 Found 232 hits with Last Name = 'marcus' and Initial = 'ap'
Found 232 hits with Last Name = 'marcus' and Initial = 'ap' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192752
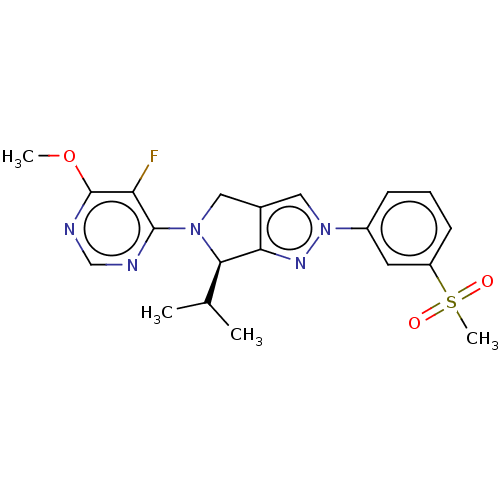
(CHEMBL3905741)Show SMILES COc1ncnc(N2Cc3cn(nc3[C@H]2C(C)C)-c2cccc(c2)S(C)(=O)=O)c1F |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(9-25(18)19-16(21)20(29-3)23-11-22-19)10-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-8,10-12,18H,9H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50177015

(CHEMBL3814206 | US10144715, Compound 19-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H27F3N4O4S/c1-13(2)17-10-27(16-5-4-14(11-29)18(8-16)33(3,31)32)6-7-28(17)20-25-9-15(12-30)19(26-20)21(22,23)24/h4-5,8-9,13,17,29-30H,6-7,10-12H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192753
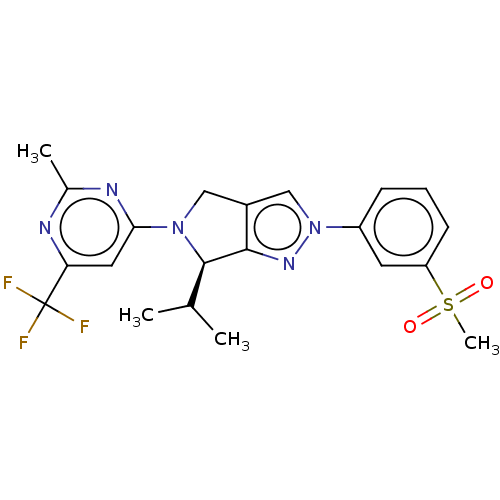
(CHEMBL3985591)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(nc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O2S/c1-12(2)20-19-14(10-28(20)18-9-17(21(22,23)24)25-13(3)26-18)11-29(27-19)15-6-5-7-16(8-15)32(4,30)31/h5-9,11-12,20H,10H2,1-4H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229378
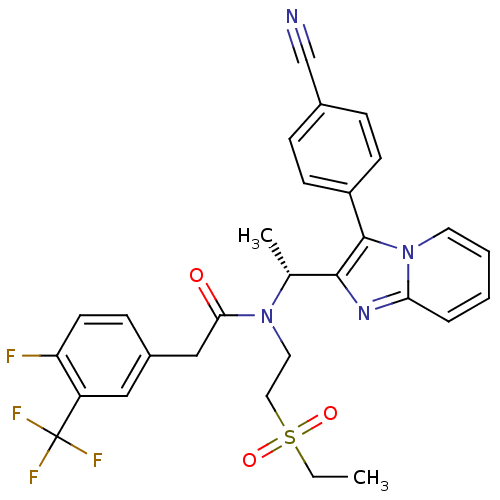
((R)-N-(1-(3-(4-cyanophenyl)H-imidazo[1,2-a]pyridin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H26F4N4O3S/c1-3-41(39,40)15-14-36(26(38)17-21-9-12-24(30)23(16-21)29(31,32)33)19(2)27-28(22-10-7-20(18-34)8-11-22)37-13-5-4-6-25(37)35-27/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192757
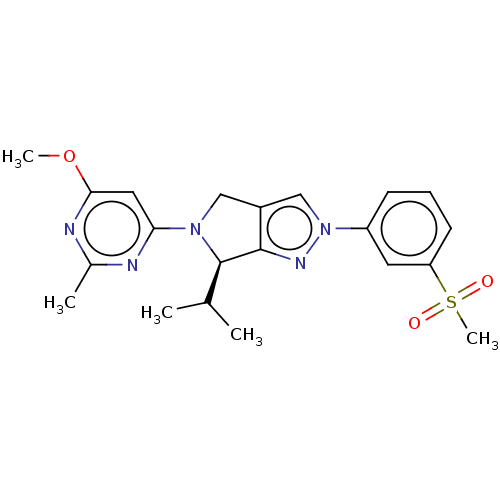
(CHEMBL3914727)Show SMILES COc1cc(nc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H25N5O3S/c1-13(2)21-20-15(11-25(21)18-10-19(29-4)23-14(3)22-18)12-26(24-20)16-7-6-8-17(9-16)30(5,27)28/h6-10,12-13,21H,11H2,1-5H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192750
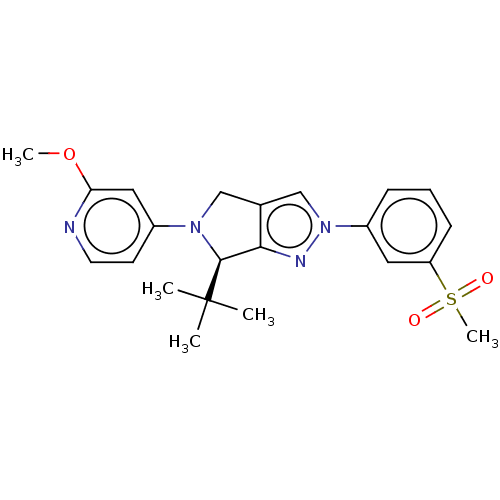
(CHEMBL3940521)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-22(2,3)21-20-15(13-25(21)16-9-10-23-19(12-16)29-4)14-26(24-20)17-7-6-8-18(11-17)30(5,27)28/h6-12,14,21H,13H2,1-5H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192756
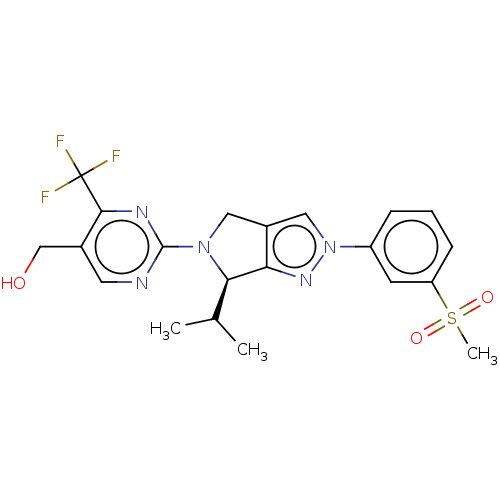
(CHEMBL3932169)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O3S/c1-12(2)18-17-14(10-29(27-17)15-5-4-6-16(7-15)33(3,31)32)9-28(18)20-25-8-13(11-30)19(26-20)21(22,23)24/h4-8,10,12,18,30H,9,11H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192758
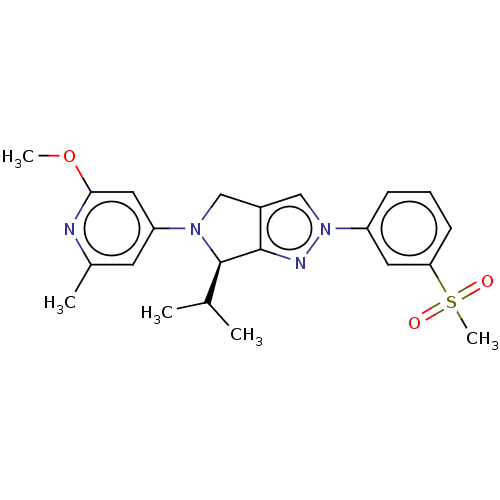
(CHEMBL3976470)Show SMILES COc1cc(cc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-14(2)22-21-16(12-25(22)18-9-15(3)23-20(11-18)29-4)13-26(24-21)17-7-6-8-19(10-17)30(5,27)28/h6-11,13-14,22H,12H2,1-5H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192761
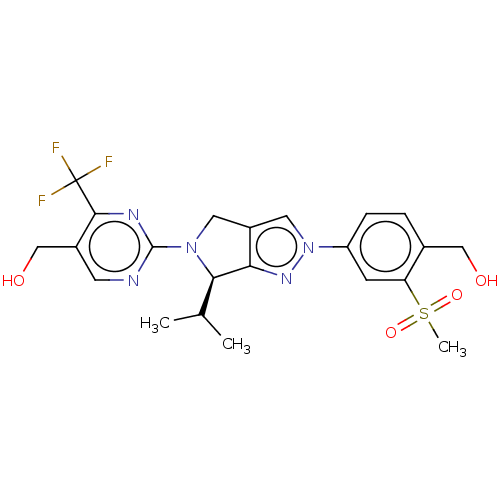
(CHEMBL3978980)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192759

(CHEMBL3960195)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(ncn1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)19-18-13(9-27(19)17-8-16(20(21,22)23)24-11-25-17)10-28(26-18)14-5-4-6-15(7-14)31(3,29)30/h4-8,10-12,19H,9H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192762
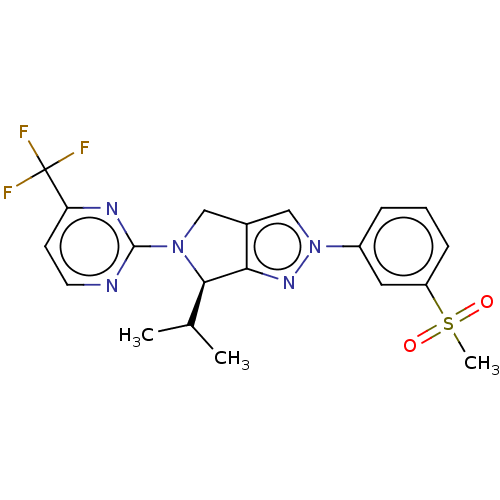
(CHEMBL3959681)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1nccc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)19-24-8-7-16(25-19)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192752

(CHEMBL3905741)Show SMILES COc1ncnc(N2Cc3cn(nc3[C@H]2C(C)C)-c2cccc(c2)S(C)(=O)=O)c1F |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(9-25(18)19-16(21)20(29-3)23-11-22-19)10-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-8,10-12,18H,9H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192754
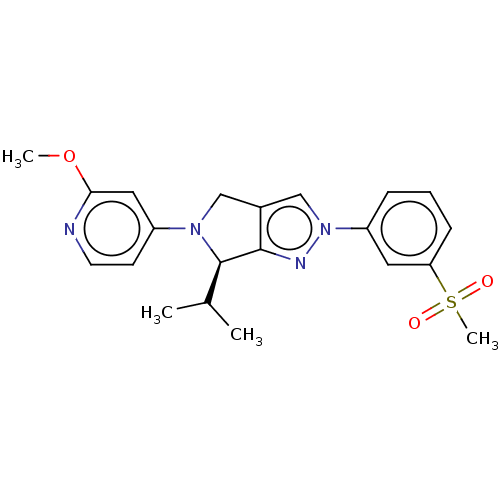
(CHEMBL3913348)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14(2)21-20-15(12-24(21)16-8-9-22-19(11-16)28-3)13-25(23-20)17-6-5-7-18(10-17)29(4,26)27/h5-11,13-14,21H,12H2,1-4H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192760
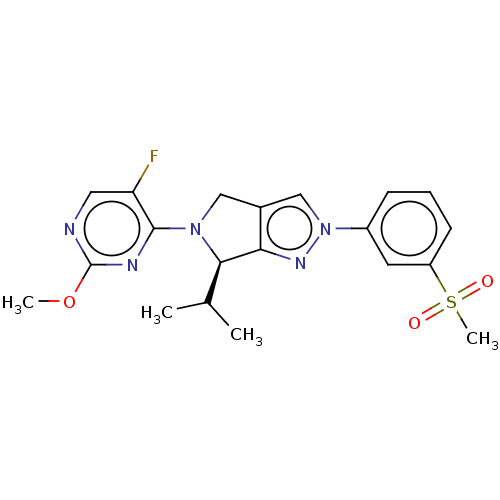
(CHEMBL3972392)Show SMILES COc1ncc(F)c(n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(10-25(18)19-16(21)9-22-20(23-19)29-3)11-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-9,11-12,18H,10H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192763
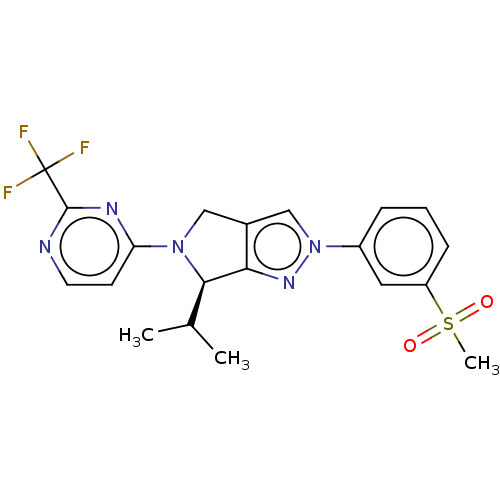
(CHEMBL3933543)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ccnc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)16-7-8-24-19(25-16)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192755
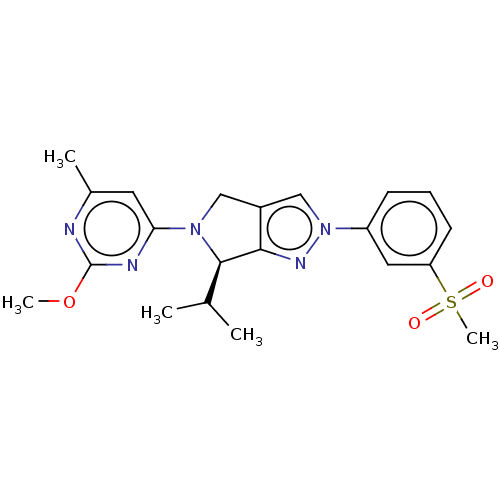
(CHEMBL3956923)Show SMILES COc1nc(C)cc(n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H25N5O3S/c1-13(2)20-19-15(11-25(20)18-9-14(3)22-21(23-18)29-4)12-26(24-19)16-7-6-8-17(10-16)30(5,27)28/h6-10,12-13,20H,11H2,1-5H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192762

(CHEMBL3959681)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1nccc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)19-24-8-7-16(25-19)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192757

(CHEMBL3914727)Show SMILES COc1cc(nc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H25N5O3S/c1-13(2)21-20-15(11-25(21)18-10-19(29-4)23-14(3)22-18)12-26(24-20)16-7-6-8-17(9-16)30(5,27)28/h6-10,12-13,21H,11H2,1-5H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192751
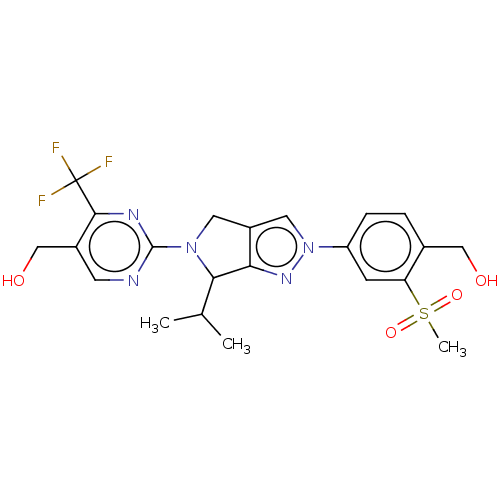
(CHEMBL3889601)Show SMILES CC(C)C1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50192751

(CHEMBL3889601)Show SMILES CC(C)C1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192753

(CHEMBL3985591)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(nc(C)n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O2S/c1-12(2)20-19-14(10-28(20)18-9-17(21(22,23)24)25-13(3)26-18)11-29(27-19)15-6-5-7-16(8-15)32(4,30)31/h5-9,11-12,20H,10H2,1-4H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192750

(CHEMBL3940521)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-22(2,3)21-20-15(13-25(21)16-9-10-23-19(12-16)29-4)14-26(24-20)17-7-6-8-18(11-17)30(5,27)28/h6-12,14,21H,13H2,1-5H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192758

(CHEMBL3976470)Show SMILES COc1cc(cc(C)n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H26N4O3S/c1-14(2)22-21-16(12-25(22)18-9-15(3)23-20(11-18)29-4)13-26(24-21)17-7-6-8-19(10-17)30(5,27)28/h6-11,13-14,22H,12H2,1-5H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192756

(CHEMBL3932169)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F3N5O3S/c1-12(2)18-17-14(10-29(27-17)15-5-4-6-16(7-15)33(3,31)32)9-28(18)20-25-8-13(11-30)19(26-20)21(22,23)24/h4-8,10,12,18,30H,9,11H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192761

(CHEMBL3978980)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50177015

(CHEMBL3814206 | US10144715, Compound 19-1)Show SMILES CC(C)[C@@H]1CN(CCN1c1ncc(CO)c(n1)C(F)(F)F)c1ccc(CO)c(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H27F3N4O4S/c1-13(2)17-10-27(16-5-4-14(11-29)18(8-16)33(3,31)32)6-7-28(17)20-25-9-15(12-30)19(26-20)21(22,23)24/h4-5,8-9,13,17,29-30H,6-7,10-12H2,1-3H3/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192754

(CHEMBL3913348)Show SMILES COc1cc(ccn1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14(2)21-20-15(12-24(21)16-8-9-22-19(11-16)28-3)13-25(23-20)17-6-5-7-18(10-17)29(4,26)27/h5-11,13-14,21H,12H2,1-4H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192760

(CHEMBL3972392)Show SMILES COc1ncc(F)c(n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C20H22FN5O3S/c1-12(2)18-17-13(10-25(18)19-16(21)9-22-20(23-19)29-3)11-26(24-17)14-6-5-7-15(8-14)30(4,27)28/h5-9,11-12,18H,10H2,1-4H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192759

(CHEMBL3960195)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1cc(ncn1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)19-18-13(9-27(19)17-8-16(20(21,22)23)24-11-25-17)10-28(26-18)14-5-4-6-15(7-14)31(3,29)30/h4-8,10-12,19H,9H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192763

(CHEMBL3933543)Show SMILES CC(C)[C@H]1N(Cc2cn(nc12)-c1cccc(c1)S(C)(=O)=O)c1ccnc(n1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F3N5O2S/c1-12(2)18-17-13(10-27(18)16-7-8-24-19(25-16)20(21,22)23)11-28(26-17)14-5-4-6-15(9-14)31(3,29)30/h4-9,11-12,18H,10H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192755

(CHEMBL3956923)Show SMILES COc1nc(C)cc(n1)N1Cc2cn(nc2[C@H]1C(C)C)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H25N5O3S/c1-13(2)20-19-15(11-25(20)18-9-14(3)22-21(23-18)29-4)12-26(24-19)16-7-6-8-17(10-16)30(5,27)28/h6-10,12-13,20H,11H2,1-5H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192751

(CHEMBL3889601)Show SMILES CC(C)C1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 383 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50192751

(CHEMBL3889601)Show SMILES CC(C)C1N(Cc2cn(nc12)-c1ccc(CO)c(c1)S(C)(=O)=O)c1ncc(CO)c(n1)C(F)(F)F Show InChI InChI=1S/C22H24F3N5O4S/c1-12(2)19-18-15(8-29(19)21-26-7-14(11-32)20(27-21)22(23,24)25)9-30(28-18)16-5-4-13(10-31)17(6-16)35(3,33)34/h4-7,9,12,19,31-32H,8,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 383 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin) |
Bioorg Med Chem Lett 26: 5044-5050 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.089
BindingDB Entry DOI: 10.7270/Q28P62GC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229381
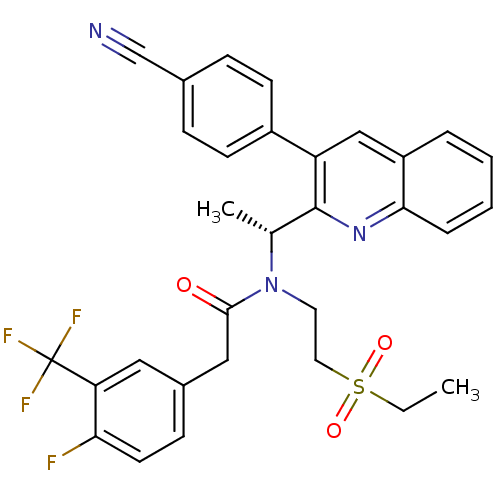
((R)-N-(1-(3-(4-cyanophenyl)quinolin-2-yl)ethyl)-N-...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccc2cc1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C31H27F4N3O3S/c1-3-42(40,41)15-14-38(29(39)17-22-10-13-27(32)26(16-22)31(33,34)35)20(2)30-25(23-11-8-21(19-36)9-12-23)18-24-6-4-5-7-28(24)37-30/h4-13,16,18,20H,3,14-15,17H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229383
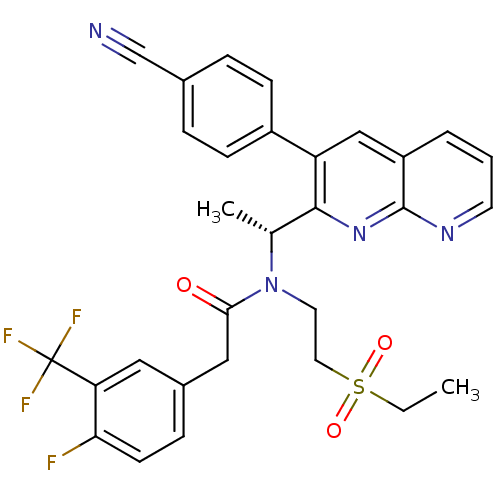
((R)-N-(1-(3-(4-cyanophenyl)-1,8-naphthyridin-2-yl)...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2cc1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C30H26F4N4O3S/c1-3-42(40,41)14-13-38(27(39)16-21-8-11-26(31)25(15-21)30(32,33)34)19(2)28-24(22-9-6-20(18-35)7-10-22)17-23-5-4-12-36-29(23)37-28/h4-12,15,17,19H,3,13-14,16H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229377
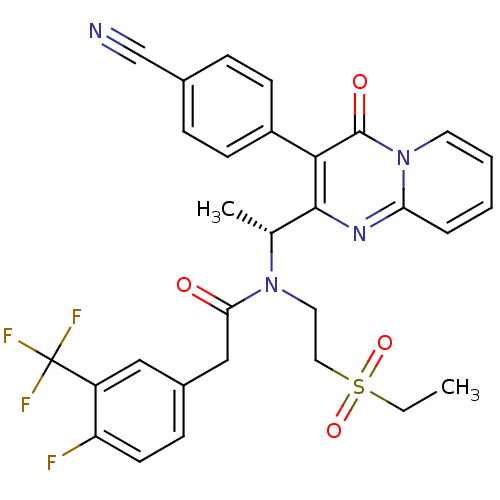
((R)-N-(1-(3-(4-cyanophenyl)-4-oxo-4H-pyrido[1,2-a]...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccn2c(=O)c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C30H26F4N4O4S/c1-3-43(41,42)15-14-37(26(39)17-21-9-12-24(31)23(16-21)30(32,33)34)19(2)28-27(22-10-7-20(18-35)8-11-22)29(40)38-13-5-4-6-25(38)36-28/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
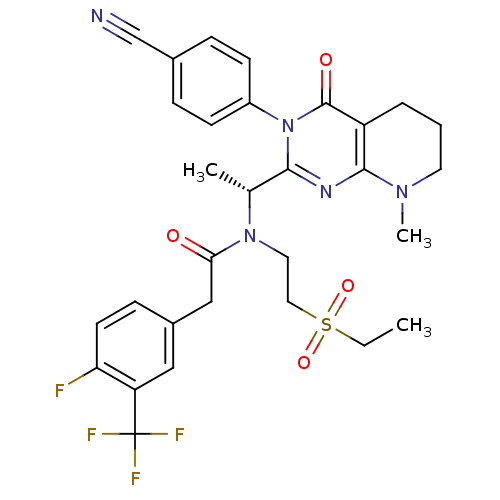
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229380
((R)-N-(1-(3-(4-cyanophenyl)-8-methyl-4-oxo-3,4,5,6...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2N(C)CCCc2c(=O)n1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C30H31F4N5O4S/c1-4-44(42,43)15-14-38(26(40)17-21-9-12-25(31)24(16-21)30(32,33)34)19(2)27-36-28-23(6-5-13-37(28)3)29(41)39(27)22-10-7-20(18-35)8-11-22/h7-12,16,19H,4-6,13-15,17H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
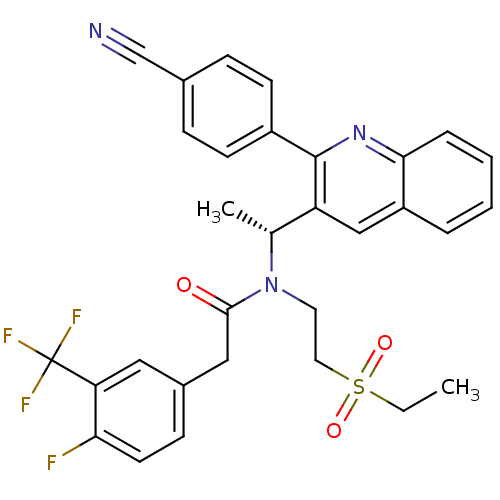
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229384
((R)-N-(1-(2-(4-cyanophenyl)quinolin-3-yl)ethyl)-N-...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1cc2ccccc2nc1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C31H27F4N3O3S/c1-3-42(40,41)15-14-38(29(39)17-22-10-13-27(32)26(16-22)31(33,34)35)20(2)25-18-24-6-4-5-7-28(24)37-30(25)23-11-8-21(19-36)9-12-23/h4-13,16,18,20H,3,14-15,17H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229379
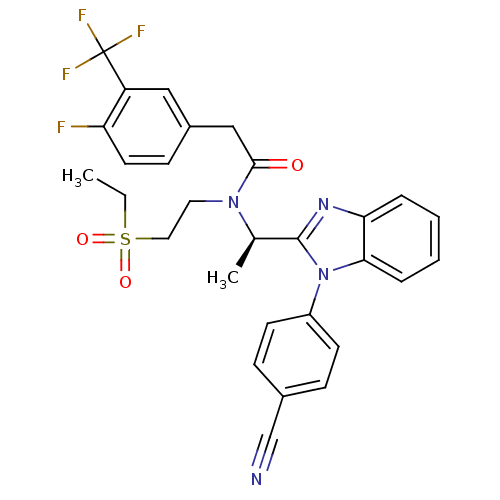
((R)-N-(1-(1-(4-cyanophenyl)-1H-benzo[d]imidazol-2-...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccc2n1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H26F4N4O3S/c1-3-41(39,40)15-14-36(27(38)17-21-10-13-24(30)23(16-21)29(31,32)33)19(2)28-35-25-6-4-5-7-26(25)37(28)22-11-8-20(18-34)9-12-22/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR3 assessed as IP-10-mediated cell migration |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ITAC from CXCR3 receptor |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229385
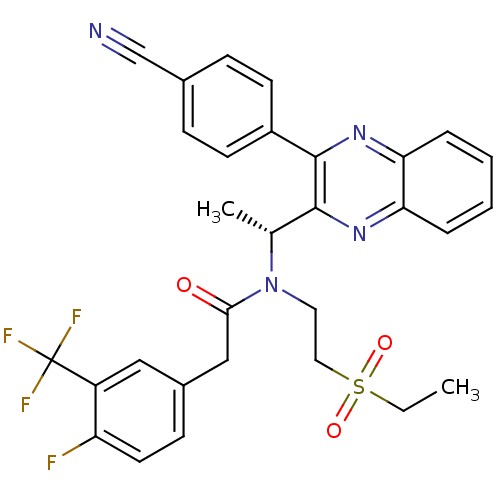
((R)-N-(1-(3-(4-cyanophenyl)quinoxalin-2-yl)ethyl)-...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccc2nc1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C30H26F4N4O3S/c1-3-42(40,41)15-14-38(27(39)17-21-10-13-24(31)23(16-21)30(32,33)34)19(2)28-29(22-11-8-20(18-35)9-12-22)37-26-7-5-4-6-25(26)36-28/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229382
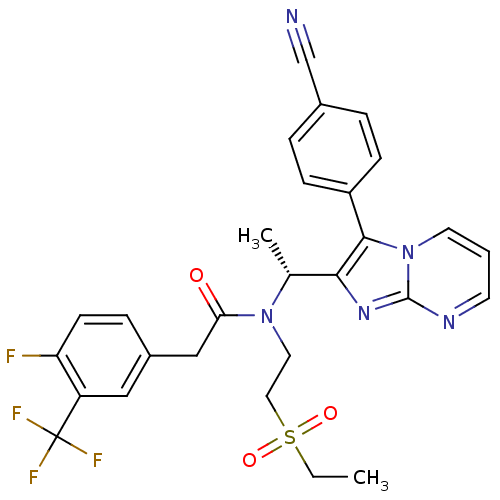
((R)-N-(1-(3-(4-cyanophenyl)imidazo[1,2-a]pyrimidin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C28H25F4N5O3S/c1-3-41(39,40)14-13-36(24(38)16-20-7-10-23(29)22(15-20)28(30,31)32)18(2)25-26(21-8-5-19(17-33)6-9-21)37-12-4-11-34-27(37)35-25/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229376
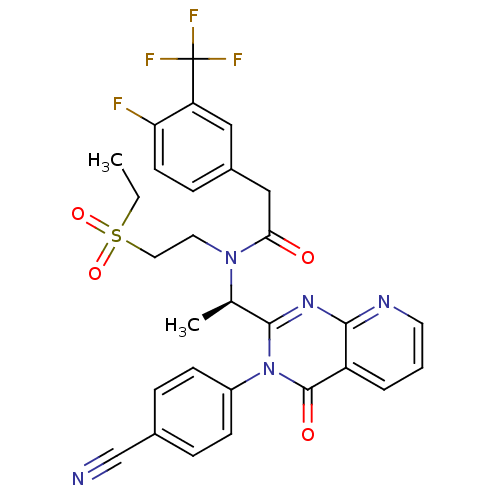
((R)-N-(1-(3-(4-cyanophenyl)-4-oxo-3,4-dihydropyrid...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H25F4N5O4S/c1-3-43(41,42)14-13-37(25(39)16-20-8-11-24(30)23(15-20)29(31,32)33)18(2)27-36-26-22(5-4-12-35-26)28(40)38(27)21-9-6-19(17-34)7-10-21/h4-12,15,18H,3,13-14,16H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CXCR3 assessed as ITAC-mediated cell migration |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229377

((R)-N-(1-(3-(4-cyanophenyl)-4-oxo-4H-pyrido[1,2-a]...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccn2c(=O)c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C30H26F4N4O4S/c1-3-43(41,42)15-14-37(26(39)17-21-9-12-24(31)23(16-21)30(32,33)34)19(2)28-27(22-10-7-20(18-35)8-11-22)29(40)38-13-5-4-6-25(38)36-28/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in EDTA-anti-coagulated human plasma |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229386
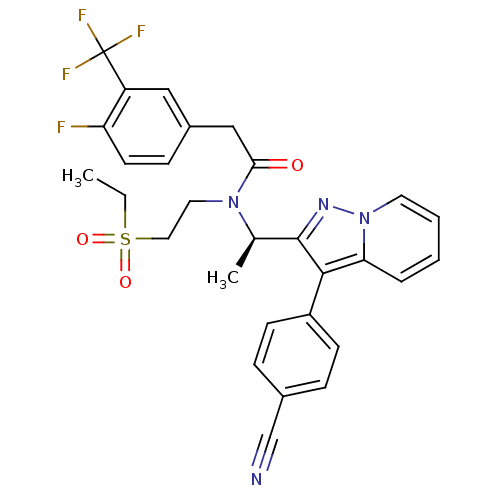
((R)-N-(1-(3-(4-cyanophenyl)H-pyrazolo[1,5-a]pyridi...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nn2ccccc2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H26F4N4O3S/c1-3-41(39,40)15-14-36(26(38)17-21-9-12-24(30)23(16-21)29(31,32)33)19(2)28-27(22-10-7-20(18-34)8-11-22)25-6-4-5-13-37(25)35-28/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data