 Found 5476 hits with Last Name = 'smith' and Initial = 'b'
Found 5476 hits with Last Name = 'smith' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576
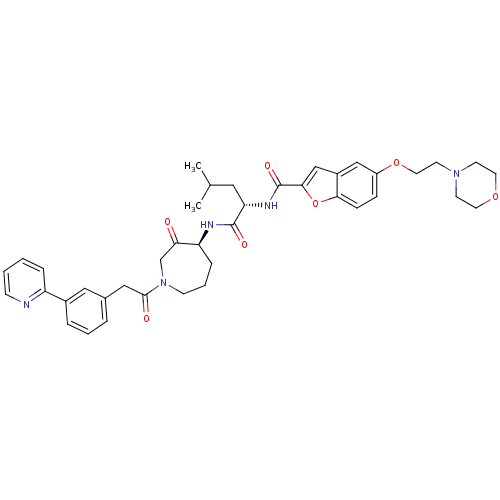
(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19770
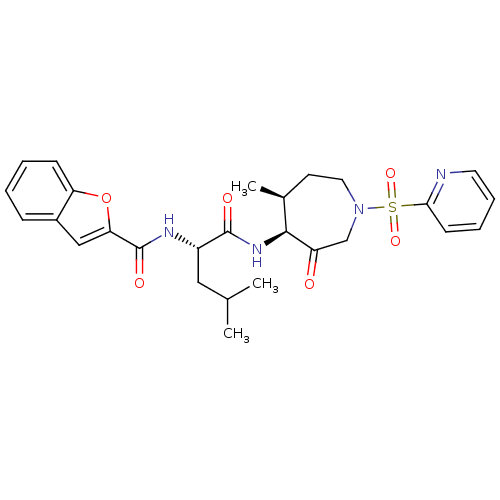
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
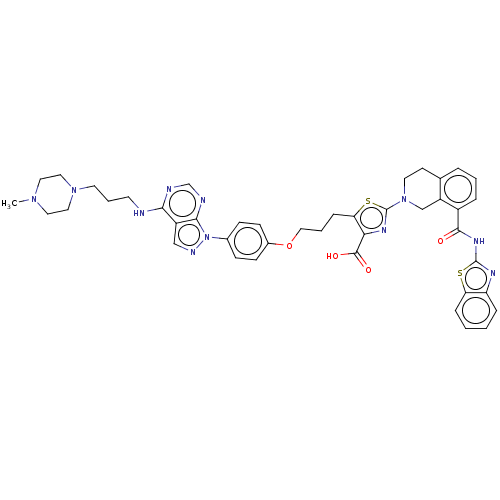
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
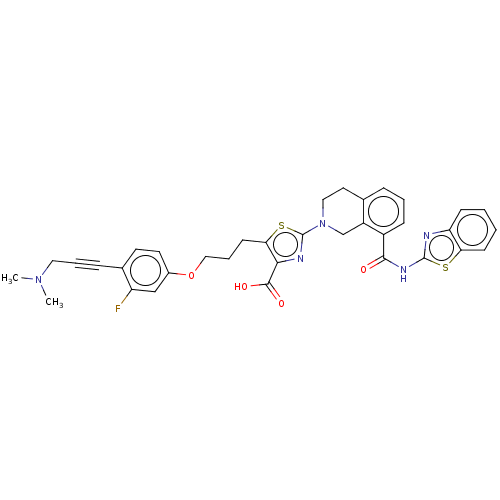
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
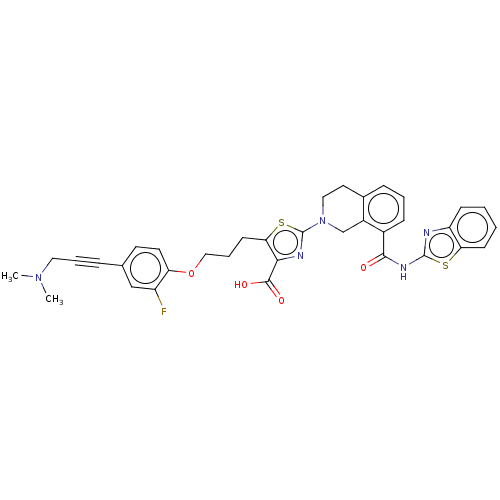
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
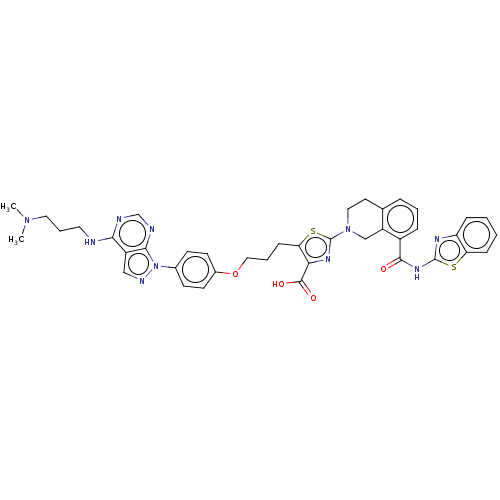
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
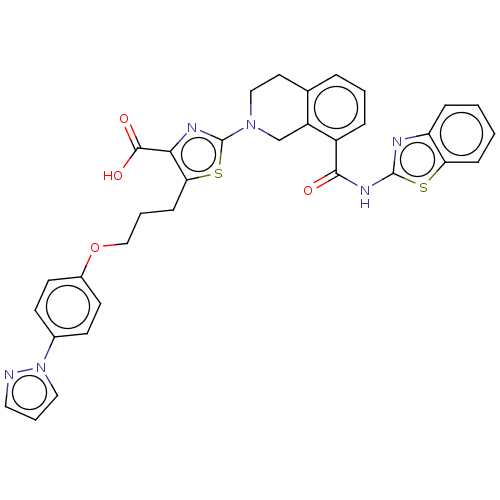
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756
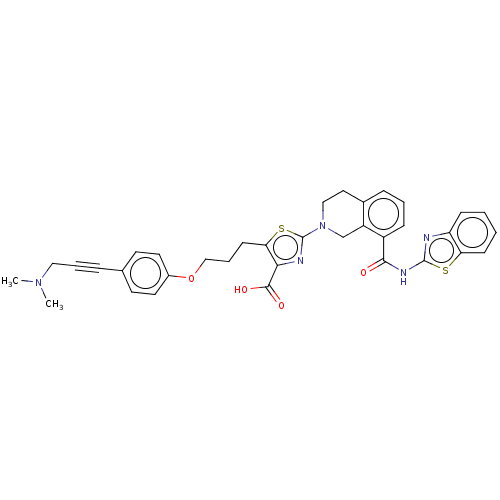
(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757

(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030751
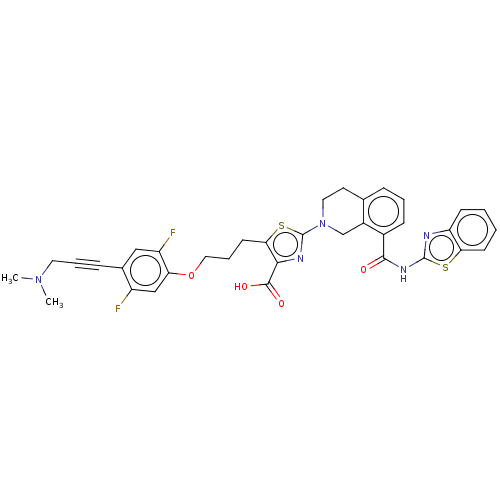
(CHEMBL3342334)Show SMILES CN(C)CC#Cc1cc(F)c(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H31F2N5O4S2/c1-41(2)15-6-9-22-18-26(37)28(19-25(22)36)46-17-7-13-30-31(33(44)45)39-35(48-30)42-16-14-21-8-5-10-23(24(21)20-42)32(43)40-34-38-27-11-3-4-12-29(27)47-34/h3-5,8,10-12,18-19H,7,13-17,20H2,1-2H3,(H,44,45)(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030755
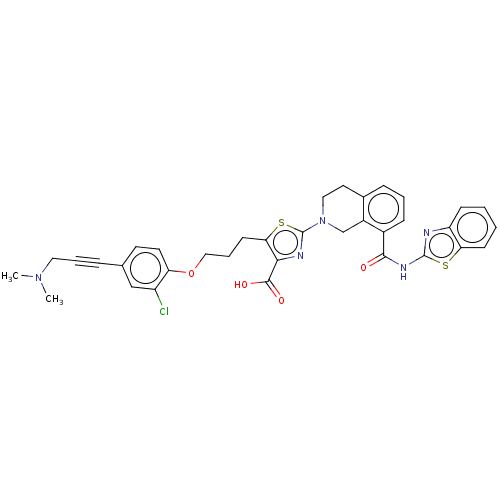
(CHEMBL3342198)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(Cl)c1 Show InChI InChI=1S/C35H32ClN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19770

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1[C@@H](C)CCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-20(29-27(34)23-15-19-8-4-5-9-22(19)37-23)26(33)30-25-18(3)11-13-31(16-21(25)32)38(35,36)24-10-6-7-12-28-24/h4-10,12,15,17-18,20,25H,11,13-14,16H2,1-3H3,(H,29,34)(H,30,33)/t18-,20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030761
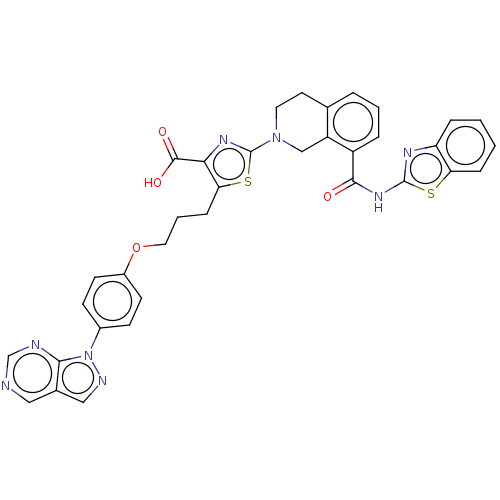
(CHEMBL3342192)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C35H28N8O4S2/c44-32(41-34-39-27-7-1-2-8-28(27)48-34)25-6-3-5-21-14-15-42(19-26(21)25)35-40-30(33(45)46)29(49-35)9-4-16-47-24-12-10-23(11-13-24)43-31-22(18-38-43)17-36-20-37-31/h1-3,5-8,10-13,17-18,20H,4,9,14-16,19H2,(H,45,46)(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758

(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19778

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CC[C@@H](C)N(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)14-21(30-27(34)24-15-19-8-4-5-9-23(19)37-24)26(33)29-20-12-11-18(3)31(16-22(20)32)38(35,36)25-10-6-7-13-28-25/h4-10,13,15,17-18,20-21H,11-12,14,16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50370582
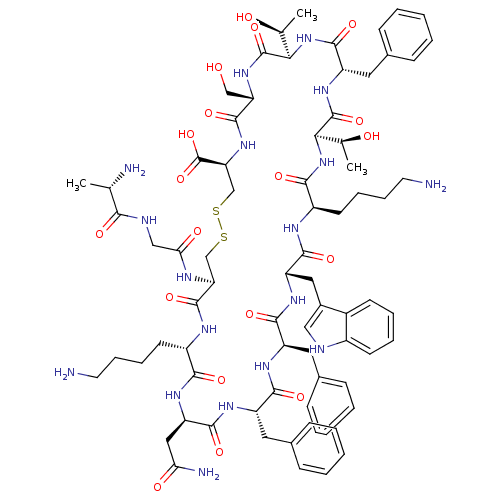
(CHEMBL1791306)Show SMILES C[C@H](O)[C@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@H](C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42-,43-,50-,51+,52-,53+,54-,55-,56+,57-,58-,59-,62+,63+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of human somatostatin receptor type 2 |
J Med Chem 48: 4025-30 (2005)
Article DOI: 10.1021/jm058184l
BindingDB Entry DOI: 10.7270/Q2736RP5 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754

(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19775
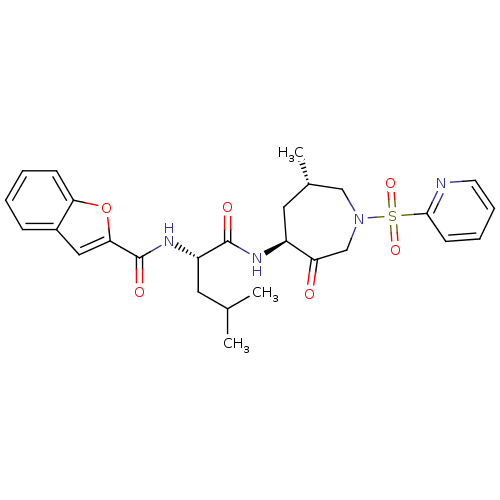
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50030760
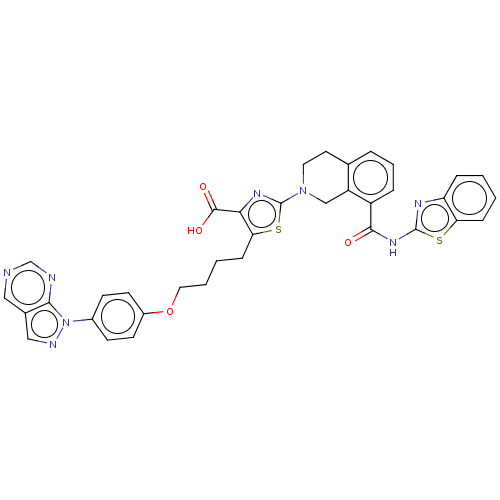
(CHEMBL3342193)Show SMILES OC(=O)c1nc(sc1CCCCOc1ccc(cc1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C36H30N8O4S2/c45-33(42-35-40-28-8-1-2-9-29(28)49-35)26-7-5-6-22-15-16-43(20-27(22)26)36-41-31(34(46)47)30(50-36)10-3-4-17-48-25-13-11-24(12-14-25)44-32-23(19-39-44)18-37-21-38-32/h1-2,5-9,11-14,18-19,21H,3-4,10,15-17,20H2,(H,46,47)(H,40,42,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-2 (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361235
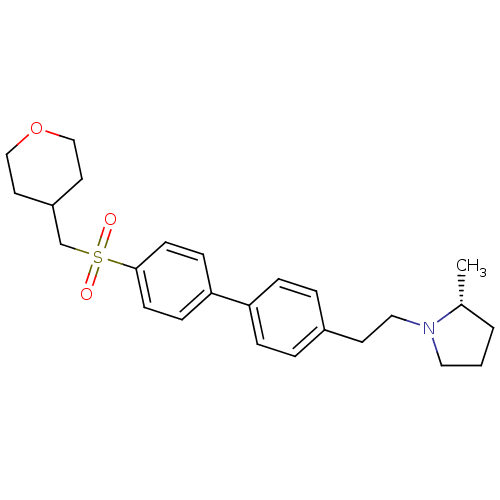
(CHEMBL1934525)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CC1CCOCC1 |r| Show InChI InChI=1S/C25H33NO3S/c1-20-3-2-15-26(20)16-12-21-4-6-23(7-5-21)24-8-10-25(11-9-24)30(27,28)19-22-13-17-29-18-14-22/h4-11,20,22H,2-3,12-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352357
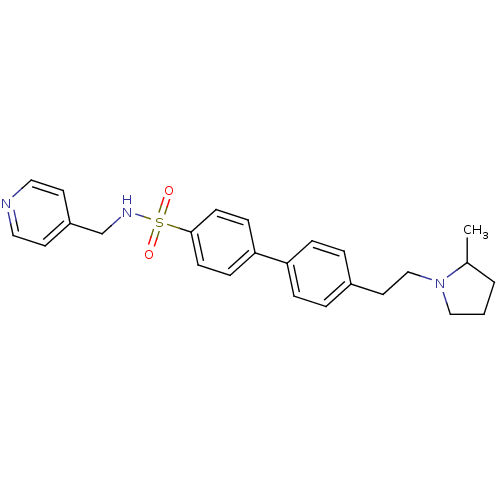
(CHEMBL558655)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NCc1ccncc1 Show InChI InChI=1S/C25H29N3O2S/c1-20-3-2-17-28(20)18-14-21-4-6-23(7-5-21)24-8-10-25(11-9-24)31(29,30)27-19-22-12-15-26-16-13-22/h4-13,15-16,20,27H,2-3,14,17-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361237
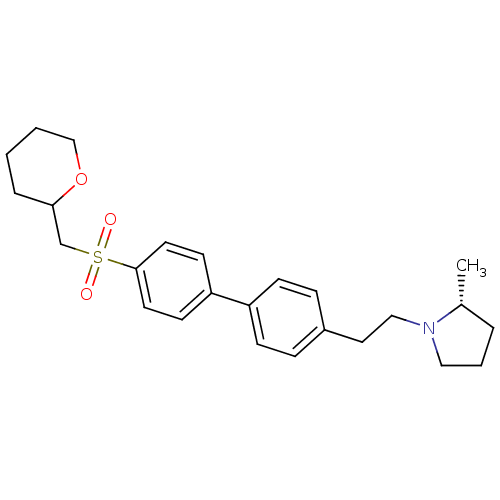
(CHEMBL1934527)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CC1CCCCO1 |r| Show InChI InChI=1S/C25H33NO3S/c1-20-5-4-16-26(20)17-15-21-7-9-22(10-8-21)23-11-13-25(14-12-23)30(27,28)19-24-6-2-3-18-29-24/h7-14,20,24H,2-6,15-19H2,1H3/t20-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756

(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352358
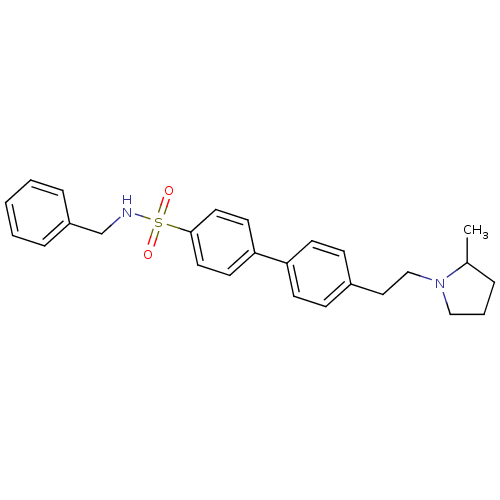
(CHEMBL558456)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NCc1ccccc1 Show InChI InChI=1S/C26H30N2O2S/c1-21-6-5-18-28(21)19-17-22-9-11-24(12-10-22)25-13-15-26(16-14-25)31(29,30)27-20-23-7-3-2-4-8-23/h2-4,7-16,21,27H,5-6,17-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030751

(CHEMBL3342334)Show SMILES CN(C)CC#Cc1cc(F)c(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H31F2N5O4S2/c1-41(2)15-6-9-22-18-26(37)28(19-25(22)36)46-17-7-13-30-31(33(44)45)39-35(48-30)42-16-14-21-8-5-10-23(24(21)20-42)32(43)40-34-38-27-11-3-4-12-29(27)47-34/h3-5,8,10-12,18-19H,7,13-17,20H2,1-2H3,(H,44,45)(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030749
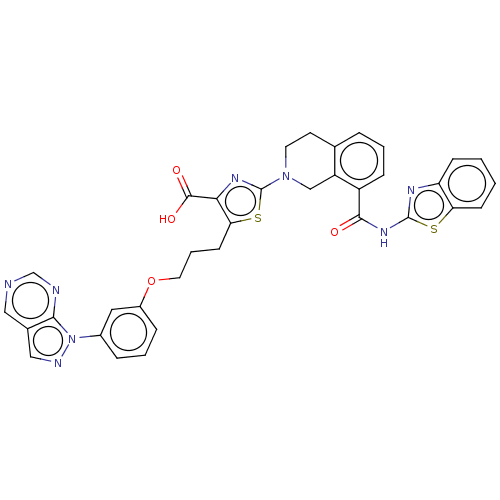
(CHEMBL3342190)Show SMILES OC(=O)c1nc(sc1CCCOc1cccc(c1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C35H28N8O4S2/c44-32(41-34-39-27-10-1-2-11-28(27)48-34)25-9-3-6-21-13-14-42(19-26(21)25)35-40-30(33(45)46)29(49-35)12-5-15-47-24-8-4-7-23(16-24)43-31-22(18-38-43)17-36-20-37-31/h1-4,6-11,16-18,20H,5,12-15,19H2,(H,45,46)(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030761

(CHEMBL3342192)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C35H28N8O4S2/c44-32(41-34-39-27-7-1-2-8-28(27)48-34)25-6-3-5-21-14-15-42(19-26(21)25)35-40-30(33(45)46)29(49-35)9-4-16-47-24-12-10-23(11-13-24)43-31-22(18-38-43)17-36-20-37-31/h1-3,5-8,10-13,17-18,20H,4,9,14-16,19H2,(H,45,46)(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030755

(CHEMBL3342198)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(Cl)c1 Show InChI InChI=1S/C35H32ClN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50085050
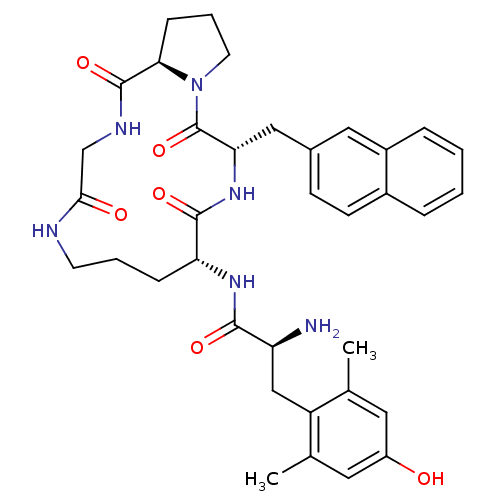
(CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-])Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCNC(=O)CNC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc3ccccc3c2)NC1=O Show InChI InChI=1S/C36H44N6O6/c1-21-15-26(43)16-22(2)27(21)19-28(37)33(45)40-29-9-5-13-38-32(44)20-39-35(47)31-10-6-14-42(31)36(48)30(41-34(29)46)18-23-11-12-24-7-3-4-8-25(24)17-23/h3-4,7-8,11-12,15-17,28-31,43H,5-6,9-10,13-14,18-20,37H2,1-2H3,(H,38,44)(H,39,47)(H,40,45)(H,41,46)/t28-,29+,30-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]- DSLET at Opioid receptor delta 1 in rat brain membrane homogenates |
J Med Chem 43: 551-9 (2000)
BindingDB Entry DOI: 10.7270/Q2WQ04H9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50085050

(CHEMBL152690 | H-Dmt-c [-D-Orn-2-Nal-D-Pro-Gly-])Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CCCNC(=O)CNC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccc3ccccc3c2)NC1=O Show InChI InChI=1S/C36H44N6O6/c1-21-15-26(43)16-22(2)27(21)19-28(37)33(45)40-29-9-5-13-38-32(44)20-39-35(47)31-10-6-14-42(31)36(48)30(41-34(29)46)18-23-11-12-24-7-3-4-8-25(24)17-23/h3-4,7-8,11-12,15-17,28-31,43H,5-6,9-10,13-14,18-20,37H2,1-2H3,(H,38,44)(H,39,47)(H,40,45)(H,41,46)/t28-,29+,30-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montr£al
Curated by ChEMBL
| Assay Description
Binding affinity was determined by displacement of [3H]- DAMGO at Opioid receptor mu 1 in rat brain membrane homogenates |
J Med Chem 43: 551-9 (2000)
BindingDB Entry DOI: 10.7270/Q2WQ04H9 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50414743
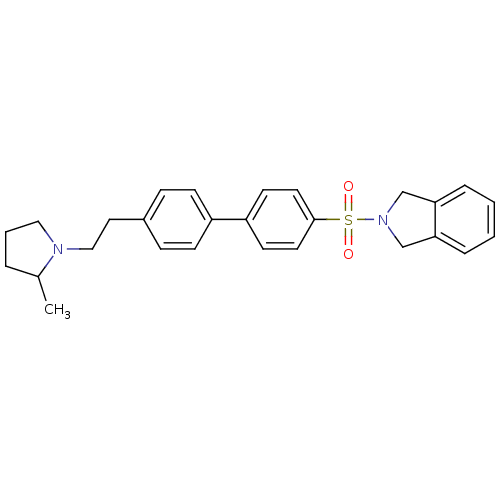
(CHEMBL564803)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N1Cc2ccccc2C1 Show InChI InChI=1S/C27H30N2O2S/c1-21-5-4-17-28(21)18-16-22-8-10-23(11-9-22)24-12-14-27(15-13-24)32(30,31)29-19-25-6-2-3-7-26(25)20-29/h2-3,6-15,21H,4-5,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361226
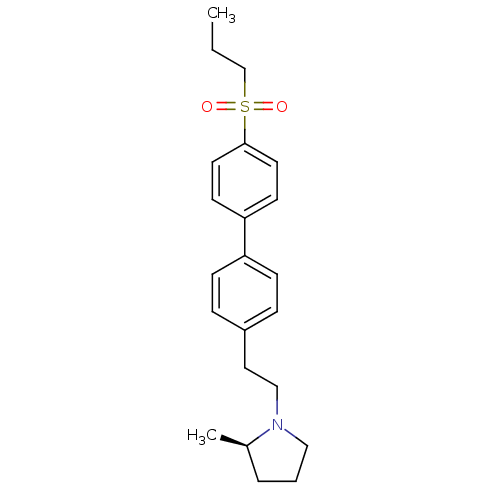
(CHEMBL1934356)Show SMILES CCCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C22H29NO2S/c1-3-17-26(24,25)22-12-10-21(11-13-22)20-8-6-19(7-9-20)14-16-23-15-4-5-18(23)2/h6-13,18H,3-5,14-17H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361228
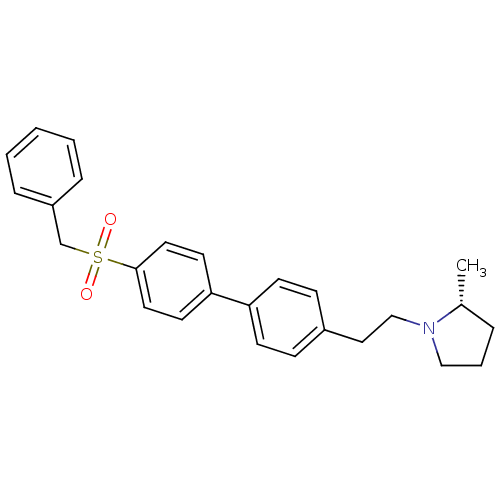
(CHEMBL1934358)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C26H29NO2S/c1-21-6-5-18-27(21)19-17-22-9-11-24(12-10-22)25-13-15-26(16-14-25)30(28,29)20-23-7-3-2-4-8-23/h2-4,7-16,21H,5-6,17-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19775

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1C[C@H](C)CN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C27H32N4O6S/c1-17(2)12-21(30-27(34)24-14-19-8-4-5-9-23(19)37-24)26(33)29-20-13-18(3)15-31(16-22(20)32)38(35,36)25-10-6-7-11-28-25/h4-11,14,17-18,20-21H,12-13,15-16H2,1-3H3,(H,29,33)(H,30,34)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | -52.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... |
J Med Chem 49: 1597-612 (2006)
Article DOI: 10.1021/jm050915u
BindingDB Entry DOI: 10.7270/Q2SQ8XP5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352354
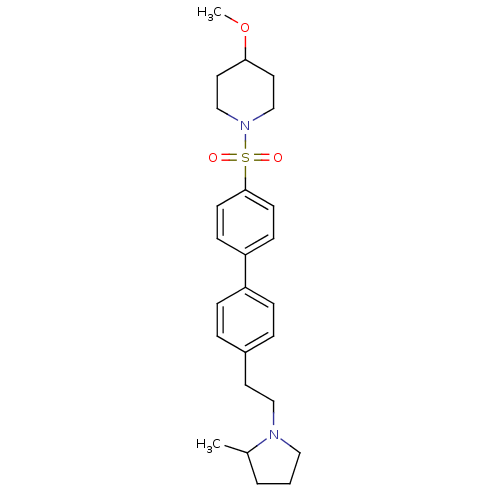
(CHEMBL554506)Show SMILES COC1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCCC2C)cc1 Show InChI InChI=1S/C25H34N2O3S/c1-20-4-3-16-26(20)17-13-21-5-7-22(8-6-21)23-9-11-25(12-10-23)31(28,29)27-18-14-24(30-2)15-19-27/h5-12,20,24H,3-4,13-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361233
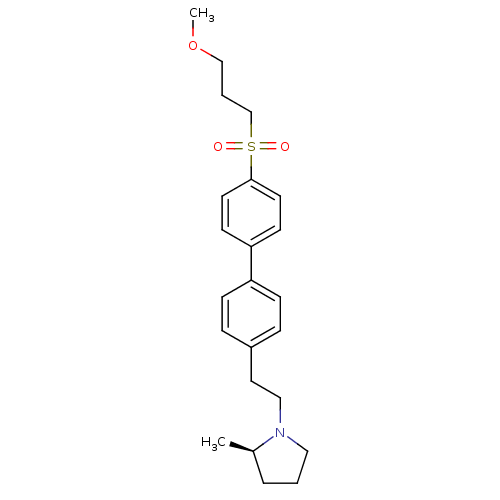
(CHEMBL1934523)Show SMILES COCCCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-19-5-3-15-24(19)16-14-20-6-8-21(9-7-20)22-10-12-23(13-11-22)28(25,26)18-4-17-27-2/h6-13,19H,3-5,14-18H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474384

(CHEMBL2113364)Show SMILES Cl.CS(=O)(=O)Oc1ccc2CCN(CC[C@H]3CC[C@@H](CC3)NC(=O)\C=C\c3ccc(F)cc3)CCc2c1 |r,wU:15.13,wD:18.20,(23.3,.02,;-6.64,-.16,;-5.58,-.78,;-6.64,-1.39,;-5.57,.45,;-4.24,-1.55,;-2.91,-.78,;-2.91,.78,;-1.53,1.57,;,.78,;.8,1.73,;2.31,1.43,;3.02,,;4.56,.01,;5.33,1.34,;6.87,1.35,;7.63,2.69,;9.17,2.69,;9.95,1.36,;9.18,.02,;7.64,.02,;11.49,1.36,;12.26,.02,;11.64,-1.04,;13.8,.03,;14.57,-1.31,;16.11,-1.31,;16.88,.03,;18.42,.03,;19.19,-1.3,;20.42,-1.3,;18.43,-2.64,;16.89,-2.64,;2.29,-1.45,;.76,-1.7,;,-.78,;-1.53,-1.57,)| Show InChI InChI=1S/C28H35FN2O4S.ClH/c1-36(33,34)35-27-12-7-23-15-18-31(19-16-24(23)20-27)17-14-22-4-10-26(11-5-22)30-28(32)13-6-21-2-8-25(29)9-3-21;/h2-3,6-9,12-13,20,22,26H,4-5,10-11,14-19H2,1H3,(H,30,32);1H/b13-6+;/t22-,26-; | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand |
J Med Chem 46: 4952-64 (2003)
Article DOI: 10.1021/jm030817d
BindingDB Entry DOI: 10.7270/Q2TT4TPR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361234
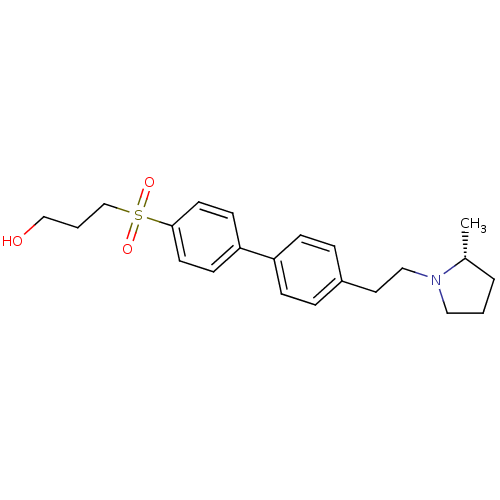
(CHEMBL1934524)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CCCO |r| Show InChI InChI=1S/C22H29NO3S/c1-18-4-2-14-23(18)15-13-19-5-7-20(8-6-19)21-9-11-22(12-10-21)27(25,26)17-3-16-24/h5-12,18,24H,2-4,13-17H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50142480

((E)-7-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)-3-hydroxy-o...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)N1C\C=C\CCCC(O)=O Show InChI InChI=1S/C19H31NO5/c1-2-3-6-9-15(21)11-12-16-17(22)14-18(23)20(16)13-8-5-4-7-10-19(24)25/h5,8,11-12,15-17,21-22H,2-4,6-7,9-10,13-14H2,1H3,(H,24,25)/b8-5+,12-11+/t15-,16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity was determined against prostanoid EP4 receptor |
Bioorg Med Chem Lett 14: 1655-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.063
BindingDB Entry DOI: 10.7270/Q2MK6CBC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361225
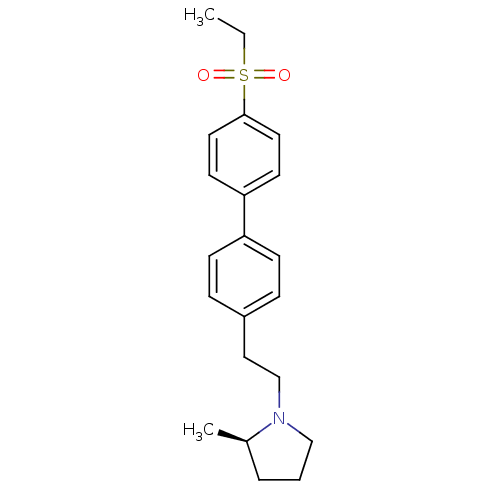
(CHEMBL1934355)Show SMILES CCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C21H27NO2S/c1-3-25(23,24)21-12-10-20(11-13-21)19-8-6-18(7-9-19)14-16-22-15-4-5-17(22)2/h6-13,17H,3-5,14-16H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083761
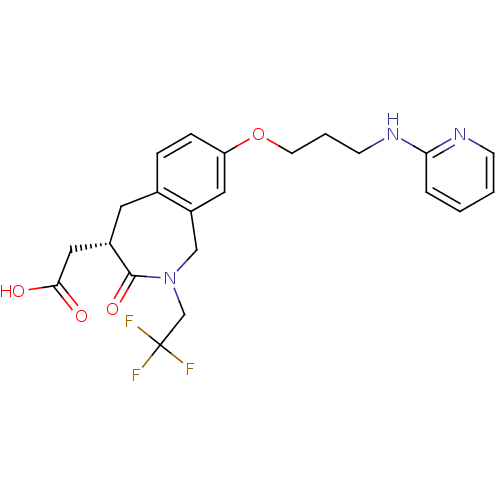
(CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352355
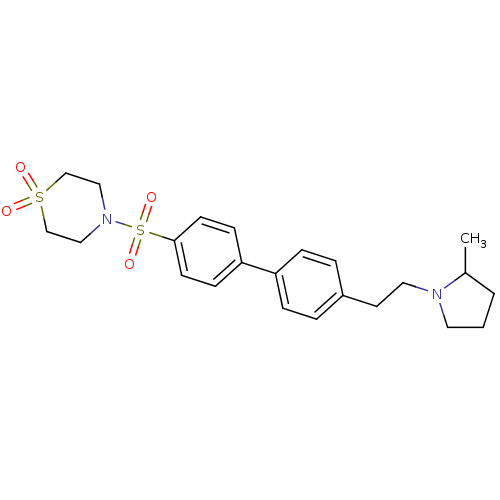
(CHEMBL560140)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C23H30N2O4S2/c1-19-3-2-13-24(19)14-12-20-4-6-21(7-5-20)22-8-10-23(11-9-22)31(28,29)25-15-17-30(26,27)18-16-25/h4-11,19H,2-3,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759

(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361232
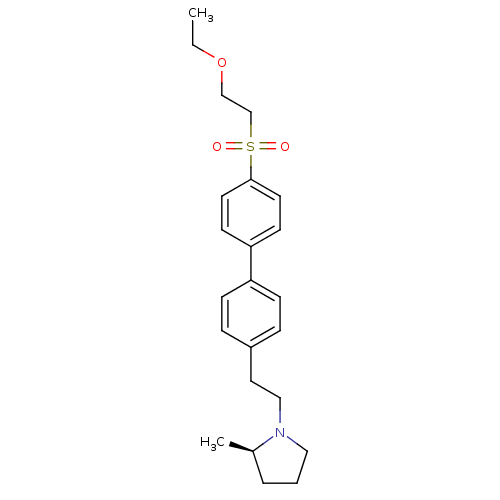
(CHEMBL1934522)Show SMILES CCOCCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-3-27-17-18-28(25,26)23-12-10-22(11-13-23)21-8-6-20(7-9-21)14-16-24-15-4-5-19(24)2/h6-13,19H,3-5,14-18H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50474396

(CHEMBL2113356)Show SMILES Cl.COc1ccc(\C=C\C(=O)N[C@H]2CC[C@H](CCN3CCc4ccc(OS(C)(=O)=O)cc4CC3)CC2)cc1 |r,wU:12.10,wD:15.14,(24.07,.02,;21.35,-2.37,;20.73,-1.3,;19.19,-1.3,;18.42,.03,;16.88,.03,;16.11,-1.31,;14.57,-1.31,;13.8,.03,;12.26,.02,;11.64,-1.04,;11.49,1.36,;9.95,1.36,;9.18,.02,;7.64,.02,;6.87,1.35,;5.33,1.34,;4.56,.01,;3.02,,;2.31,1.43,;.8,1.73,;,.78,;-1.53,1.57,;-2.91,.78,;-2.91,-.78,;-4.24,-1.55,;-5.58,-.78,;-6.64,-.16,;-6.64,-1.39,;-5.57,.45,;-1.53,-1.57,;,-.78,;.76,-1.7,;2.29,-1.45,;7.63,2.69,;9.17,2.69,;16.89,-2.64,;18.43,-2.64,)| Show InChI InChI=1S/C29H38N2O5S.ClH/c1-35-27-11-5-22(6-12-27)7-14-29(32)30-26-9-3-23(4-10-26)15-18-31-19-16-24-8-13-28(36-37(2,33)34)21-25(24)17-20-31;/h5-8,11-14,21,23,26H,3-4,9-10,15-20H2,1-2H3,(H,30,32);1H/b14-7+;/t23-,26-; | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D3 expressed in CHO cells by using [125I]iodosulpiride as radioligand |
J Med Chem 46: 4952-64 (2003)
Article DOI: 10.1021/jm030817d
BindingDB Entry DOI: 10.7270/Q2TT4TPR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data