOI: 10.1021/acs.jmedchem.6b01022 BindingDB Entry DOI: 10.7270/Q27083DF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503404 (CHEMBL4455513) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321458 (6-but-3-enyl-4-[3,4-difluoro-5-(morpholine-4-carbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM321407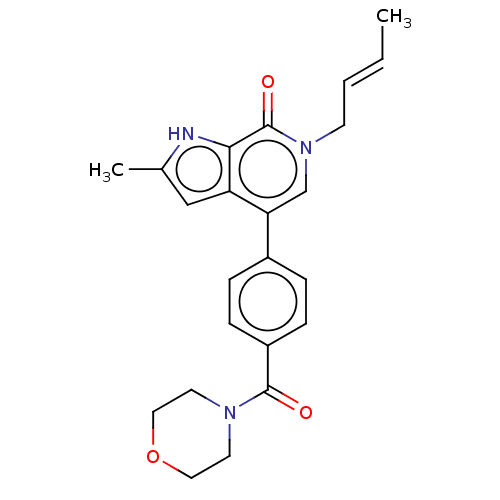 (6-[(E)-but-2-enyl]-2-methyl-4-[4-(morpholine-4-car...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31841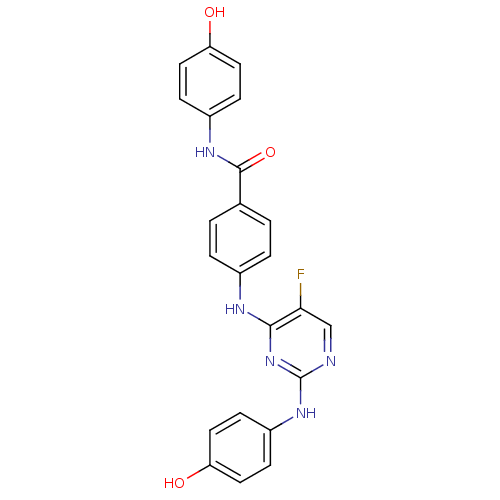 (2,4-Bisanilinopyrimidine, 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503408 (CHEMBL4459660) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31848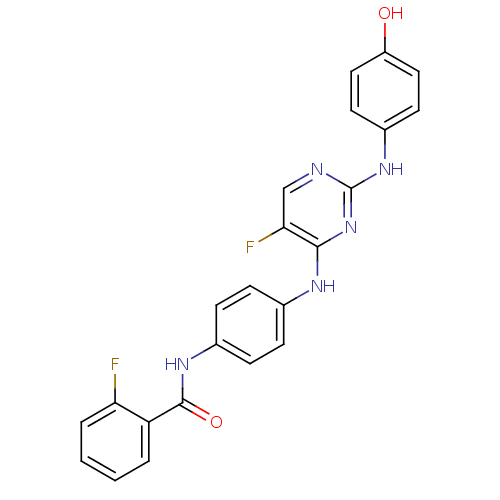 (2,4-Bisanilinopyrimidine, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM195608 (CPI-455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genentech Inc | Assay Description Time-resolved fluorescence resonance energy transfer (TR-FRET) assays were carried out using full-length KDM5 enzymes in 384-well black ProxiPlates (... | Nat Chem Biol 12: 531-8 (2016) Article DOI: 10.1038/nchembio.2085 BindingDB Entry DOI: 10.7270/Q2CF9NXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31876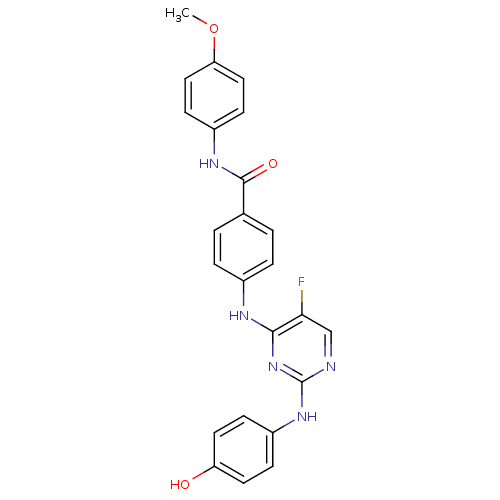 (2,4-Bisanilinopyrimidine, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31859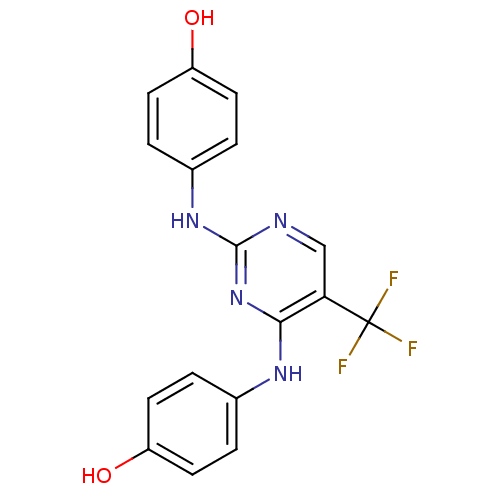 (2,4-Bisanilinopyrimidine, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321460 (3-(6-but-3-enyl-7-oxo-1H-pyrrolo[2,3-c]pyridin-4-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chromatin remodeling regulator CECR2 (Homo sapiens (Human)) | BDBM50269687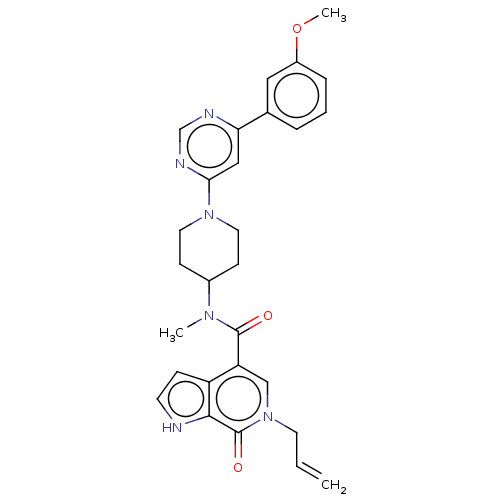 (CHEMBL4073178) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc., 1 DNA Way, South San Francisco, California 94080, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-FLAG-tagged CECR2 expressed in Rosetta2 (DE3) pLysS cells after 60 mins by TR-FRET assay | ACS Med Chem Lett 8: 737-741 (2017) Article DOI: 10.1021/acsmedchemlett.7b00132 BindingDB Entry DOI: 10.7270/Q2862JXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321464 (6-but-3-enyl-4-[7-(morpholine-4-carbonyl)-3H-benzi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503412 (CHEMBL4459431) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50503398 (CHEMBL4445482) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321465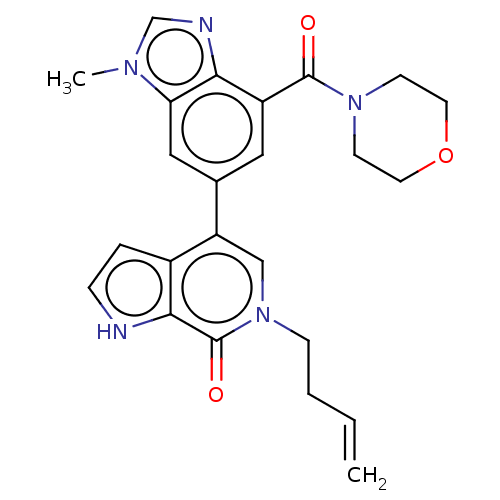 (6-but-3-enyl-4-[3-methyl-7-(morpholine-4-carbonyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM321461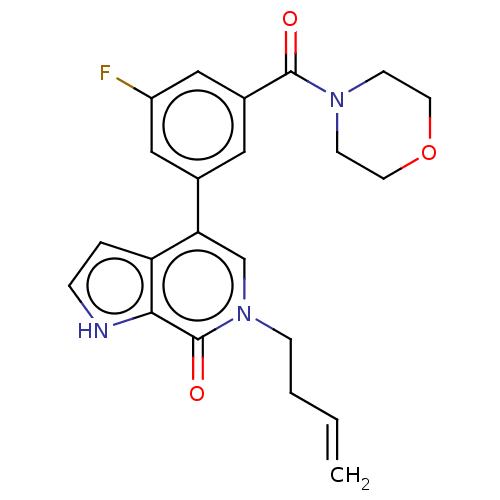 (6-but-3-enyl-4-[3-fluoro-5-(morpholine-4-carbonyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged TAF1 bromodomain 2 (unknown origin) after 10 mins by TR-FRET assay | J Med Chem 61: 9301-9315 (2018) Article DOI: 10.1021/acs.jmedchem.8b01225 BindingDB Entry DOI: 10.7270/Q2NV9NH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31847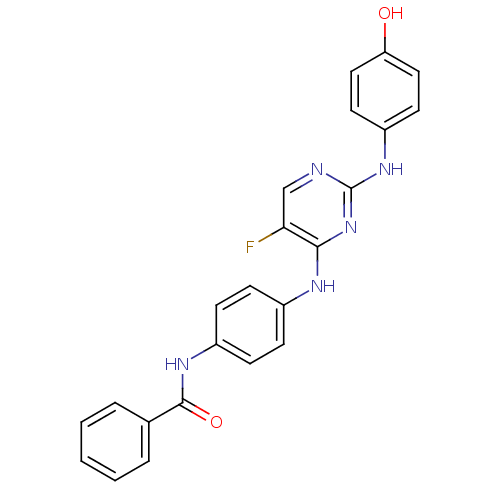 (2,4-Bisanilinopyrimidine, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31873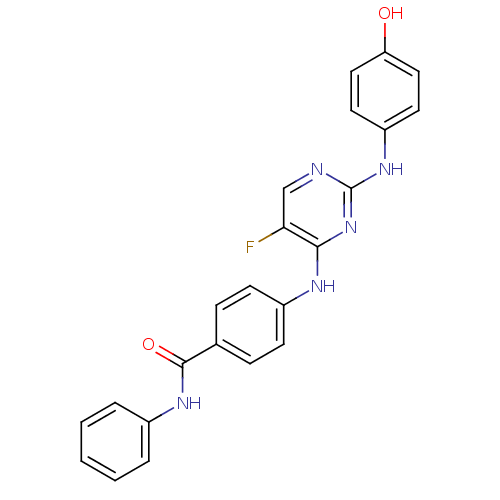 (2,4-Bisanilinopyrimidine, 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31840 (2,4-Bisanilinopyrimidine, 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Genentech | Assay Description Aurora kinase was assayed in ELISA format using a GST fusion of the N-terminus of Histone H3 as substrate. Plates were coated with substrate, and the... | J Med Chem 52: 3300-7 (2009) Article DOI: 10.1021/jm9000314 BindingDB Entry DOI: 10.7270/Q2M90706 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A (Homo sapiens (Human)) | BDBM195610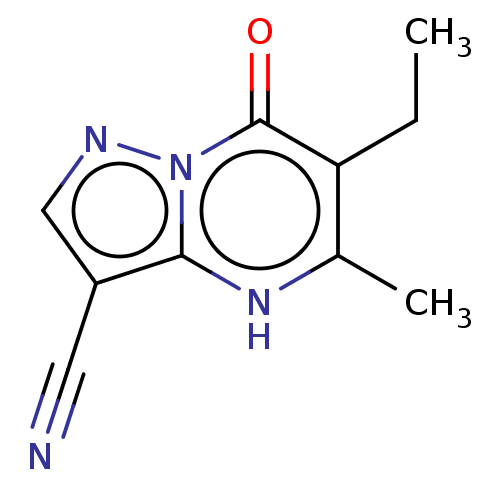 (KDM inhibitor, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Genentech Inc | Assay Description Time-resolved fluorescence resonance energy transfer (TR-FRET) assays were carried out using full-length KDM5 enzymes in 384-well black ProxiPlates (... | Nat Chem Biol 12: 531-8 (2016) Article DOI: 10.1038/nchembio.2085 BindingDB Entry DOI: 10.7270/Q2CF9NXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 991 total ) | Next | Last >> |