 Found 4514 hits with Last Name = 'anne' and Initial = 'c'
Found 4514 hits with Last Name = 'anne' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279

(CHEMBL4436207)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)B(O)O |r| Show InChI InChI=1S/C27H37BN2O9S/c1-18(2)15-30(40(35,36)21-10-8-20(9-11-21)28(33)34)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)39-25-17-38-26-22(25)12-13-37-26/h3-11,18,22-26,31,33-34H,12-17H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to wild type HIV1 protease |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737
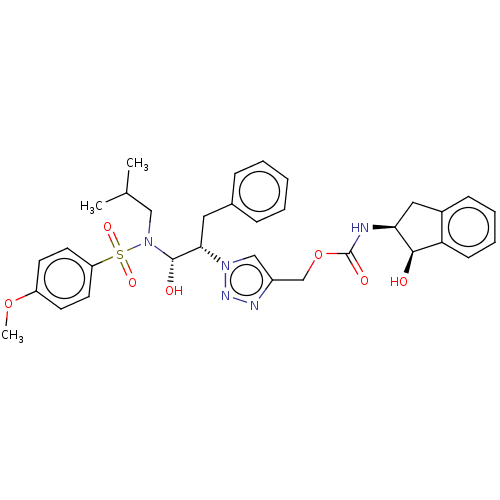
(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using Pr SF-2-WTQ7K-Pr as substrate after 24 hrs by LC/MS-MS analysis |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
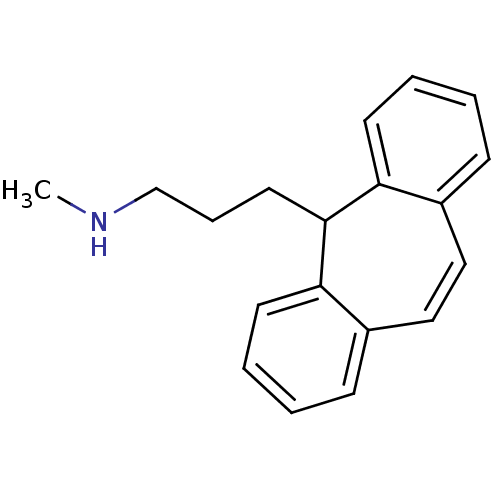
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NET expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 55: 3488-501 (2012)
Article DOI: 10.1021/jm300138g
BindingDB Entry DOI: 10.7270/Q2KW5H2V |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569716
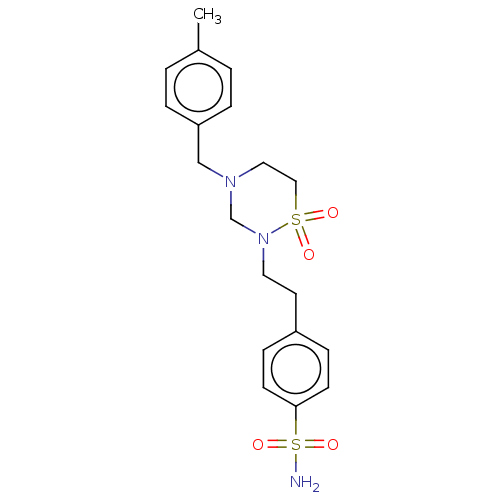
(CHEMBL4870611)Show SMILES Cc1ccc(CN2CCS(=O)(=O)N(CCc3ccc(cc3)S(N)(=O)=O)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750
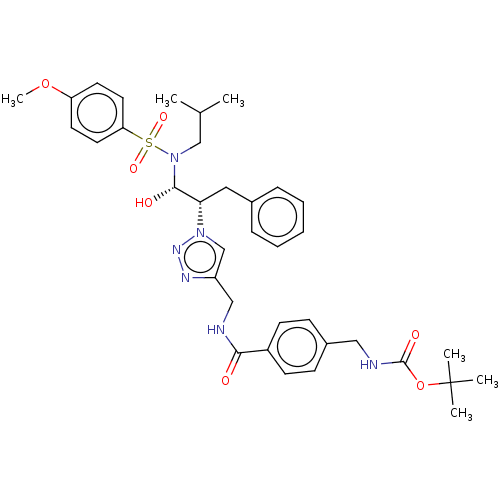
(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50255365
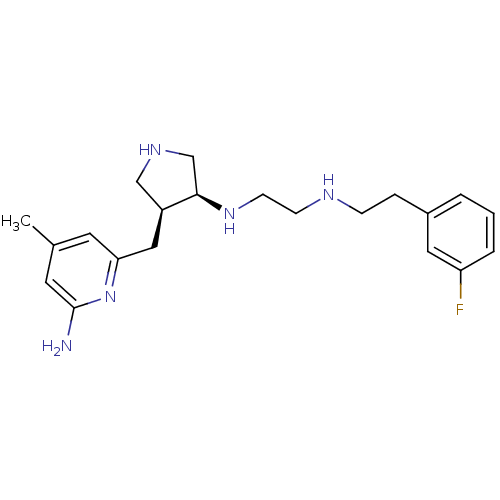
((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS |
J Med Chem 58: 1064-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00057
BindingDB Entry DOI: 10.7270/Q2348N2Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM25400
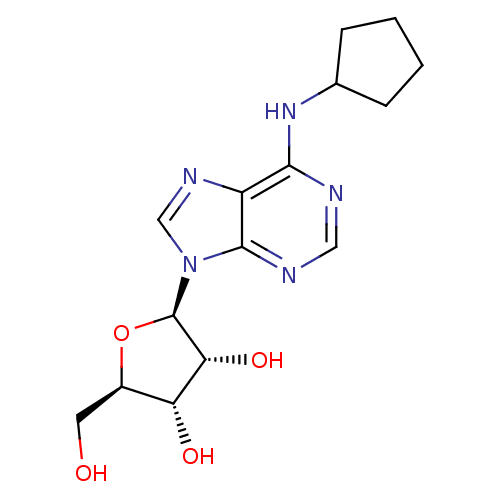
((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity for adenosine A1 receptor was assayed by displacement of [3H]DPCPX from rat cortical membranes. |
J Med Chem 43: 250-60 (2000)
BindingDB Entry DOI: 10.7270/Q2930TW1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50526946
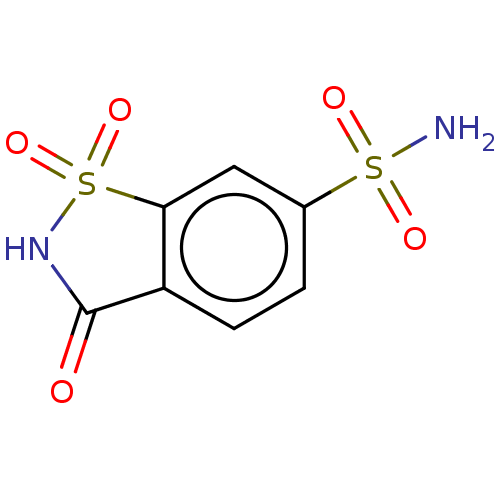
(CHEMBL4456920)Show InChI InChI=1S/C7H6N2O5S2/c8-15(11,12)4-1-2-5-6(3-4)16(13,14)9-7(5)10/h1-3H,(H,9,10)(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569722
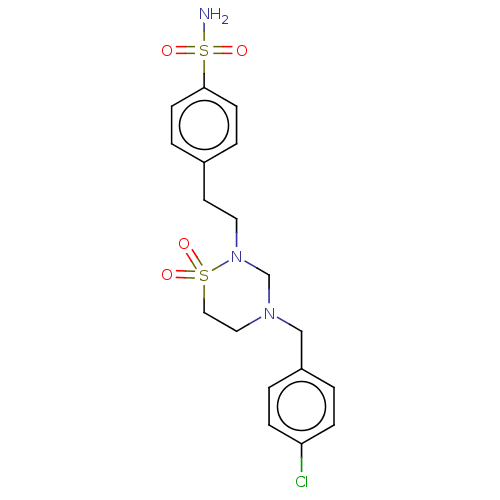
(CHEMBL4870726)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(Cl)cc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Rattus norvegicus (rat)) | BDBM50278675

((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat nNOS |
J Med Chem 58: 1064-6 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00057
BindingDB Entry DOI: 10.7270/Q2348N2Q |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM25400

((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)ncnc12 Show InChI InChI=1S/C15H21N5O4/c21-5-9-11(22)12(23)15(24-9)20-7-18-10-13(16-6-17-14(10)20)19-8-3-1-2-4-8/h6-9,11-12,15,21-23H,1-5H2,(H,16,17,19)/t9-,11-,12-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research
Curated by ChEMBL
| Assay Description
Affinity towards adenosine A1 receptor from rat cortical membranes using [3H]DPCPX |
J Med Chem 44: 2966-75 (2001)
BindingDB Entry DOI: 10.7270/Q2J38T85 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity for adenosine A1 receptor was assayed by displacement of [3H]DPCPX from rat cortical membranes. |
J Med Chem 43: 250-60 (2000)
BindingDB Entry DOI: 10.7270/Q2930TW1 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50200943

(CHEMBL269769 | cyclopentyl (2S,3S)-3-[4-([4-(5-chl...)Show SMILES Cc1ccc(Cl)cc1N1CCN(Cc2nnn([C@@H](Cc3ccccc3)[C@H](Cc3ccccc3)NC(=O)OC3CCCC3)c2CO)CC1 |r| Show InChI InChI=1S/C37H45ClN6O3/c1-27-16-17-30(38)24-34(27)43-20-18-42(19-21-43)25-33-36(26-45)44(41-40-33)35(23-29-12-6-3-7-13-29)32(22-28-10-4-2-5-11-28)39-37(46)47-31-14-8-9-15-31/h2-7,10-13,16-17,24,31-32,35,45H,8-9,14-15,18-23,25-26H2,1H3,(H,39,46)/t32-,35-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease after 48 hrs by micro-titer plate assay |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | |
Sodium- and chloride-dependent GABA transporter 1
(Rattus norvegicus) | BDBM50505281
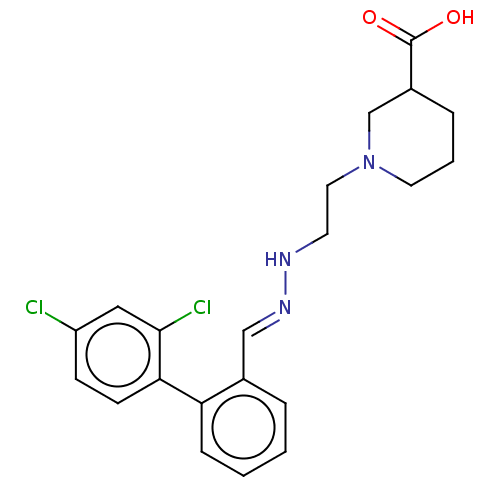
(CHEMBL2315948)Show SMILES OC(=O)C1CCCN(CCN\N=C\c2ccccc2-c2ccc(Cl)cc2Cl)C1 Show InChI InChI=1S/C21H23Cl2N3O2/c22-17-7-8-19(20(23)12-17)18-6-2-1-4-15(18)13-25-24-9-11-26-10-3-5-16(14-26)21(27)28/h1-2,4,6-8,12-13,16,24H,3,5,9-11,14H2,(H,27,28)/b25-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of NO711 binding to mouse GAT1 receptor expressed in HEK293 cell membranes incubated for 1 hr by LC-ESI-MS/MS analysis |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50569722

(CHEMBL4870726)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(Cl)cc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569706
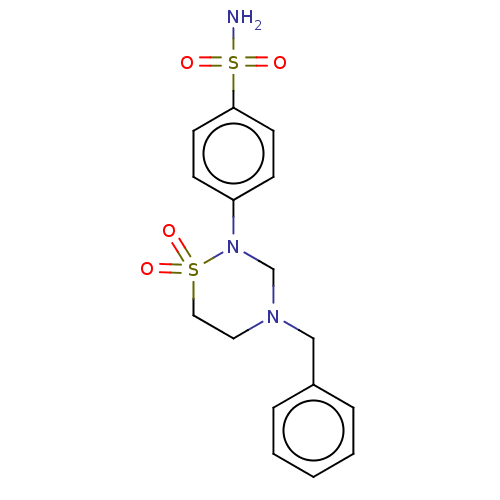
(CHEMBL4872649)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CN(Cc2ccccc2)CCS1(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50569717
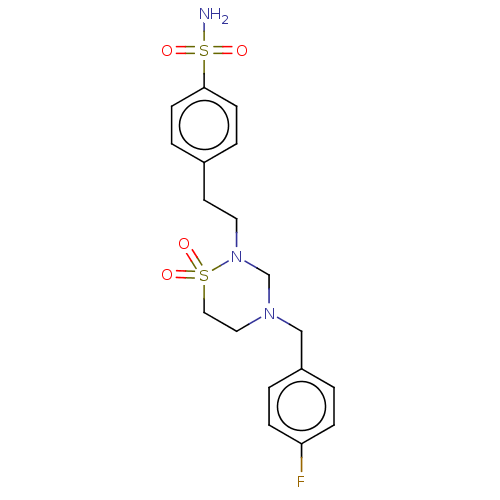
(CHEMBL4853346)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(F)cc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569712

(CHEMBL4863712)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CN(Cc2c(F)c(F)c(F)c(F)c2F)CCS1(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research
Curated by ChEMBL
| Assay Description
Affinity towards adenosine A1 receptor from rat cortical membranes using [3H]DPCPX |
J Med Chem 44: 2966-75 (2001)
BindingDB Entry DOI: 10.7270/Q2J38T85 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50569720
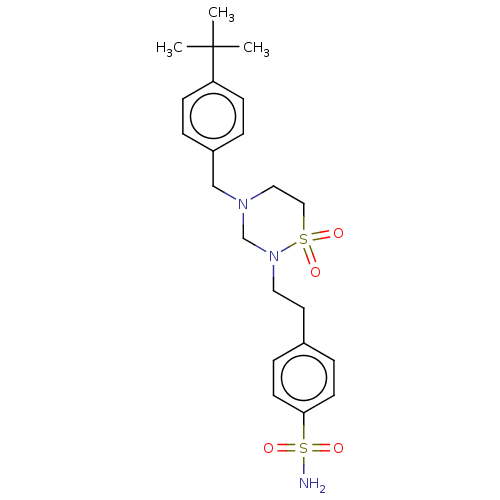
(CHEMBL4848588)Show SMILES CC(C)(C)c1ccc(CN2CCS(=O)(=O)N(CCc3ccc(cc3)S(N)(=O)=O)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50599475
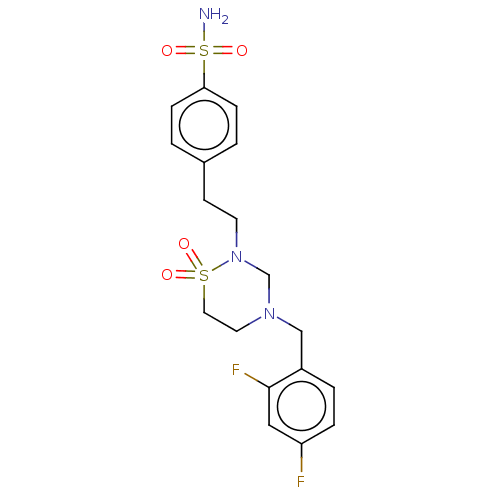
(CHEMBL5171574)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(F)cc3F)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483
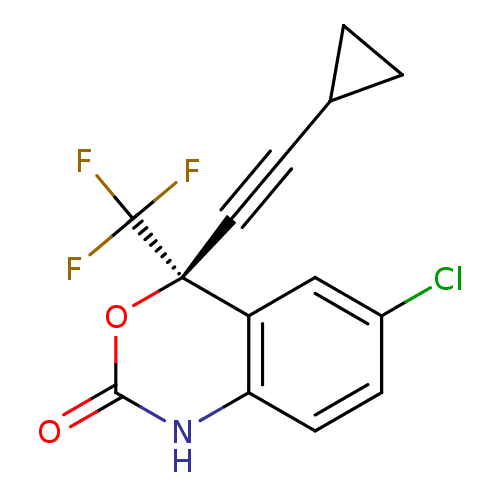
((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Volgograd State Medical University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of wild type HIV1 reverse transcriptase p66/p51 after 30 mins by liquid scintillation counting |
Bioorg Med Chem 21: 1150-8 (2013)
Article DOI: 10.1016/j.bmc.2012.12.027
BindingDB Entry DOI: 10.7270/Q2NZ8BKZ |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82F mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163752
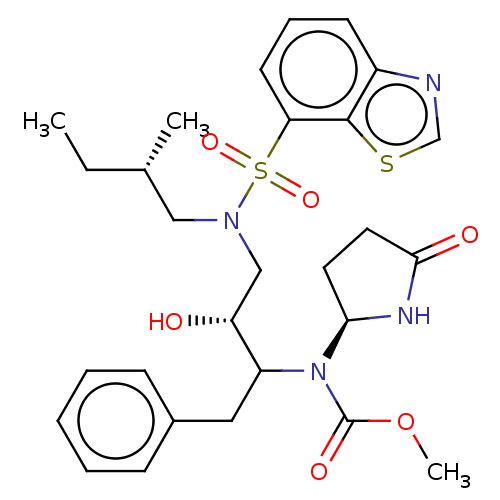
(CHEMBL3799500)Show SMILES CC[C@H](C)CN(C[C@@H](O)C(Cc1ccccc1)N([C@H]1CCC(=O)N1)C(=O)OC)S(=O)(=O)c1cccc2ncsc12 |r| Show InChI InChI=1S/C28H36N4O6S2/c1-4-19(2)16-31(40(36,37)24-12-8-11-21-27(24)39-18-29-21)17-23(33)22(15-20-9-6-5-7-10-20)32(28(35)38-3)25-13-14-26(34)30-25/h5-12,18-19,22-23,25,33H,4,13-17H2,1-3H3,(H,30,34)/t19-,22?,23+,25-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Binding affinity to wild type HIV1 protease |
J Med Chem 59: 2849-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00497
BindingDB Entry DOI: 10.7270/Q2JH3P3H |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease G48V mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569708
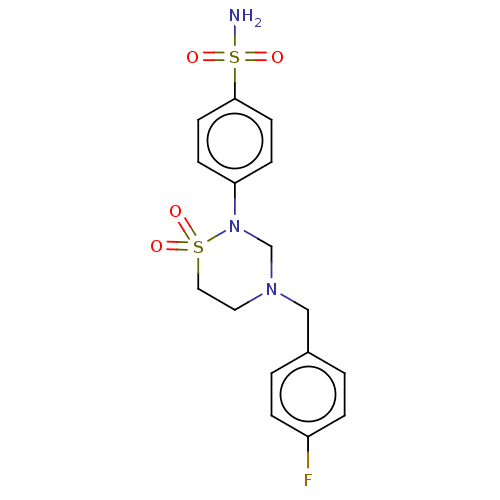
(CHEMBL4856228)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CN(Cc2ccc(F)cc2)CCS1(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50569719
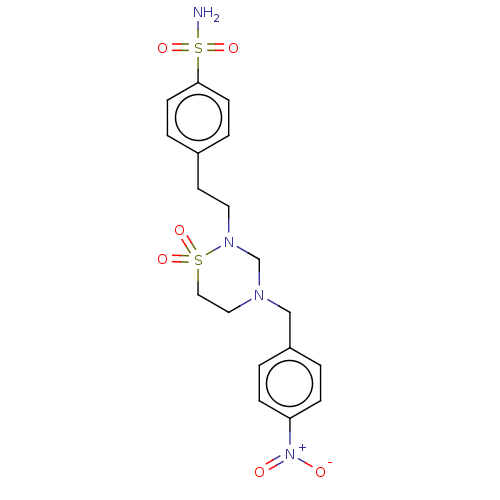
(CHEMBL4858317)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(cc3)[N+]([O-])=O)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737

(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569707
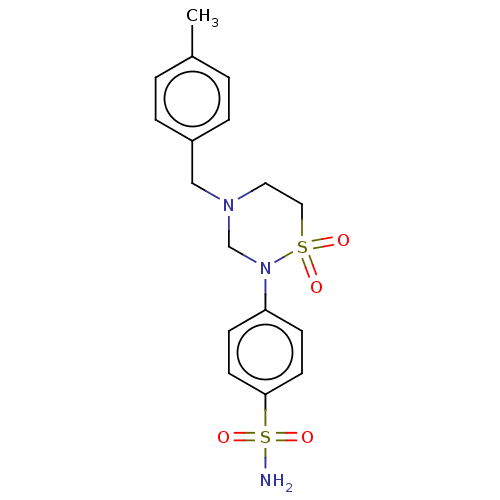
(CHEMBL4871439)Show SMILES Cc1ccc(CN2CCS(=O)(=O)N(C2)c2ccc(cc2)S(N)(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163750

(CHEMBL3797292)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(CNC(=O)c2ccc(CNC(=O)OC(C)(C)C)cc2)nn1 |r| Show InChI InChI=1S/C36H46N6O7S/c1-25(2)23-42(50(46,47)31-18-16-30(48-6)17-19-31)34(44)32(20-26-10-8-7-9-11-26)41-24-29(39-40-41)22-37-33(43)28-14-12-27(13-15-28)21-38-35(45)49-36(3,4)5/h7-19,24-25,32,34,44H,20-23H2,1-6H3,(H,37,43)(H,38,45)/t32-,34-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease V82A mutant |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569720

(CHEMBL4848588)Show SMILES CC(C)(C)c1ccc(CN2CCS(=O)(=O)N(CCc3ccc(cc3)S(N)(=O)=O)C2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50569715
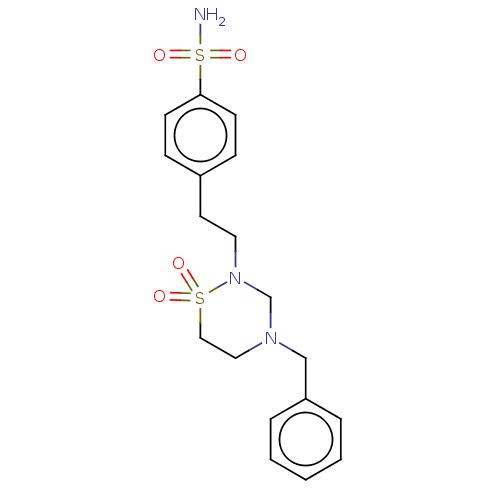
(CHEMBL4862757)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccccc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Hydroxycarboxylic acid receptor 2
(Rattus norvegicus) | BDBM23515
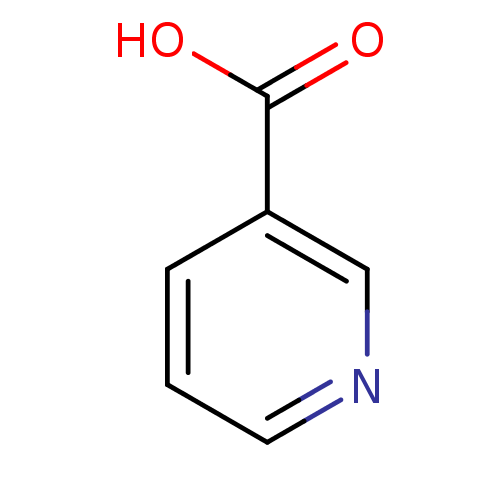
(CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...)Show InChI InChI=1S/C6H5NO2/c8-6(9)5-2-1-3-7-4-5/h1-4H,(H,8,9) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden/Amsterdam Center for Drug Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-nicotinic acid (20 nM) binding to nicotinic acid receptor in rat spleen membrane. |
J Med Chem 46: 3945-51 (2003)
Article DOI: 10.1021/jm030888c
BindingDB Entry DOI: 10.7270/Q2V988TK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569709
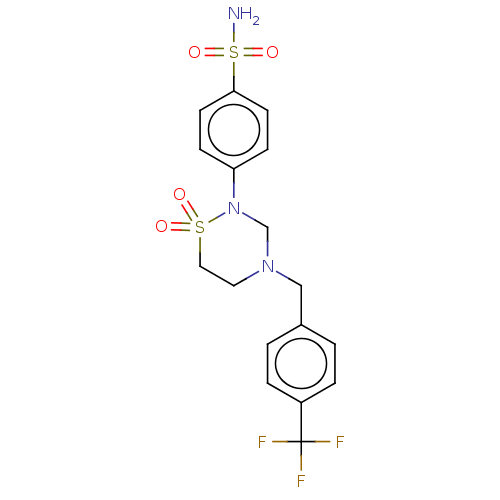
(CHEMBL4855465)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CN(Cc2ccc(cc2)C(F)(F)F)CCS1(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50569715

(CHEMBL4862757)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccccc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50569718
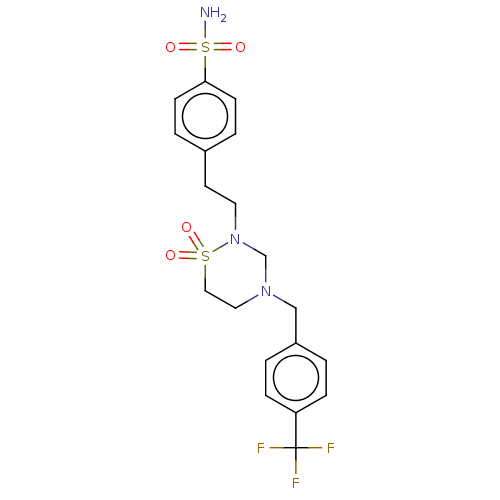
(CHEMBL4865453)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(cc3)C(F)(F)F)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50369627

(CHEMBL608306)Show SMILES OC[C@H]1OC([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C17H24ClN5O4/c18-17-21-14(20-9-5-3-1-2-4-6-9)11-15(22-17)23(8-19-11)16-13(26)12(25)10(7-24)27-16/h8-10,12-13,16,24-26H,1-7H2,(H,20,21,22)/t10-,12-,13-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 37.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Binding affinity for adenosine A1 receptor was assayed by displacement of [3H]DPCPX from rat cortical membranes. |
J Med Chem 43: 250-60 (2000)
BindingDB Entry DOI: 10.7270/Q2930TW1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50569709

(CHEMBL4855465)Show SMILES NS(=O)(=O)c1ccc(cc1)N1CN(Cc2ccc(cc2)C(F)(F)F)CCS1(=O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50569722

(CHEMBL4870726)Show SMILES NS(=O)(=O)c1ccc(CCN2CN(Cc3ccc(Cl)cc3)CCS2(=O)=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113956
BindingDB Entry DOI: 10.7270/Q2X06C4M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data