

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094539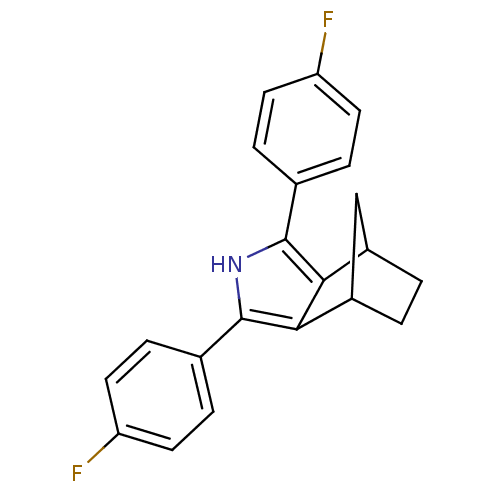 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Tested in vitro for inhibition against Prostaglandin G/H synthase 2 in human blood (95% confidence limits) | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051495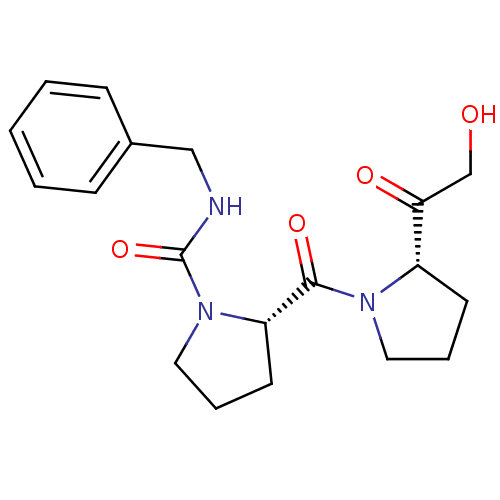 ((S)-2-[(S)-2-(2-Hydroxy-acetyl)-pyrrolidine-1-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat brain | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50058495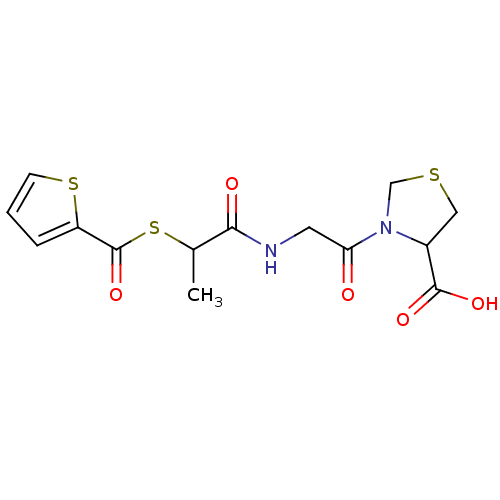 (3-{2-[2-(Thiophene-2-carbonylsulfanyl)-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory concentration against Human Leukocyte Elastase from human sputum | J Med Chem 40: 1906-18 (1997) Article DOI: 10.1021/jm960772z BindingDB Entry DOI: 10.7270/Q2MS3RW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50094551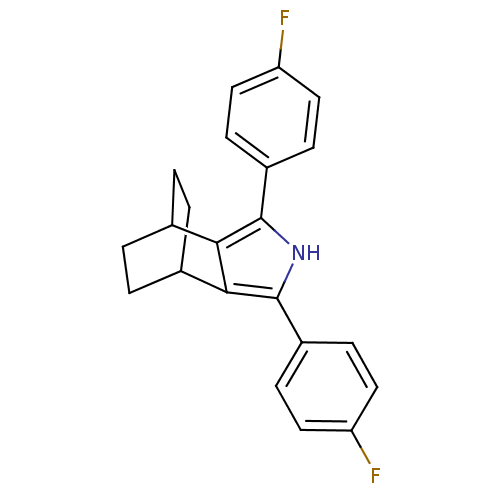 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.2.0*2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Tested in vitro for inhibition against Prostaglandin G/H synthase 2 in human blood (95% confidence limits) | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094551 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.2.0*2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094542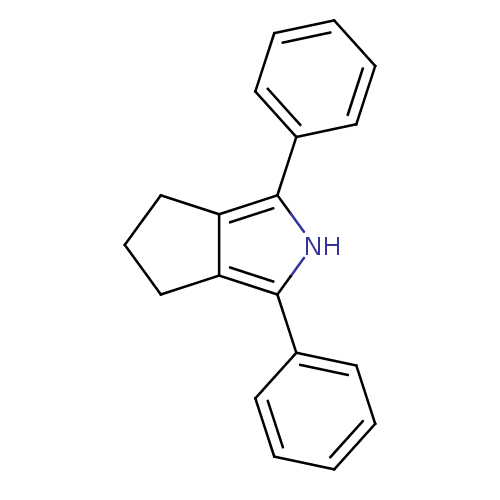 (1,3-Diphenyl-2,4,5,6-tetrahydro-cyclopenta[c]pyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Mus musculus) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Tested for inhibition against Prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051520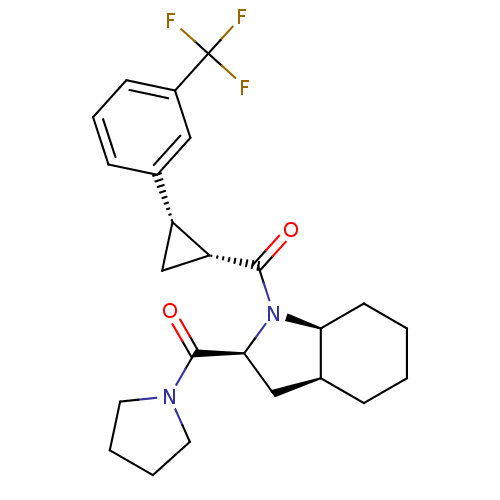 (CHEMBL305681 | [(2S,3aS,7aS)-2-(Pyrrolidine-1-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50094539 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Tested in vitro for inhibition against Prostaglandin G/H synthase 1 in human blood | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051511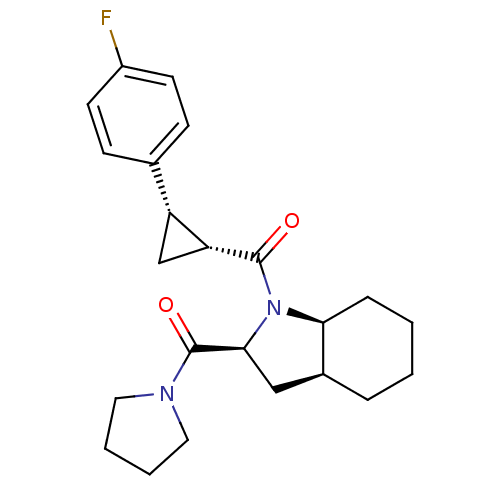 (CHEMBL80684 | [(1R,2S)-2-(4-Fluoro-phenyl)-cyclopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094539 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051523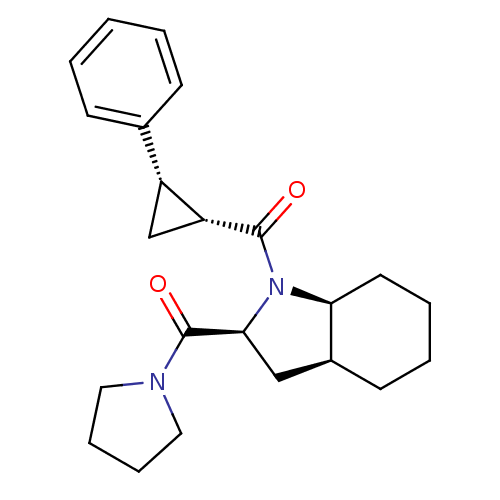 (((1R,2S)-2-Phenyl-cyclopropyl)-[(2S,3aS,7aS)-2-(py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051504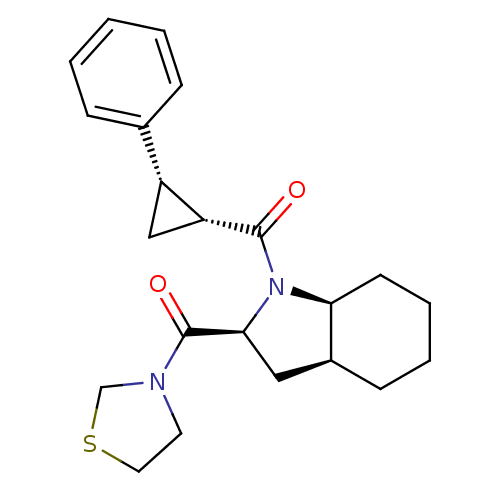 (((1R,2S)-2-Phenyl-cyclopropyl)-[(2S,3aS,7aS)-2-(th...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051515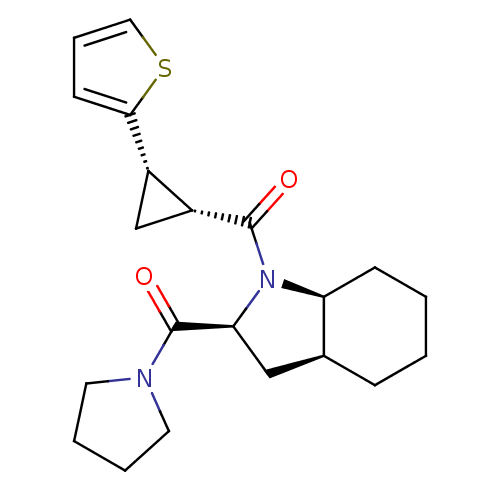 (CHEMBL80698 | [(2S,3aS,7aS)-2-(Pyrrolidine-1-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051506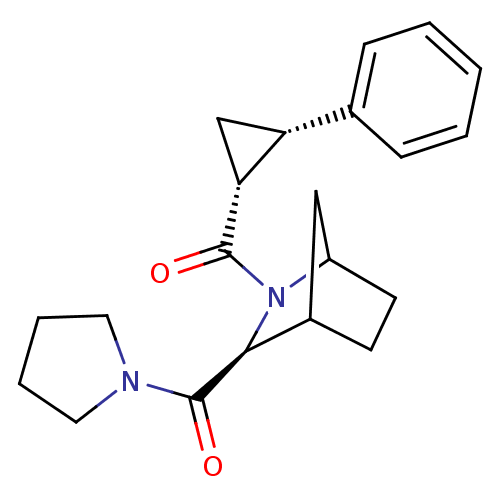 (((1R,2S)-2-Phenyl-cyclopropyl)-[(S)-3-(pyrrolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051516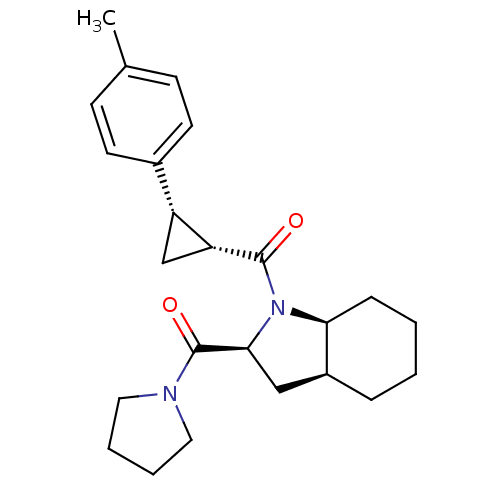 (CHEMBL79213 | [(2S,3aS,7aS)-2-(Pyrrolidine-1-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011365 (1-[2-(1-Carboxy-butylamino)-propionyl]-octahydro-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011362 (1-(3-Mercapto-2-methyl-propionyl)-pyrrolidine-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094540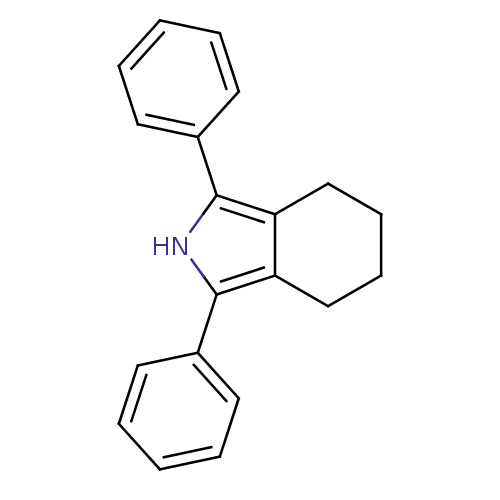 (1,3-diphenyl-4,5,6,7-tetrahydro-2H-isoindole | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094546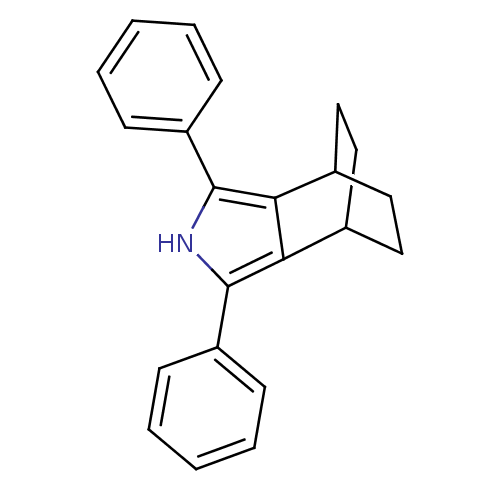 (3,5-Diphenyl-4-aza-tricyclo[5.2.2.0*2,6*]undeca-2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094563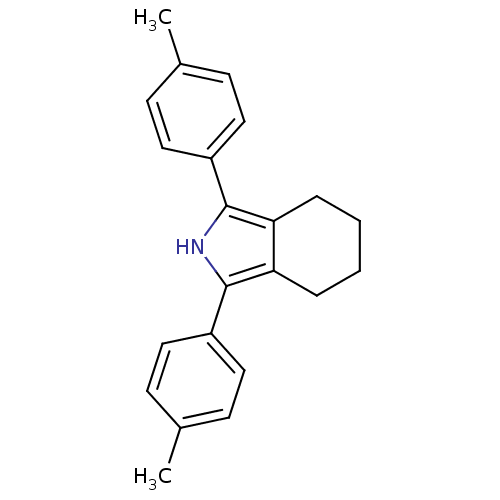 (1,3-Di-p-tolyl-4,5,6,7-tetrahydro-2H-isoindole | 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094534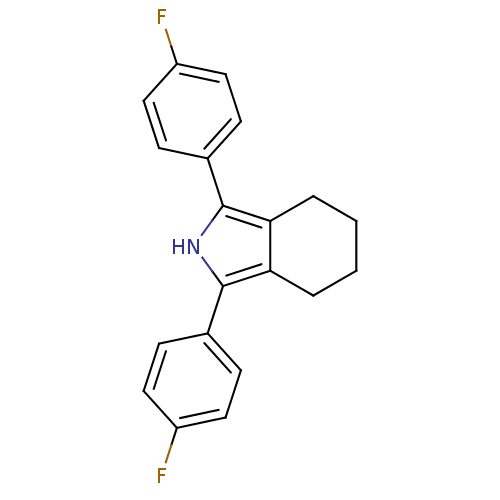 (1,3-Bis-(4-fluoro-phenyl)-4,5,6,7-tetrahydro-2H-is...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094532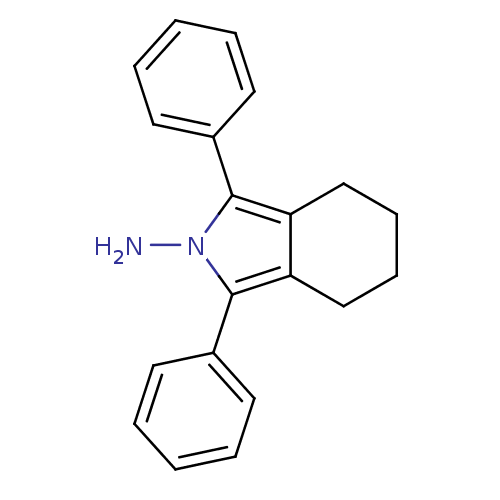 (1,3-Diphenyl-4,5,6,7-tetrahydro-isoindol-2-ylamine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011361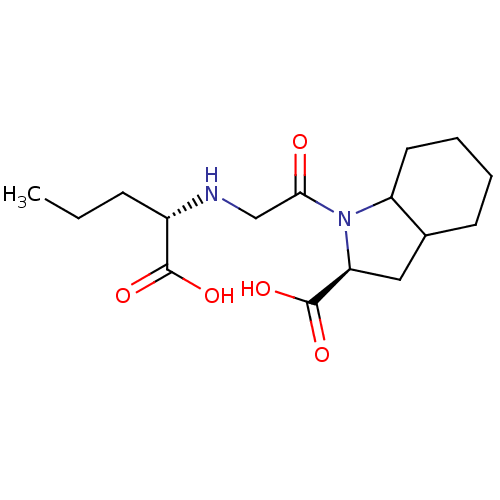 (1-[2-(1-Carboxy-butylamino)-acetyl]-octahydro-indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051508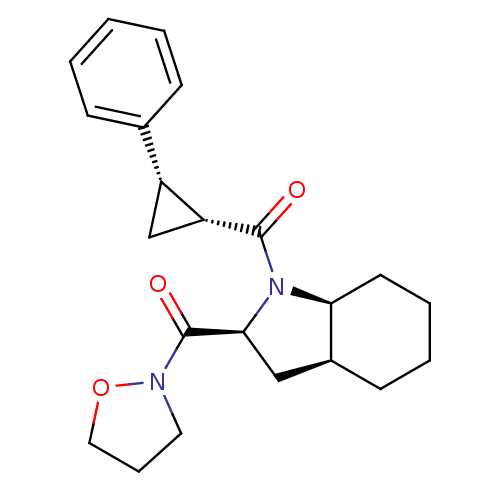 (CHEMBL75968 | [(2S,3aS,7aS)-2-(Isoxazolidine-2-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051489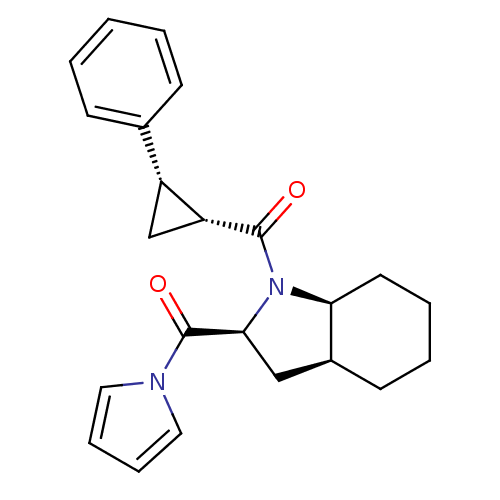 (((1R,2S)-2-Phenyl-cyclopropyl)-[(2S,3aS,7aS)-2-(py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051483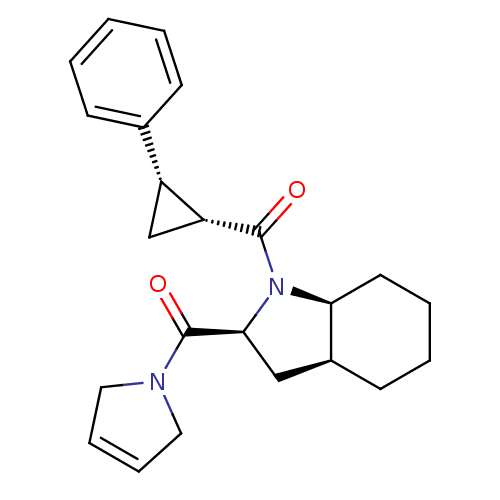 (CHEMBL80865 | [(2S,3aS,7aS)-2-(2,5-Dihydro-pyrrole...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094558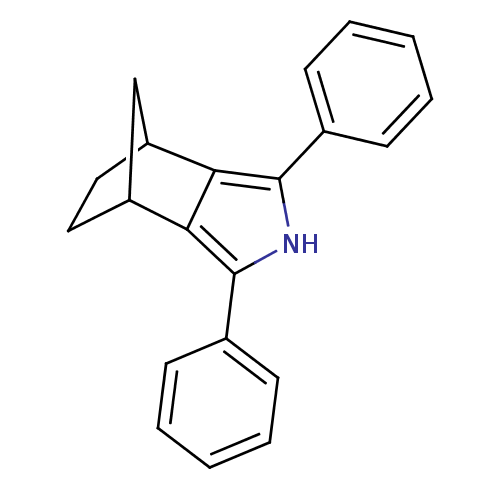 (3,5-Diphenyl-4-aza-tricyclo[5.2.1.0*2,6*]deca-2,5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051544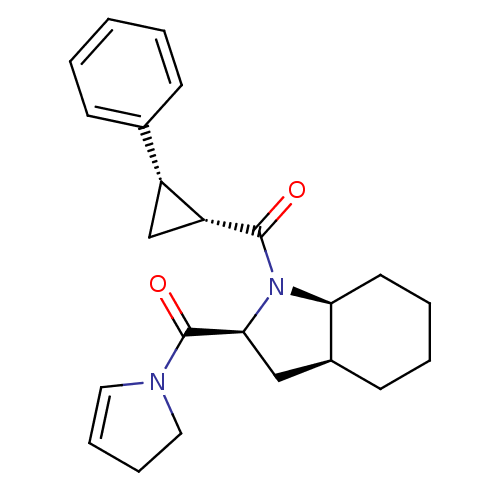 (CHEMBL311848 | [(2S,3aS,7aS)-2-(2,3-Dihydro-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094548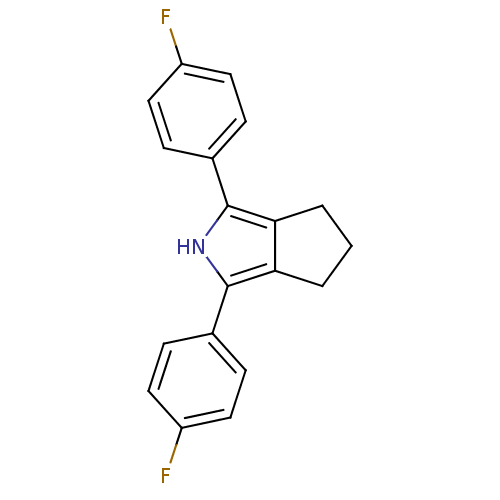 (1,3-Bis-(4-fluoro-phenyl)-2,4,5,6-tetrahydro-cyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50058491 (3-{4-[2-(4-{1-[4-(2-Carboxy-2-methyl-propane-1-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory concentration against Human Leukocyte Elastase from human sputum | J Med Chem 40: 1906-18 (1997) Article DOI: 10.1021/jm960772z BindingDB Entry DOI: 10.7270/Q2MS3RW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094528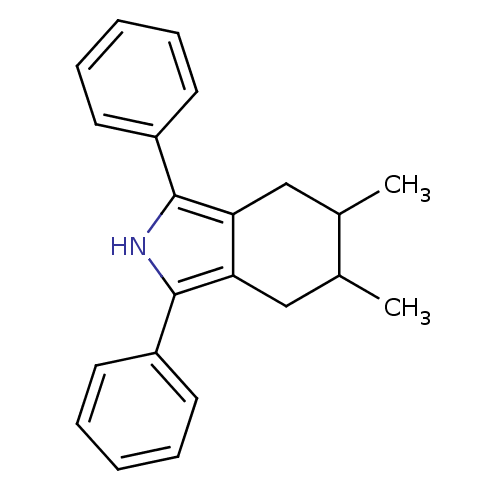 (5,6-Dimethyl-1,3-diphenyl-4,5,6,7-tetrahydro-2H-is...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051513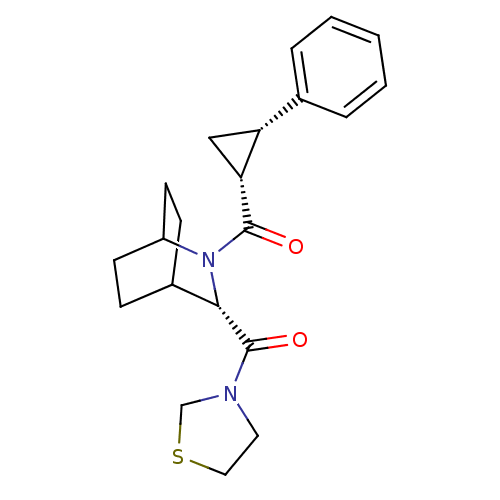 (((1R,2S)-2-Phenyl-cyclopropyl)-[(S)-3-(thiazolidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051496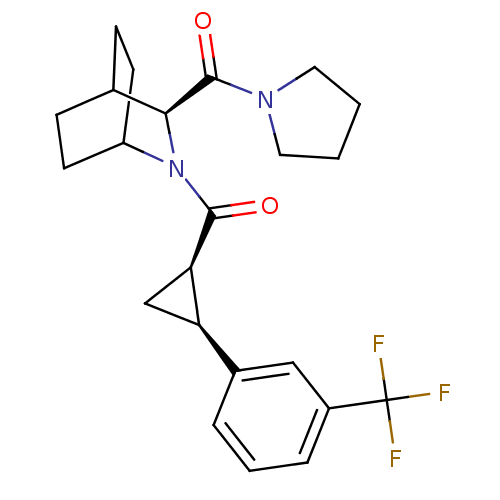 (CHEMBL310228 | [(S)-3-(Pyrrolidine-1-carbonyl)-2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094535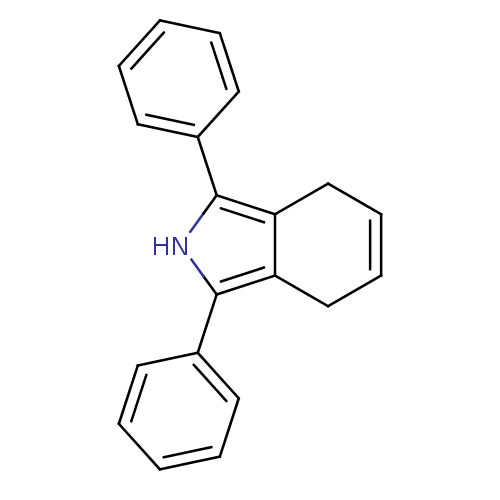 (1,3-diphenyl-4,7-dihydro-2H-isoindole | CHEMBL1408...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051537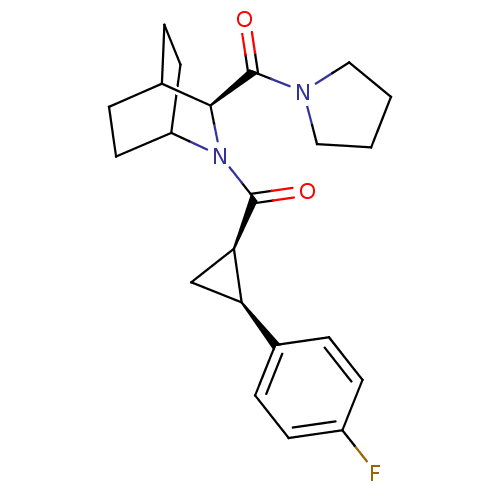 (CHEMBL80136 | [(1R,2S)-2-(4-Fluoro-phenyl)-cyclopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051543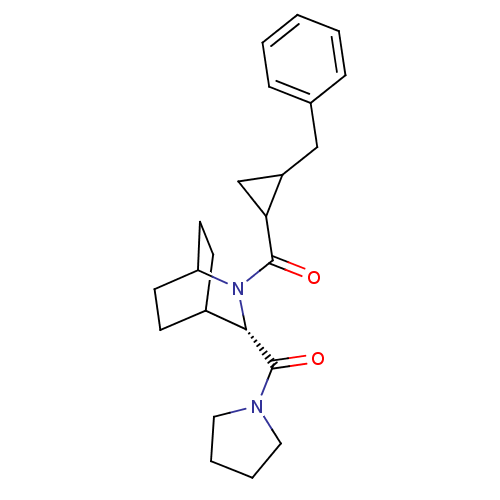 ((2-Benzyl-cyclopropyl)-[(S)-3-(pyrrolidine-1-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094561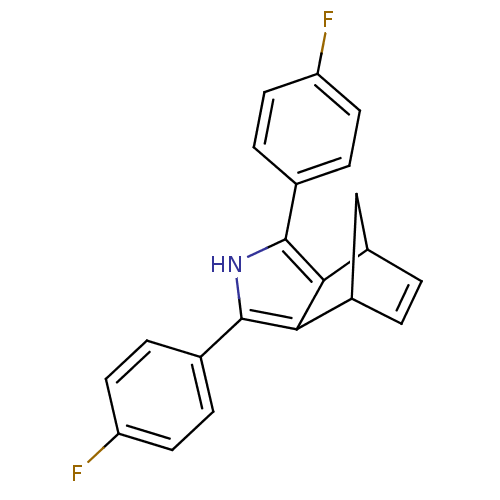 (3,5-Bis-(4-fluoro-phenyl)-4-aza-tricyclo[5.2.1.0*2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051484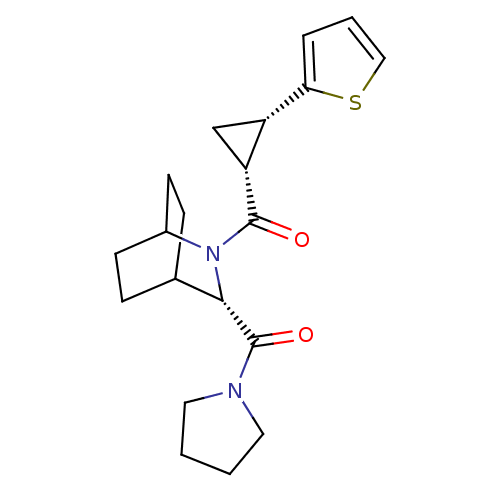 (CHEMBL307938 | [(S)-3-(Pyrrolidine-1-carbonyl)-2-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051484 (CHEMBL307938 | [(S)-3-(Pyrrolidine-1-carbonyl)-2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from Flavobacterium meningosepticam | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094541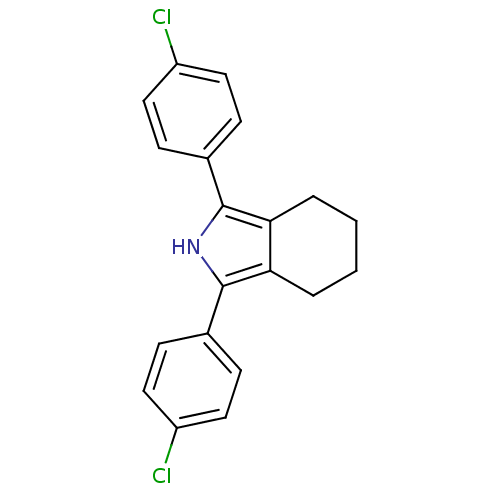 (1,3-Bis-(4-chloro-phenyl)-4,5,6,7-tetrahydro-2H-is...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051486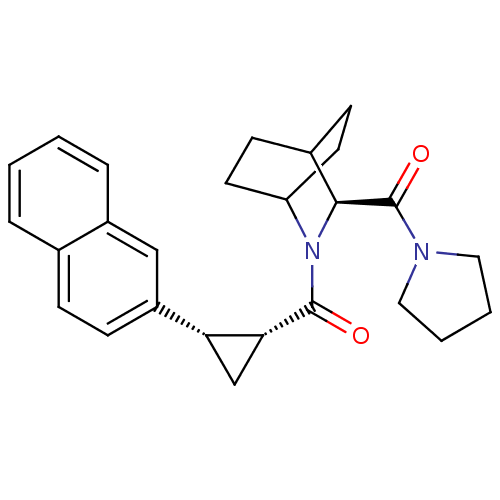 (((1R,2S)-2-Naphthalen-2-yl-cyclopropyl)-[(S)-3-(py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051518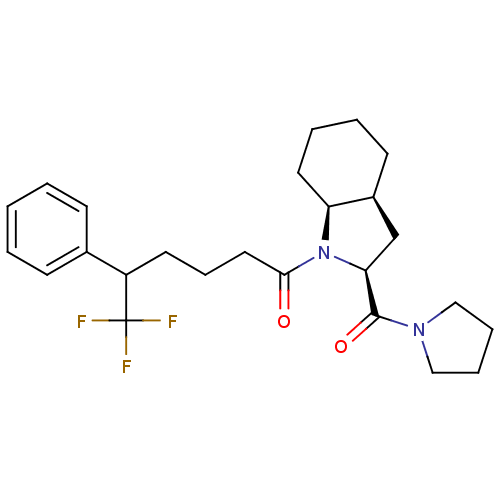 (6,6,6-Trifluoro-5-phenyl-1-[(2S,3aS,7aS)-2-(pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity evaluated against Post-proline cleaving enzyme from Flavobacterium meningosepticum | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Rattus norvegicus) | BDBM50051488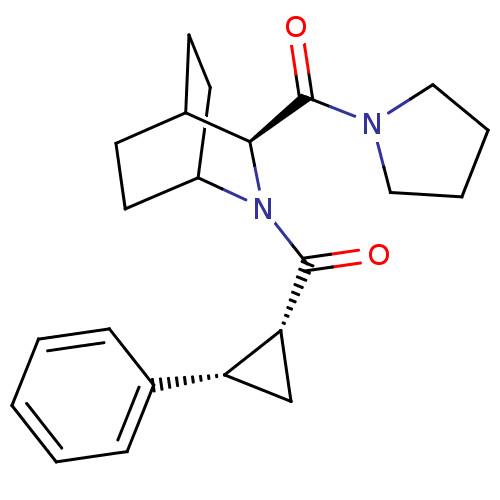 (((1R,2S)-2-Phenyl-cyclopropyl)-[(S)-3-(pyrrolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against Post-proline cleaving enzyme from rat cortex | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051540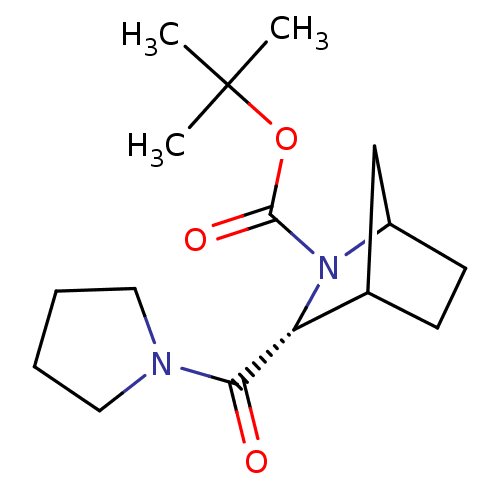 ((R)-3-(Pyrrolidine-1-carbonyl)-2-aza-bicyclo[2.2.1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity evaluated against Post-proline cleaving enzyme from Flavobacterium meningosepticum | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50454200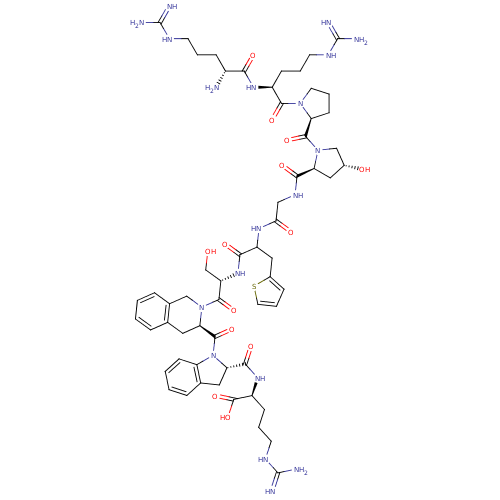 (CHEMBL2369824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity evaluated against Post-proline cleaving enzyme from Flavobacterium meningosepticum | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50051487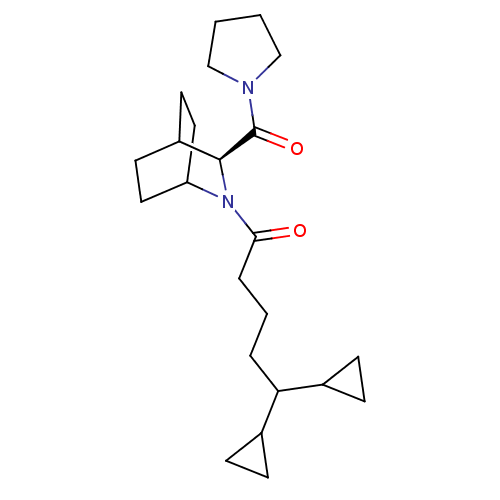 (5,5-Dicyclopropyl-1-[(S)-3-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibitory activity evaluated against Post-proline cleaving enzyme from Flavobacterium meningosepticum | J Med Chem 39: 2379-91 (1996) Article DOI: 10.1021/jm950858c BindingDB Entry DOI: 10.7270/Q2RF5T4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Tested for inhibition against Prostaglandin G/H synthase 1 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50094536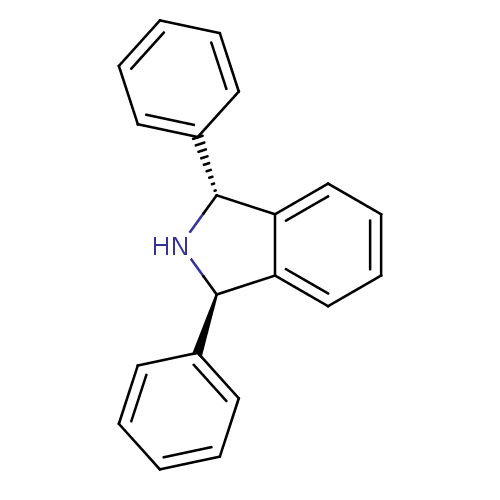 (1,3-Diphenyl-2,3-dihydro-1H-isoindole | CHEMBL1412...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherche Servier Curated by ChEMBL | Assay Description Inhibition against prostaglandin G/H synthase 2 from mouse resident macrophages | J Med Chem 43: 4582-93 (2001) BindingDB Entry DOI: 10.7270/Q23B60T1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 214 total ) | Next | Last >> |