 Found 1666 hits with Last Name = 'berry' and Initial = 'c'
Found 1666 hits with Last Name = 'berry' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50283607
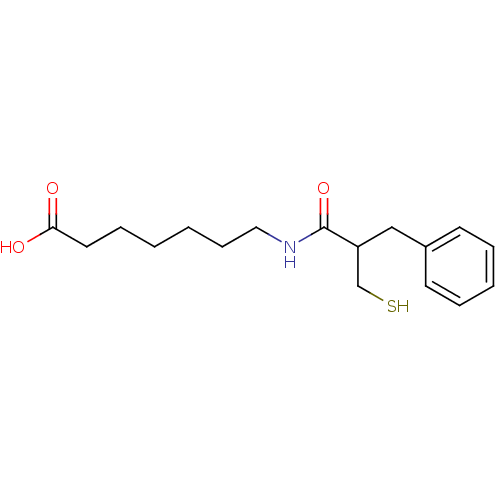
(7-(2-Mercaptomethyl-3-phenyl-propionylamino)-hepta...)Show InChI InChI=1S/C17H25NO3S/c19-16(20)10-6-1-2-7-11-18-17(21)15(13-22)12-14-8-4-3-5-9-14/h3-5,8-9,15,22H,1-2,6-7,10-13H2,(H,18,21)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition against neutral endopeptidase 24.11 (NEP) in rat kidney cortex membrane |
Bioorg Med Chem Lett 4: 2673-2676 (1994)
Article DOI: 10.1016/S0960-894X(01)80694-6
BindingDB Entry DOI: 10.7270/Q2X34XXT |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027081
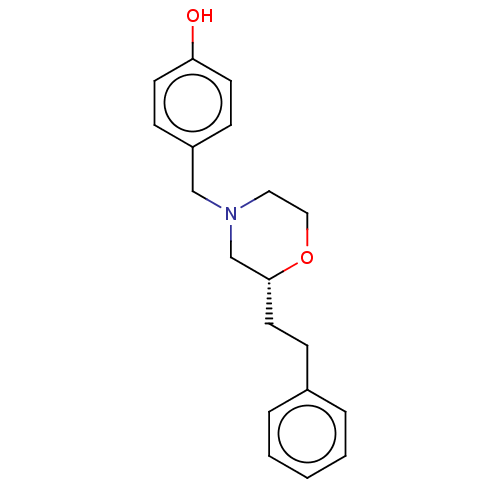
(CHEMBL3335555)Show InChI InChI=1S/C19H23NO2/c21-18-9-6-17(7-10-18)14-20-12-13-22-19(15-20)11-8-16-4-2-1-3-5-16/h1-7,9-10,19,21H,8,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027083
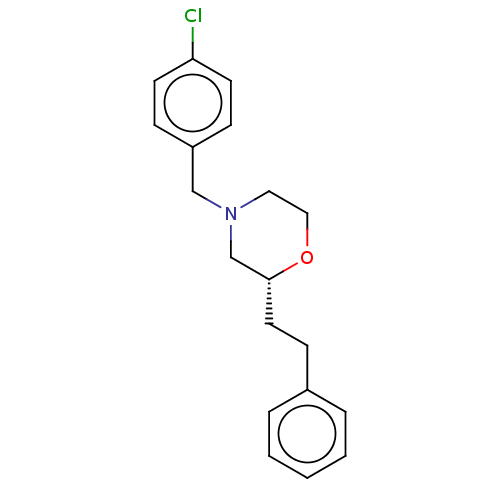
(CHEMBL3335556)Show InChI InChI=1S/C19H22ClNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078
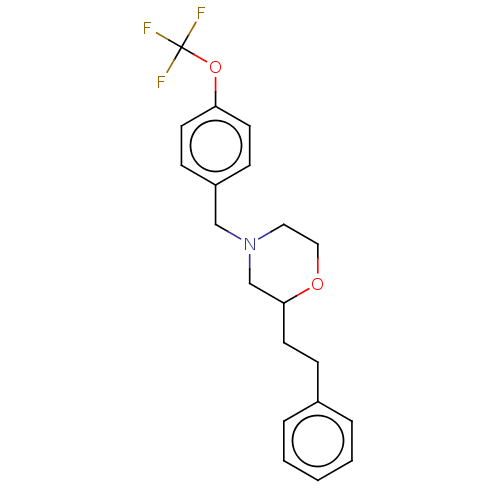
(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027079
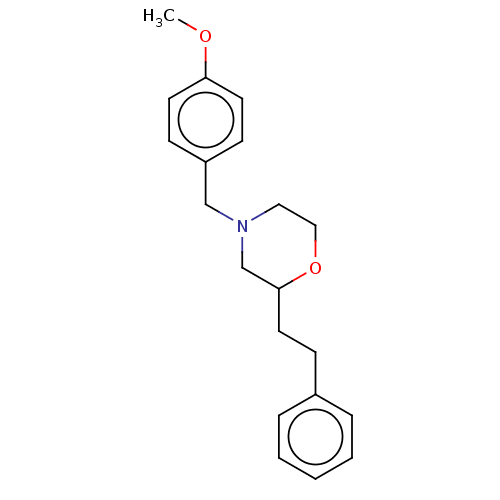
(CHEMBL3335539)Show InChI InChI=1S/C20H25NO2/c1-22-19-10-8-18(9-11-19)15-21-13-14-23-20(16-21)12-7-17-5-3-2-4-6-17/h2-6,8-11,20H,7,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080
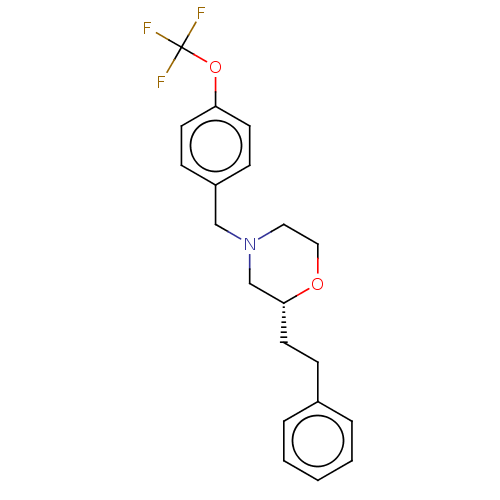
(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074
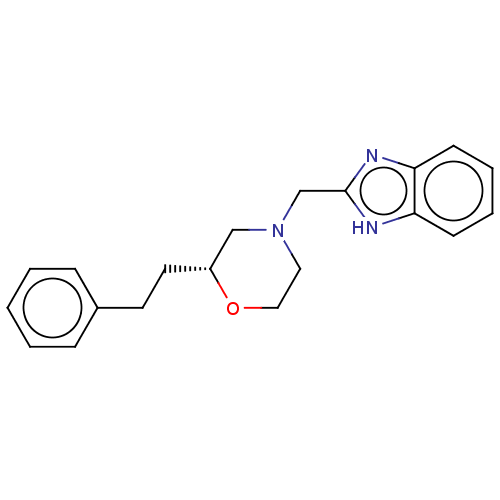
(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027092
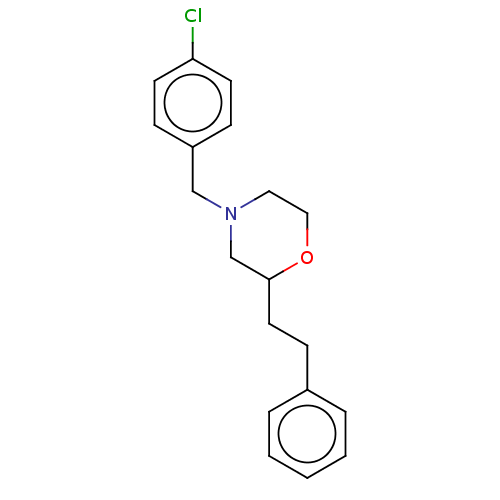
(CHEMBL3335540)Show InChI InChI=1S/C19H22ClNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027187
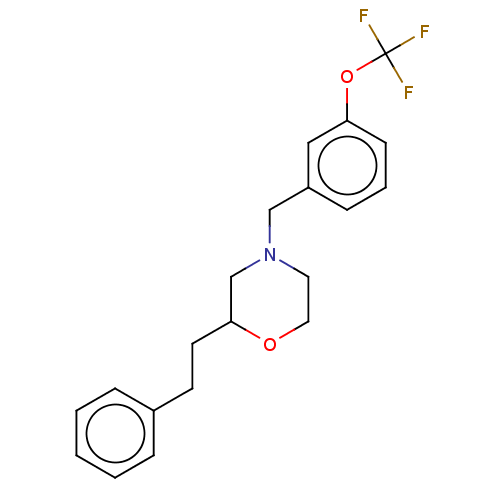
(CHEMBL3335542)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-8-4-7-17(13-18)14-24-11-12-25-19(15-24)10-9-16-5-2-1-3-6-16/h1-8,13,19H,9-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072
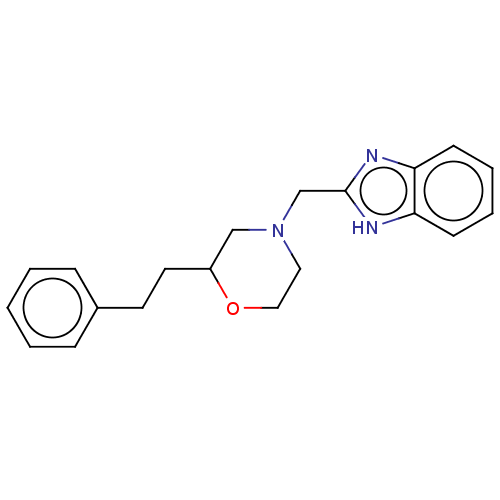
(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027257
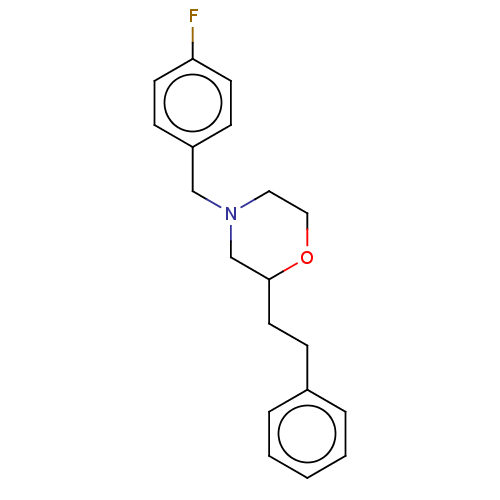
(CHEMBL3335543)Show InChI InChI=1S/C19H22FNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027258
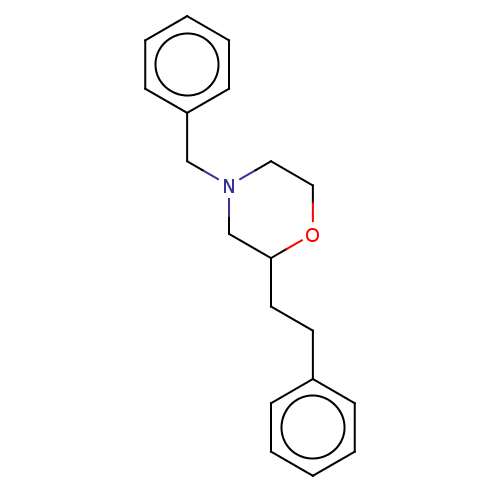
(CHEMBL3335544)Show InChI InChI=1S/C19H23NO/c1-3-7-17(8-4-1)11-12-19-16-20(13-14-21-19)15-18-9-5-2-6-10-18/h1-10,19H,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027182
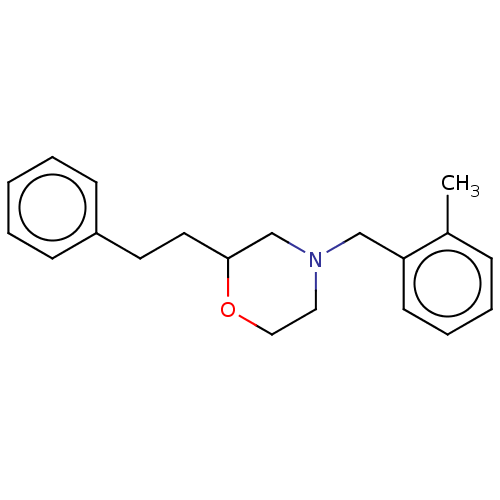
(CHEMBL3335541)Show InChI InChI=1S/C20H25NO/c1-17-7-5-6-10-19(17)15-21-13-14-22-20(16-21)12-11-18-8-3-2-4-9-18/h2-10,20H,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027261
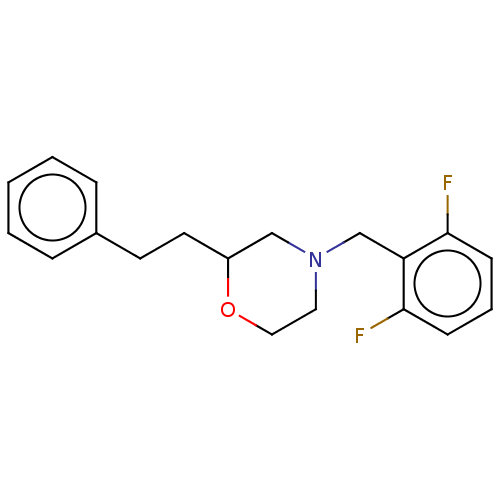
(CHEMBL3335545)Show InChI InChI=1S/C19H21F2NO/c20-18-7-4-8-19(21)17(18)14-22-11-12-23-16(13-22)10-9-15-5-2-1-3-6-15/h1-8,16H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344448
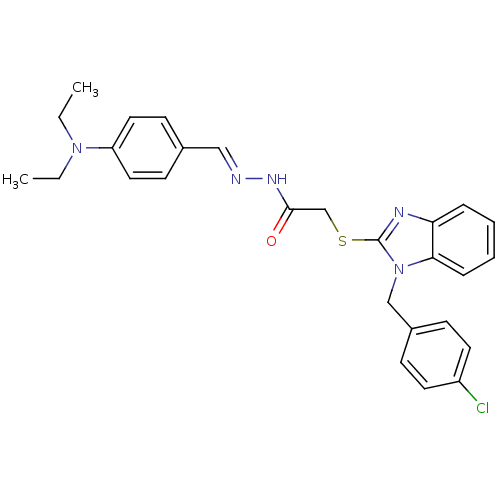
(2-(1-(4-chlorobenzyl)-1H-benzo[d]imidazol-2-ylthio...)Show SMILES CCN(CC)c1ccc(\C=N\NC(=O)CSc2nc3ccccc3n2Cc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C27H28ClN5OS/c1-3-32(4-2)23-15-11-20(12-16-23)17-29-31-26(34)19-35-27-30-24-7-5-6-8-25(24)33(27)18-21-9-13-22(28)14-10-21/h5-17H,3-4,18-19H2,1-2H3,(H,31,34)/b29-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344444
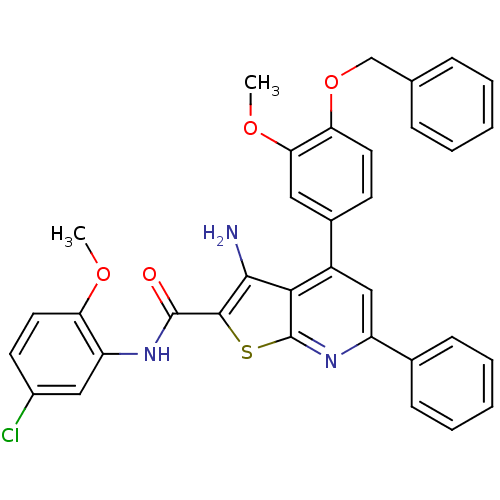
(3-amino-4-(4-(benzyloxy)-3-methoxyphenyl)-N-(5-chl...)Show SMILES COc1ccc(Cl)cc1NC(=O)c1sc2nc(cc(-c3ccc(OCc4ccccc4)c(OC)c3)c2c1N)-c1ccccc1 Show InChI InChI=1S/C35H28ClN3O4S/c1-41-28-16-14-24(36)18-27(28)38-34(40)33-32(37)31-25(19-26(39-35(31)44-33)22-11-7-4-8-12-22)23-13-15-29(30(17-23)42-2)43-20-21-9-5-3-6-10-21/h3-19H,20,37H2,1-2H3,(H,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344447
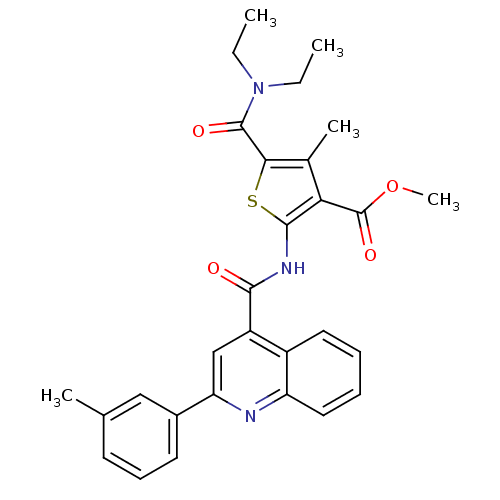
(CHEMBL1780016 | methyl-5-(diethylcarbamoyl)-4-meth...)Show SMILES CCN(CC)C(=O)c1sc(NC(=O)c2cc(nc3ccccc23)-c2cccc(C)c2)c(C(=O)OC)c1C Show InChI InChI=1S/C29H29N3O4S/c1-6-32(7-2)28(34)25-18(4)24(29(35)36-5)27(37-25)31-26(33)21-16-23(19-12-10-11-17(3)15-19)30-22-14-9-8-13-20(21)22/h8-16H,6-7H2,1-5H3,(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080

(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078

(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027079

(CHEMBL3335539)Show InChI InChI=1S/C20H25NO2/c1-22-19-10-8-18(9-11-19)15-21-13-14-23-20(16-21)12-7-17-5-3-2-4-6-17/h2-6,8-11,20H,7,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027081

(CHEMBL3335555)Show InChI InChI=1S/C19H23NO2/c21-18-9-6-17(7-10-18)14-20-12-13-22-19(15-20)11-8-16-4-2-1-3-5-16/h1-7,9-10,19,21H,8,11-15H2/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344449
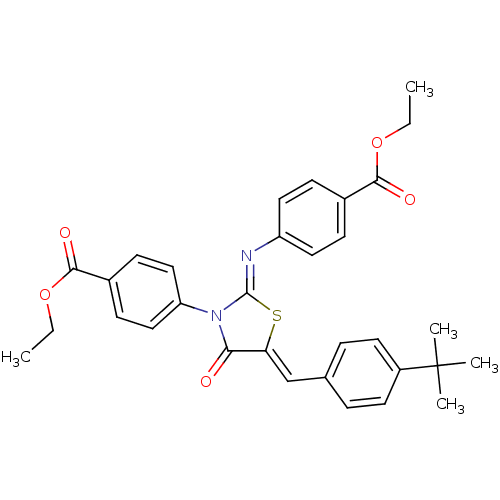
(CHEMBL1780018 | ethyl 4-(5-(4-tert-butylbenzyliden...)Show SMILES CCOC(=O)c1ccc(cc1)\N=C1/S\C(=C/c2ccc(cc2)C(C)(C)C)C(=O)N1c1ccc(cc1)C(=O)OCC Show InChI InChI=1S/C32H32N2O5S/c1-6-38-29(36)22-10-16-25(17-11-22)33-31-34(26-18-12-23(13-19-26)30(37)39-7-2)28(35)27(40-31)20-21-8-14-24(15-9-21)32(3,4)5/h8-20H,6-7H2,1-5H3/b27-20-,33-31- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344450

(1-(benzo[d][1,3]dioxol-5-ylmethyl)-1-((8-methyl-2-...)Show SMILES Cc1ccc(NC(=S)N(Cc2ccc3OCOc3c2)Cc2cc3cccc(C)c3[nH]c2=O)cc1 Show InChI InChI=1S/C27H25N3O3S/c1-17-6-9-22(10-7-17)28-27(34)30(14-19-8-11-23-24(12-19)33-16-32-23)15-21-13-20-5-3-4-18(2)25(20)29-26(21)31/h3-13H,14-16H2,1-2H3,(H,28,34)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078

(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2L receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080

(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2L receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344446
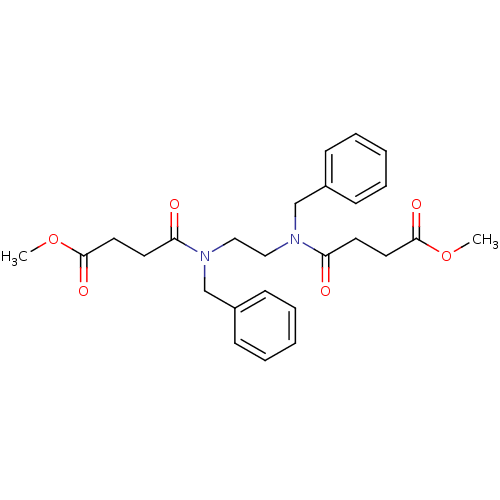
(CHEMBL1699130 | dimethyl-4,4'-(ethane-1,2-diylbis(...)Show SMILES COC(=O)CCC(=O)N(CCN(Cc1ccccc1)C(=O)CCC(=O)OC)Cc1ccccc1 Show InChI InChI=1S/C26H32N2O6/c1-33-25(31)15-13-23(29)27(19-21-9-5-3-6-10-21)17-18-28(20-22-11-7-4-8-12-22)24(30)14-16-26(32)34-2/h3-12H,13-20H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344445

(CHEMBL1780014 | N-(3-(1-(2-(2-(3-butoxyphenyl)quin...)Show SMILES CCCCOc1cccc(c1)-c1cc(C(=O)NN=C(C)c2cccc(NC(=O)C(C)(C)C)c2)c2ccccc2n1 |w:17.17| Show InChI InChI=1S/C33H36N4O3/c1-6-7-18-40-26-15-11-13-24(20-26)30-21-28(27-16-8-9-17-29(27)35-30)31(38)37-36-22(2)23-12-10-14-25(19-23)34-32(39)33(3,4)5/h8-17,19-21H,6-7,18H2,1-5H3,(H,34,39)(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50344451
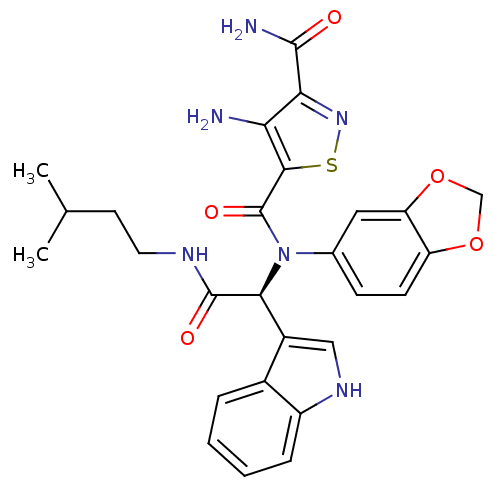
((S)-N5-(1-(1H-indol-3-yl)-2-(isopentylamino)-2-oxo...)Show SMILES CC(C)CCNC(=O)[C@@H](N(C(=O)c1snc(C(N)=O)c1N)c1ccc2OCOc2c1)c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C27H28N6O5S/c1-14(2)9-10-30-26(35)23(17-12-31-18-6-4-3-5-16(17)18)33(15-7-8-19-20(11-15)38-13-37-19)27(36)24-21(28)22(25(29)34)32-39-24/h3-8,11-12,14,23,31H,9-10,13,28H2,1-2H3,(H2,29,34)(H,30,35)/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 using DABCYL-Glu-Arg-Nle-Phe-Leu- Ser-Phe-Pro-EDANS fluorogenic substrate by fluorogenic assay |
Bioorg Med Chem Lett 21: 3335-41 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.015
BindingDB Entry DOI: 10.7270/Q24J0FFK |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077
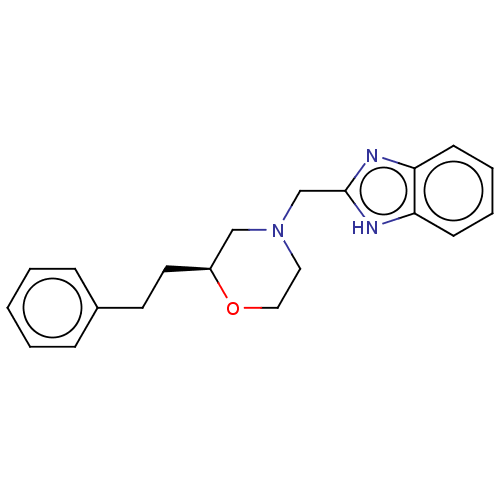
(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551191
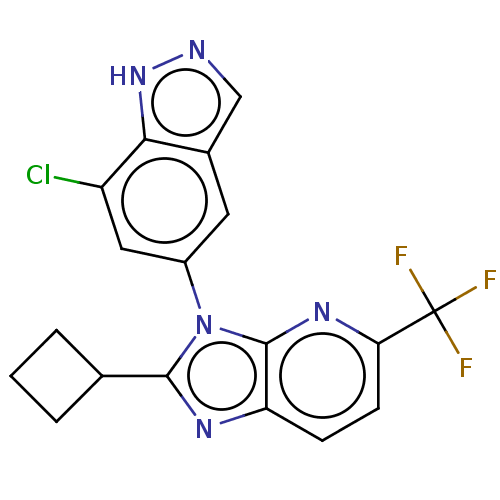
(3-(7-Chloro-1H-indazol-5-yl)-2-cyclobutyl-5-(trifl...)Show SMILES FC(F)(F)c1ccc2nc(C3CCC3)n(-c3cc(Cl)c4[nH]ncc4c3)c2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551190
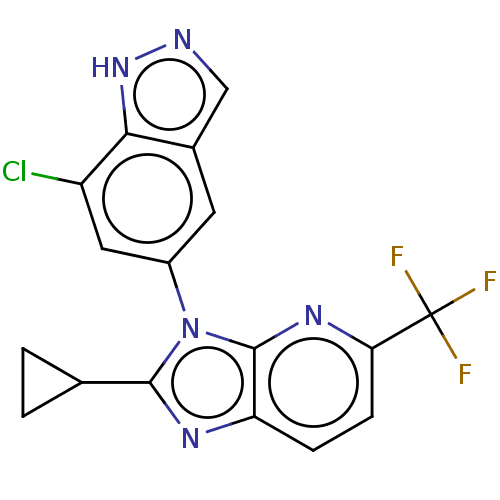
(3-(7-Chloro-1H-indazol-5-yl)-2-cyclopropyl-5-(trif...)Show SMILES FC(F)(F)c1ccc2nc(C3CC3)n(-c3cc(Cl)c4[nH]ncc4c3)c2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551115
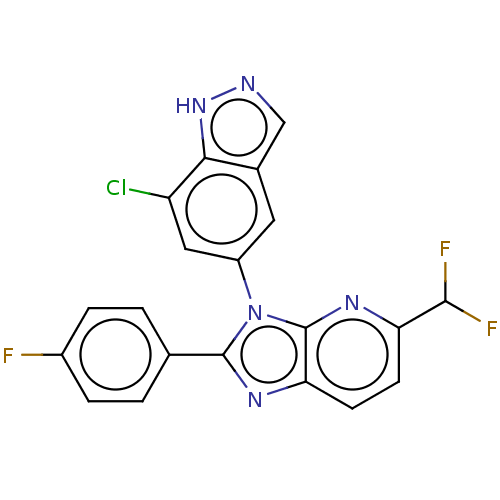
(3-(7-Chloro-1H-indazol-5-yl)-5-(difluoromethyl)-2-...)Show SMILES FC(F)c1ccc2nc(-c3ccc(F)cc3)n(-c3cc(Cl)c4[nH]ncc4c3)c2n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50582221

(CHEMBL5090028 | US11708359, Example 60)Show SMILES CC(C)(C)c1cccc(c1)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:21.31,19.21,(59.02,-28.21,;59.02,-26.67,;60.35,-25.91,;60.34,-27.44,;57.69,-25.9,;56.35,-26.67,;55.02,-25.89,;55.03,-24.36,;56.37,-23.61,;57.69,-24.37,;56.38,-22.07,;57.7,-21.3,;56.37,-20.54,;55.04,-19.76,;53.72,-20.54,;53.7,-22.08,;55.03,-22.85,;52.38,-19.77,;52.38,-18.23,;51.05,-20.54,;50.65,-22.03,;49.16,-21.63,;49.56,-20.14,;48.84,-23.14,;47.3,-23.3,;46.68,-21.89,;45.17,-21.57,;47.82,-20.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551251
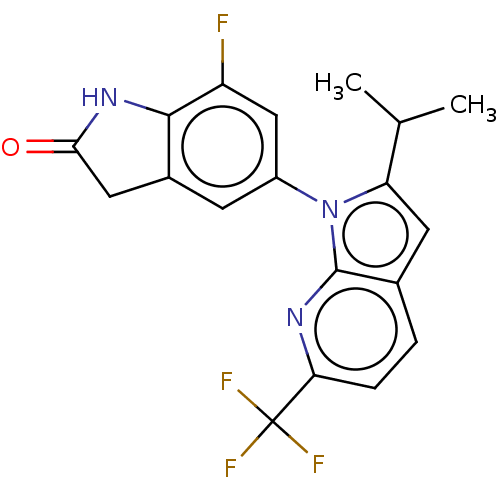
(7-Fluoro-5-[2-isopropyl-6-(trifluoromethyl)pyrrolo...)Show SMILES CC(C)c1cc2ccc(nc2n1-c1cc2CC(=O)Nc2c(F)c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551252
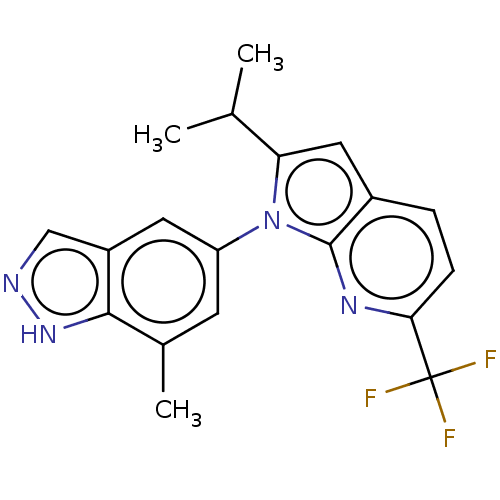
(2-Isopropyl-1-(7-methyl-1H-indazol-5-yl)-6-(triflu...)Show SMILES CC(C)c1cc2ccc(nc2n1-c1cc(C)c2[nH]ncc2c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551073
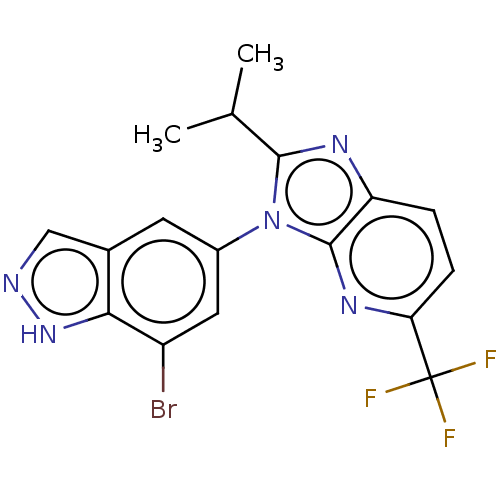
(3-(7-Bromo-1H-indazol-5-yl)-2-isopropyl-5-(trifluo...)Show SMILES CC(C)c1nc2ccc(nc2n1-c1cc(Br)c2[nH]ncc2c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551075
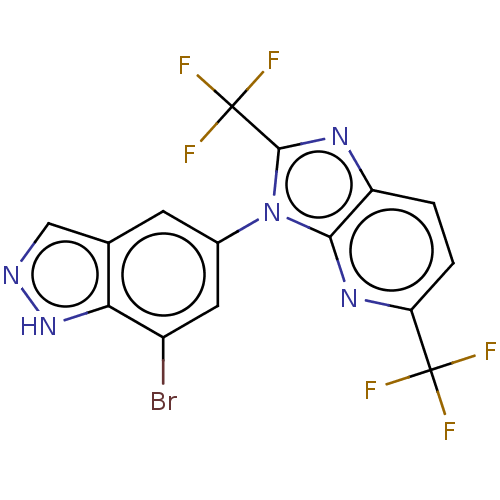
(3-(7-Bromo-1H-indazol-5-yl)-2,5-bis(trifluoromethy...)Show SMILES FC(F)(F)c1nc2ccc(nc2n1-c1cc(Br)c2[nH]ncc2c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551213
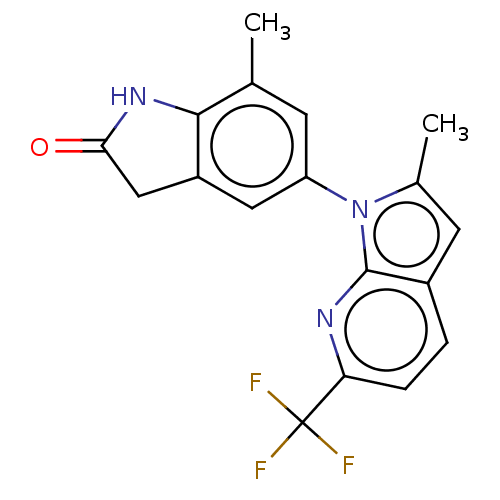
(7-Methyl-5-(2-methyl-6-(trifluoromethyl)-1H-pyrrol...)Show SMILES Cc1cc2ccc(nc2n1-c1cc2CC(=O)Nc2c(C)c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551218
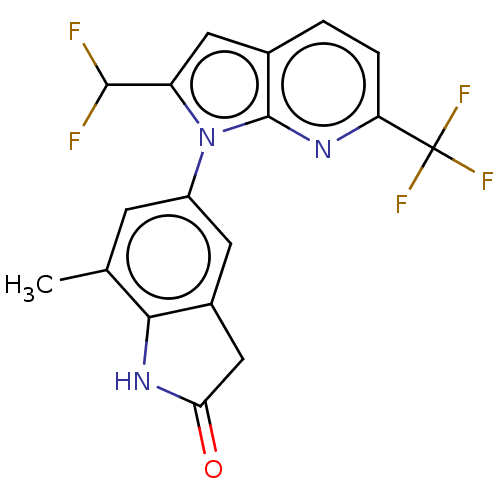
(5-[2-(Difluoromethyl)-6-(trifluoromethyl)pyrrolo[2...)Show SMILES Cc1cc(cc2CC(=O)Nc12)-n1c(cc2ccc(nc12)C(F)(F)F)C(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551256
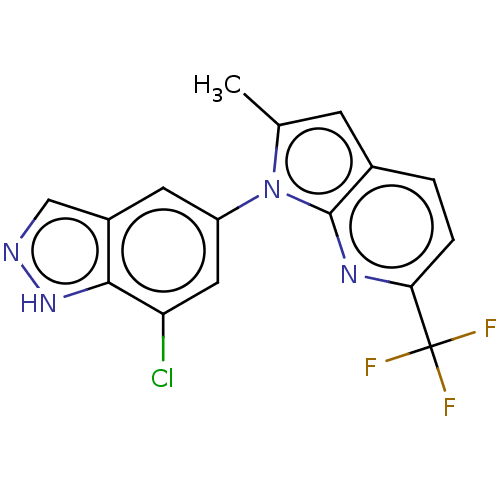
(1-(7-Chloro-1H-indazol-5-yl)-2-methyl-6-(trifluoro...)Show SMILES Cc1cc2ccc(nc2n1-c1cc(Cl)c2[nH]ncc2c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |
Voltage-dependent calcium channel gamma-8 subunit
(Homo sapiens (Human)) | BDBM551083
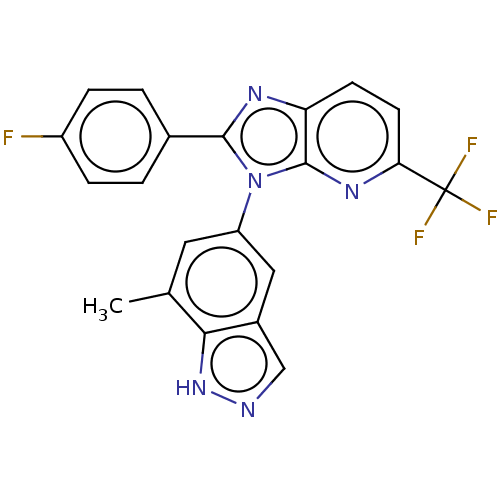
(2-(4-Fluorophenyl)-3-(7-methyl-1H-indazol-5-yl)-5-...)Show SMILES Cc1cc(cc2cn[nH]c12)-n1c(nc2ccc(nc12)C(F)(F)F)-c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
An in vitro assay was used to determine the potency of test compounds as inhibitors of the glutamate response of the channel formed by GluA1o-γ8... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2K93BR4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data