

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50167296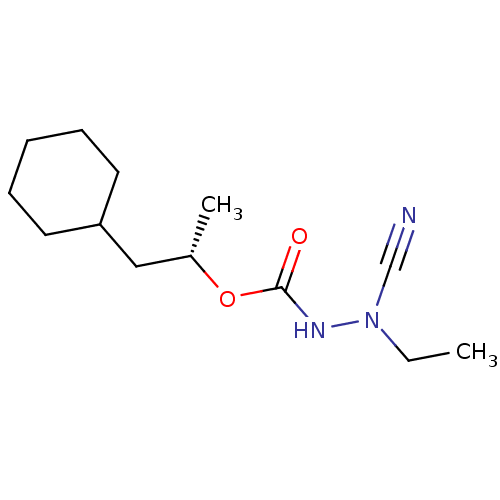 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167295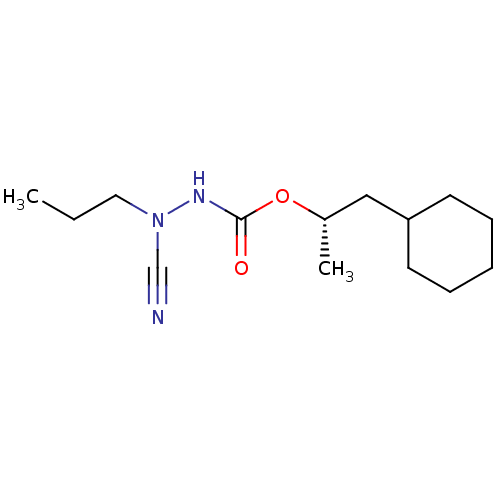 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167302 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167298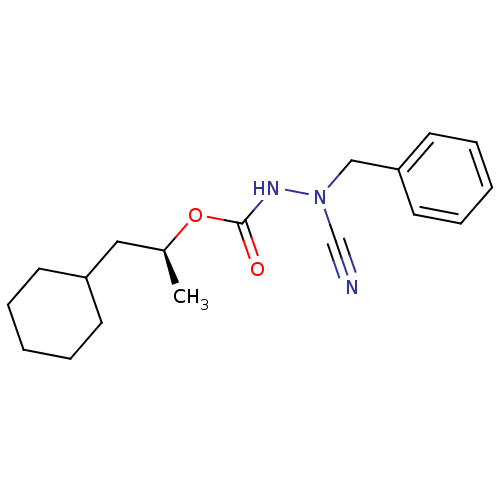 ((1S)-2-cyclohexyl-1-methylethyl 2-benzyl-2-cyanohy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167303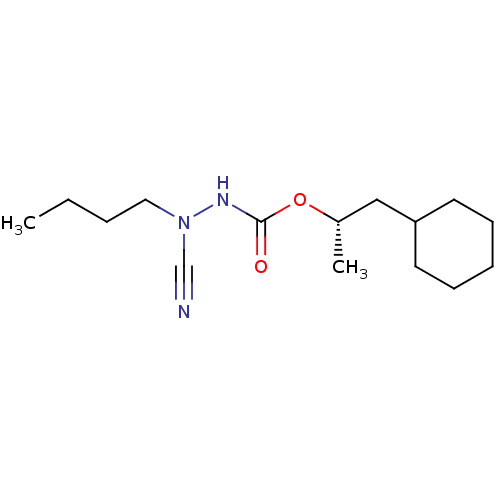 ((1S)-2-cyclohexyl-1-methylethyl 2-butyl-2-cyanohyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167289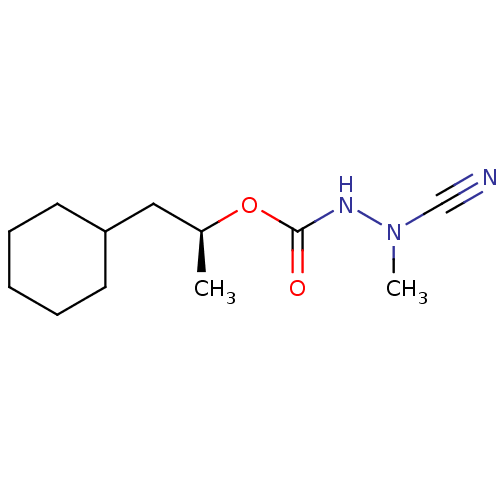 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167290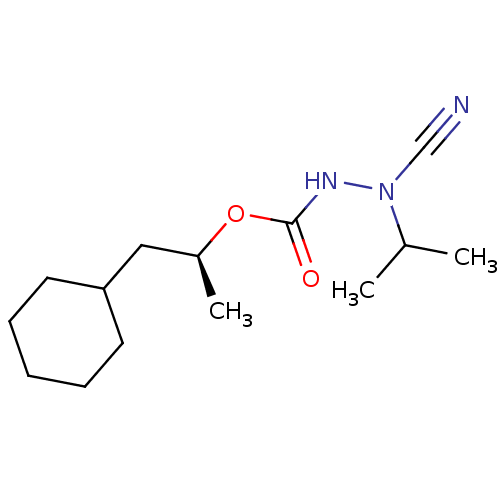 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167289 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31822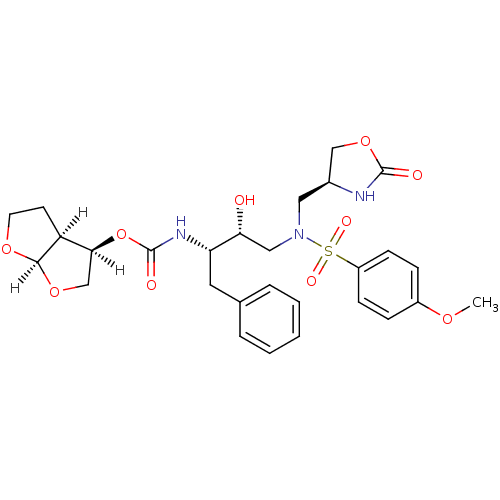 (oxazolidinone, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50417287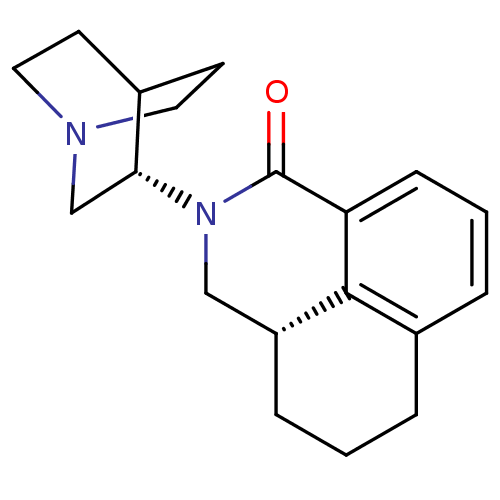 (Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Rattus norvegicus) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against rat cathepsin K | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50167289 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-methylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31817 (GRL-02031 | methyl-2-pyrrolidinone, 19b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0990 | -57.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Mus musculus) | BDBM50064089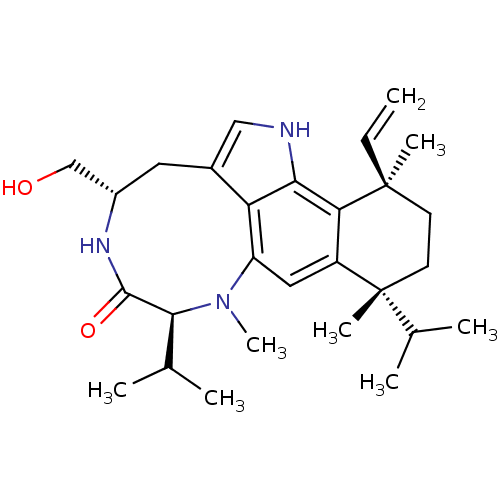 (4-hydroxymethyl-7,10-diisopropyl-8,10,13-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]- PDBu binding to peptide D of mouse skin Protein kinase C eta | Bioorg Med Chem Lett 6: 353-356 (1996) Article DOI: 10.1016/0960-894X(96)00026-1 BindingDB Entry DOI: 10.7270/Q2NG4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50231938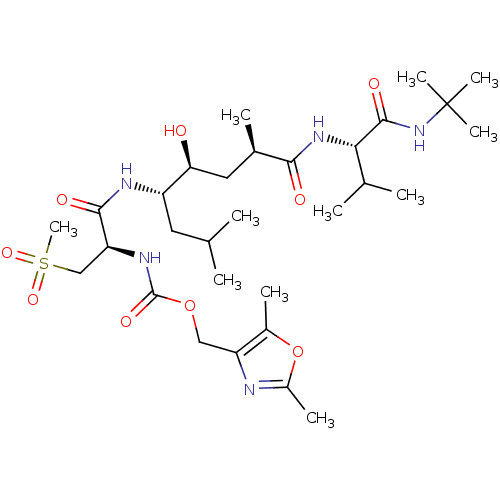 ((2,5-dimethyloxazol-4-yl)methyl (R)-1-((4S,5S,7R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to recombinant memapsin 2 | Bioorg Med Chem Lett 18: 1031-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.028 BindingDB Entry DOI: 10.7270/Q2513XZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31816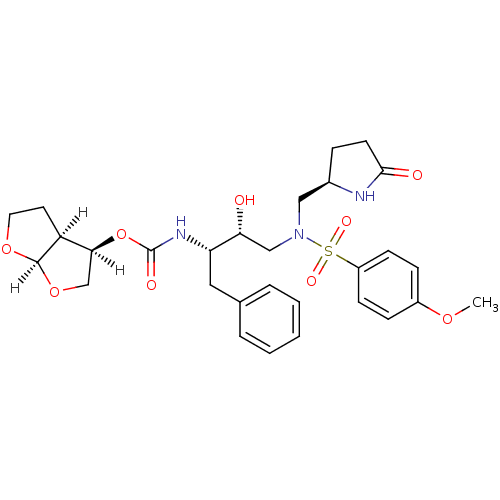 (methyl-2-pyrrolidinone, 19a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000523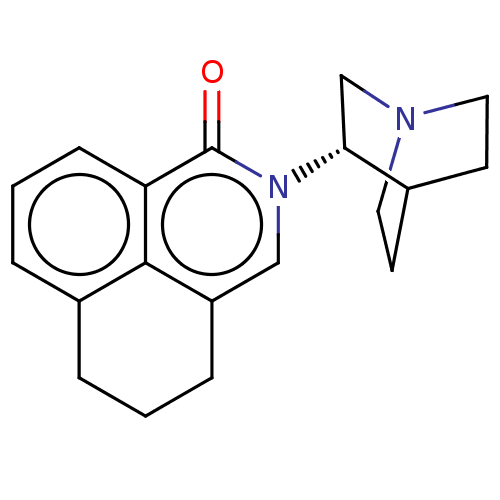 (CHEMBL544784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000453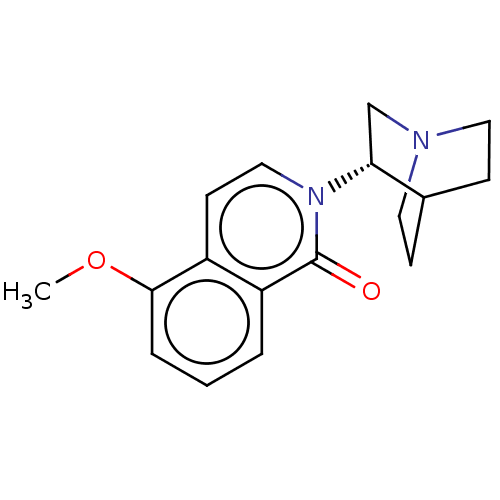 (CHEMBL540055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000450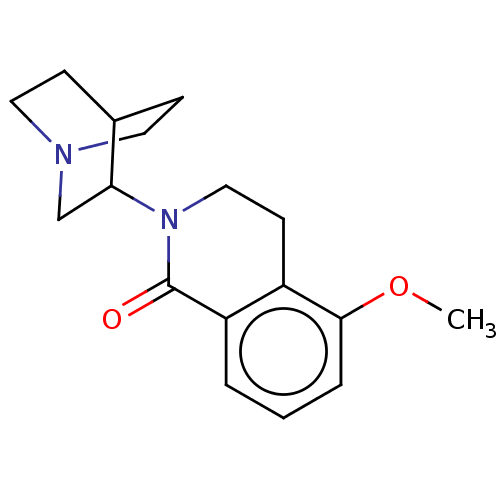 (CHEMBL542669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000471 (CHEMBL542900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000457 (CHEMBL542904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31823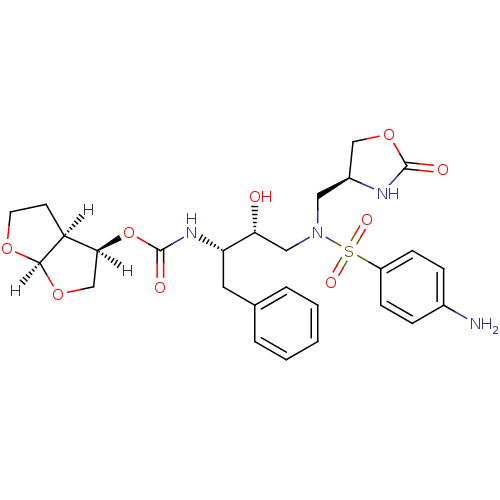 (oxazolidinone, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | -54.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50056419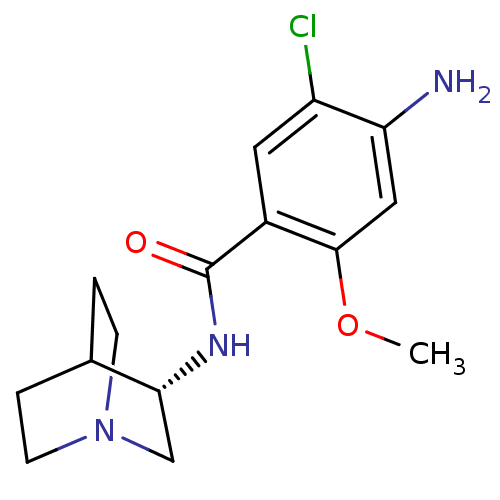 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50167288 ((1S)-1-benzylpropyl 2-cyano-2-methylhydrazinecarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin B in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50411736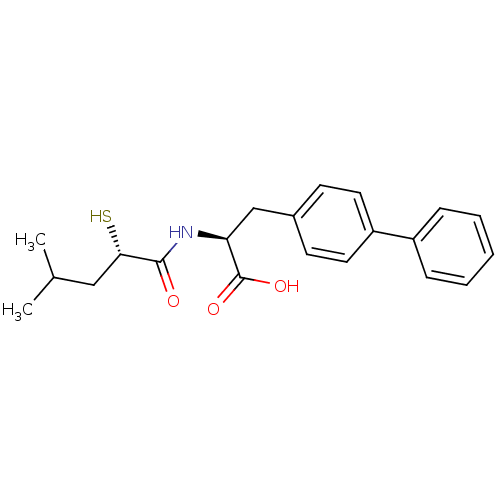 (CHEMBL271225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP by fluorescence assay | Bioorg Med Chem Lett 18: 732-7 (2008) Article DOI: 10.1016/j.bmcl.2007.11.048 BindingDB Entry DOI: 10.7270/Q2GT5PCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31814 (methyl-2-pyrrolidinone, 18a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31820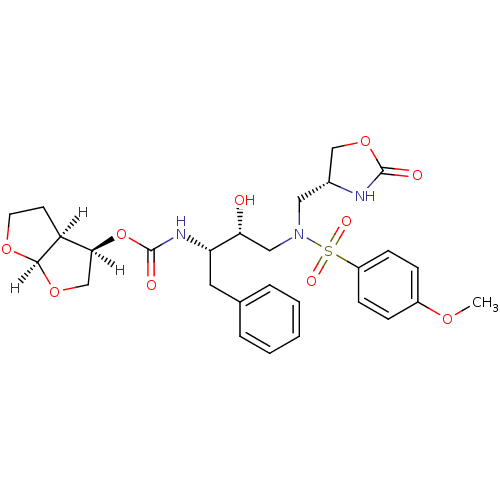 (oxazolidinone, 29) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31819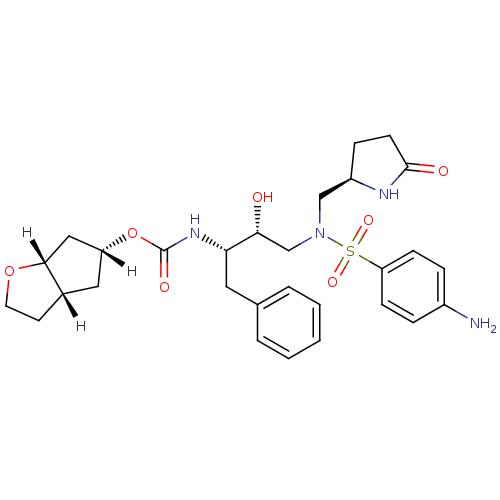 (methyl-2-pyrrolidinone, 20b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31813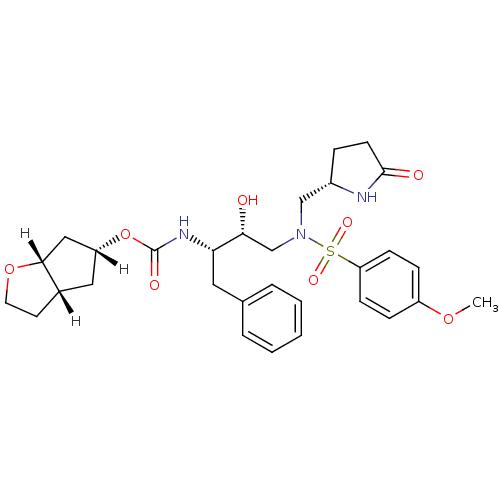 (methyl-2-pyrrolidinone, 17b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31821 (oxazolidinone, 30) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | -54.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000475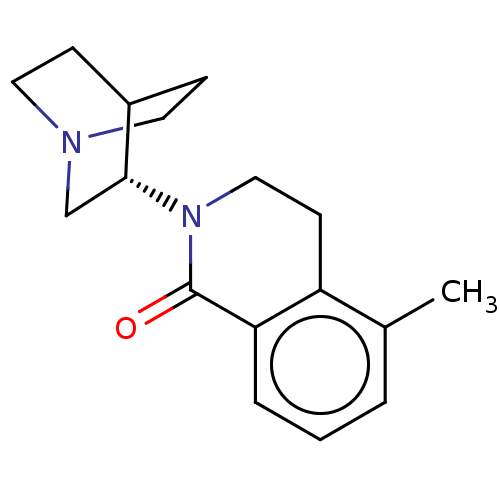 (CHEMBL555068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000460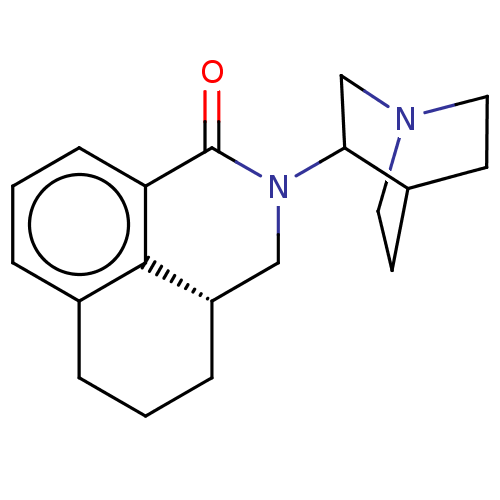 (CHEMBL540057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000455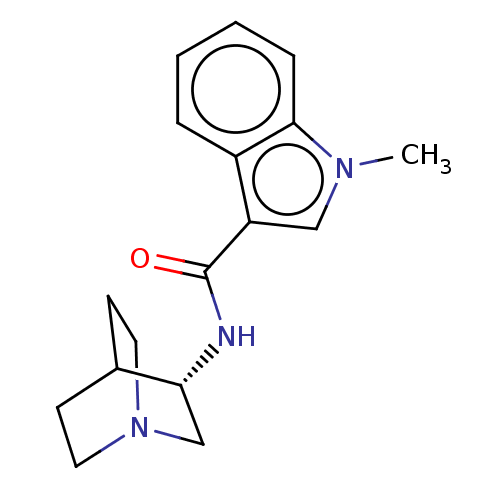 (CHEMBL2093898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000469 (CHEMBL542903) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) | Bioorg Med Chem Lett 8: 3053-8 (1998) BindingDB Entry DOI: 10.7270/Q2PV6NK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay | Bioorg Med Chem Lett 8: 1867-72 (1998) BindingDB Entry DOI: 10.7270/Q2CJ8GPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pro-cathepsin H (Homo sapiens (Human)) | BDBM50167290 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin H using L-Arg-b-naphthalamide | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Mus musculus) | BDBM50057512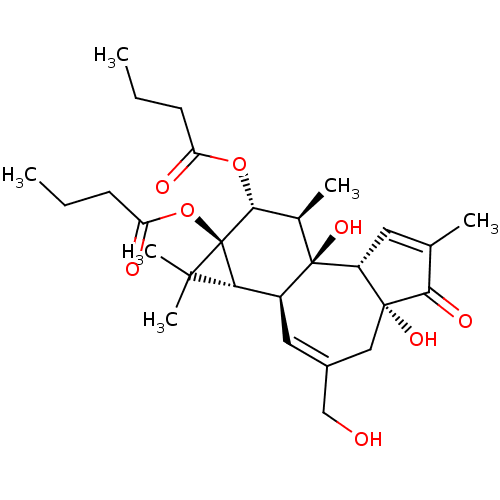 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]- PDBu binding to peptide D of mouse skin Protein kinase C eta | Bioorg Med Chem Lett 6: 353-356 (1996) Article DOI: 10.1016/0960-894X(96)00026-1 BindingDB Entry DOI: 10.7270/Q2NG4QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000445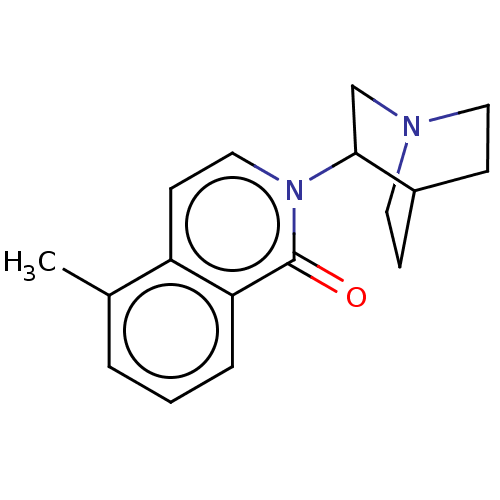 (CHEMBL555038) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000462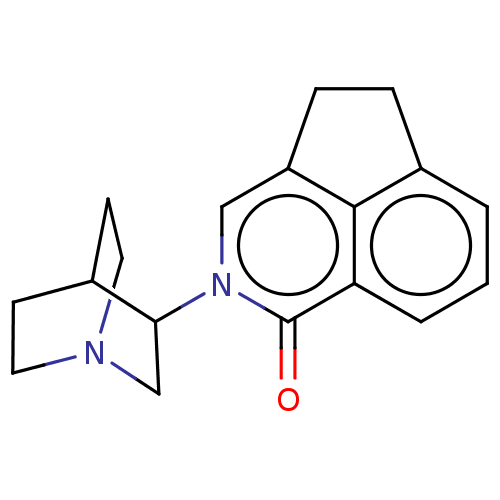 (CHEMBL545719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50167290 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin L using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000446 (CHEMBL88565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50449636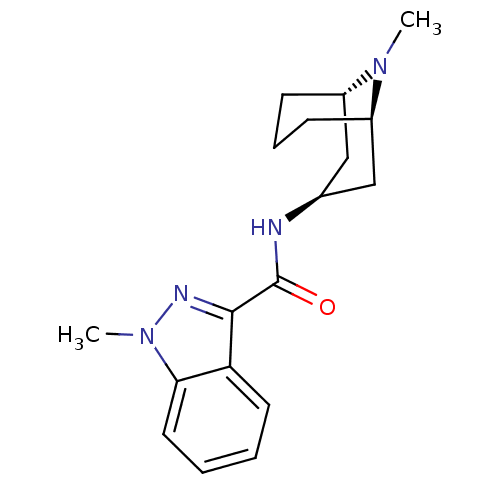 (BRL-43694 | GRANISETRON | Kytril | LY-278584 | San...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50411731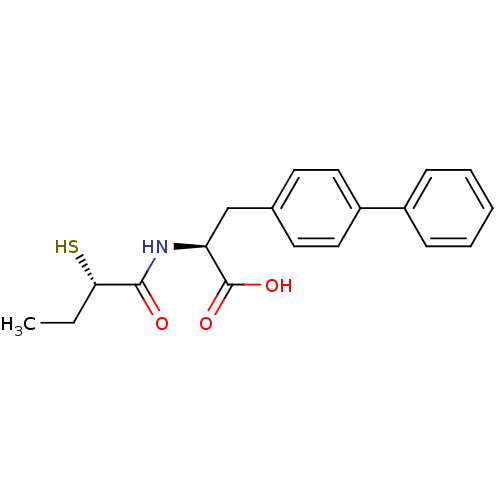 (CHEMBL257726) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP by fluorescence assay | Bioorg Med Chem Lett 18: 732-7 (2008) Article DOI: 10.1016/j.bmcl.2007.11.048 BindingDB Entry DOI: 10.7270/Q2GT5PCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31818 (methyl-2-pyrrolidinone, 20a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.850 | -51.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM31812 (methyl-2-pyrrolidinone, 17a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.850 | -51.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University | Assay Description The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... | J Med Chem 52: 3902-14 (2009) Article DOI: 10.1021/jm900303m BindingDB Entry DOI: 10.7270/Q20G3HHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50322229 (4-(4-Iodophenyl)-1-((4-methoxy-1H-indol-3-yl)methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human D2L receptor expressed in HEK cell membrane after 90 mins by scintillation counting analysis | J Med Chem 57: 3450-63 (2014) Article DOI: 10.1021/jm500126s BindingDB Entry DOI: 10.7270/Q2MW2JN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000468 (CHEMBL539020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2075 total ) | Next | Last >> |