 Found 102 hits with Last Name = 'seto' and Initial = 'ct'
Found 102 hits with Last Name = 'seto' and Initial = 'ct' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50102186

((Difluoro-phenyl-methyl)-phosphonic acid | CHEMBL5...)Show InChI InChI=1S/C7H7F2O3P/c8-7(9,13(10,11)12)6-4-2-1-3-5-6/h1-5H,(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Yersinia Protein-tyrosine phosphatase 1B |
Bioorg Med Chem Lett 11: 1935-8 (2001)
BindingDB Entry DOI: 10.7270/Q23B5ZDV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Plasminogen
(Homo sapiens (Human)) | BDBM50081422
(2-((R)-3-{(R)-3-[(S)-2-((S)-Benzyloxycarbonylamino...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C39H41N5O7/c45-35(42-34(38(48)49)20-27-22-41-31-15-7-5-13-29(27)31)18-17-25-11-8-16-32(36(25)46)43-37(47)33(19-26-21-40-30-14-6-4-12-28(26)30)44-39(50)51-23-24-9-2-1-3-10-24/h1-7,9-10,12-15,21-22,25,32-34,40-41H,8,11,16-20,23H2,(H,42,45)(H,43,47)(H,44,50)(H,48,49)/t25-,32-,33+,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
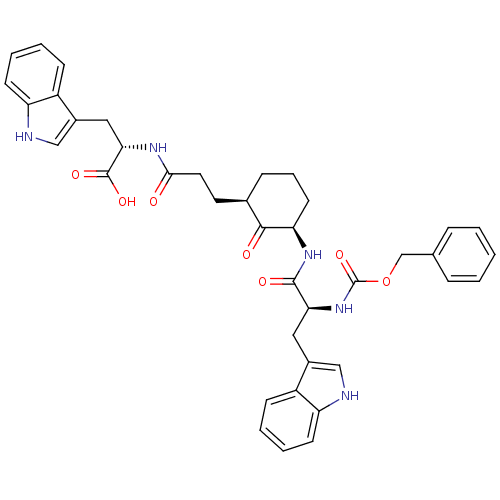
Plasminogen
(Homo sapiens (Human)) | BDBM50081422

(2-((R)-3-{(R)-3-[(S)-2-((S)-Benzyloxycarbonylamino...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C39H41N5O7/c45-35(42-34(38(48)49)20-27-22-41-31-15-7-5-13-29(27)31)18-17-25-11-8-16-32(36(25)46)43-37(47)33(19-26-21-40-30-14-6-4-12-28(26)30)44-39(50)51-23-24-9-2-1-3-10-24/h1-7,9-10,12-15,21-22,25,32-34,40-41H,8,11,16-20,23H2,(H,42,45)(H,43,47)(H,44,50)(H,48,49)/t25-,32-,33+,34+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079381
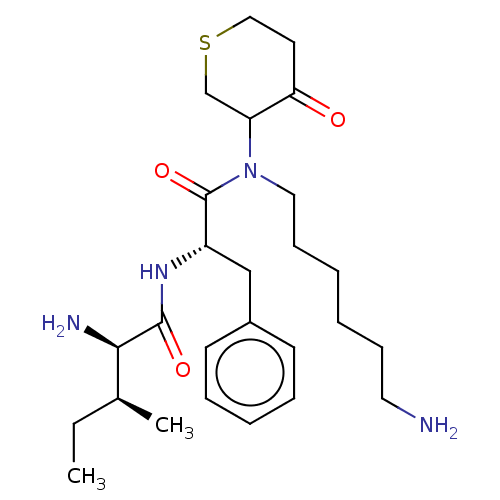
((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50102187
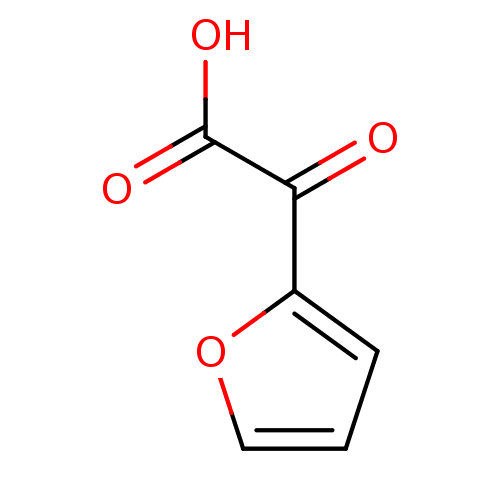
(CHEMBL299446 | Furan-2-yl-oxo-acetic acid)Show InChI InChI=1S/C6H4O4/c7-5(6(8)9)4-2-1-3-10-4/h1-3H,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Yersinia Protein-tyrosine phosphatase 1B |
Bioorg Med Chem Lett 11: 1935-8 (2001)
BindingDB Entry DOI: 10.7270/Q23B5ZDV |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081425

(2-((R)-3-{(R)-3-[(S)-2-Benzyloxycarbonylamino-3-(1...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C37H40N4O7/c42-33(39-32(36(45)46)20-24-10-3-1-4-11-24)19-18-26-14-9-17-30(34(26)43)40-35(44)31(21-27-22-38-29-16-8-7-15-28(27)29)41-37(47)48-23-25-12-5-2-6-13-25/h1-8,10-13,15-16,22,26,30-32,38H,9,14,17-21,23H2,(H,39,42)(H,40,44)(H,41,47)(H,45,46)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081425

(2-((R)-3-{(R)-3-[(S)-2-Benzyloxycarbonylamino-3-(1...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C37H40N4O7/c42-33(39-32(36(45)46)20-24-10-3-1-4-11-24)19-18-26-14-9-17-30(34(26)43)40-35(44)31(21-27-22-38-29-16-8-7-15-28(27)29)41-37(47)48-23-25-12-5-2-6-13-25/h1-8,10-13,15-16,22,26,30-32,38H,9,14,17-21,23H2,(H,39,42)(H,40,44)(H,41,47)(H,45,46)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50075312

((Difluoro-naphthalen-2-yl-methyl)-phosphonic acid ...)Show InChI InChI=1S/C11H9F2O3P/c12-11(13,17(14,15)16)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H2,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Yersinia Protein-tyrosine phosphatase 1B |
Bioorg Med Chem Lett 11: 1935-8 (2001)
BindingDB Entry DOI: 10.7270/Q23B5ZDV |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Papain
(Carica papaya) | BDBM50081425

(2-((R)-3-{(R)-3-[(S)-2-Benzyloxycarbonylamino-3-(1...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C37H40N4O7/c42-33(39-32(36(45)46)20-24-10-3-1-4-11-24)19-18-26-14-9-17-30(34(26)43)40-35(44)31(21-27-22-38-29-16-8-7-15-28(27)29)41-37(47)48-23-25-12-5-2-6-13-25/h1-8,10-13,15-16,22,26,30-32,38H,9,14,17-21,23H2,(H,39,42)(H,40,44)(H,41,47)(H,45,46)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against papain |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50462601
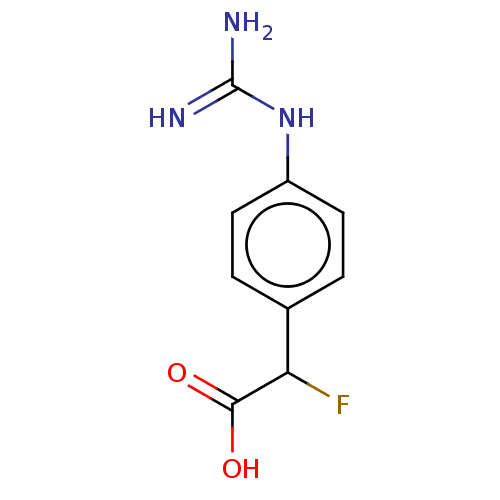
(CHEMBL4250607)Show InChI InChI=1S/C9H10FN3O2/c10-7(8(14)15)5-1-3-6(4-2-5)13-9(11)12/h1-4,7H,(H,14,15)(H4,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462601

(CHEMBL4250607)Show InChI InChI=1S/C9H10FN3O2/c10-7(8(14)15)5-1-3-6(4-2-5)13-9(11)12/h1-4,7H,(H,14,15)(H4,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50081422

(2-((R)-3-{(R)-3-[(S)-2-((S)-Benzyloxycarbonylamino...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C39H41N5O7/c45-35(42-34(38(48)49)20-27-22-41-31-15-7-5-13-29(27)31)18-17-25-11-8-16-32(36(25)46)43-37(47)33(19-26-21-40-30-14-6-4-12-28(26)30)44-39(50)51-23-24-9-2-1-3-10-24/h1-7,9-10,12-15,21-22,25,32-34,40-41H,8,11,16-20,23H2,(H,42,45)(H,43,47)(H,44,50)(H,48,49)/t25-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against papain |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Arginase-1
(Homo sapiens (Human)) | BDBM50462600
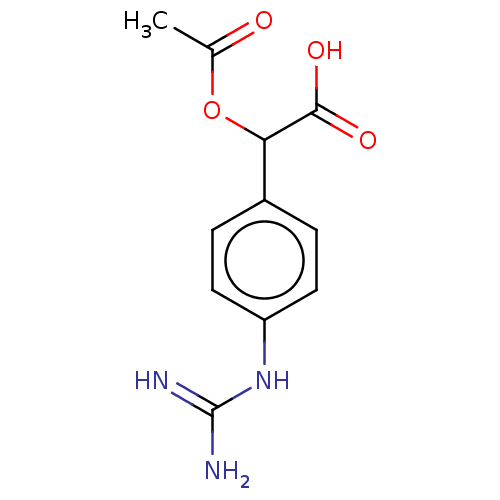
(CHEMBL4238387)Show InChI InChI=1S/C11H13N3O4/c1-6(15)18-9(10(16)17)7-2-4-8(5-3-7)14-11(12)13/h2-5,9H,1H3,(H,16,17)(H4,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 1 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079380
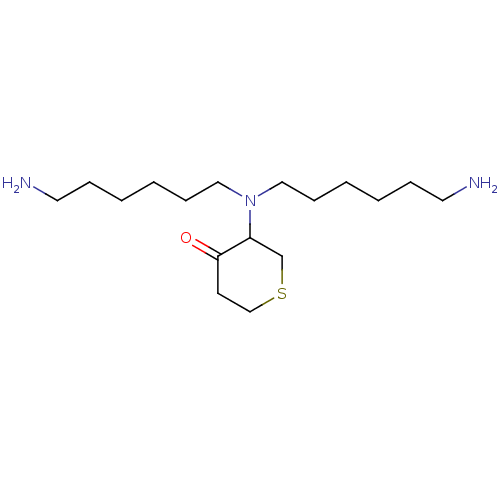
(3-[Bis-(6-amino-hexyl)-amino]-tetrahydro-thiopyran...)Show InChI InChI=1S/C17H35N3OS/c18-10-5-1-3-7-12-20(13-8-4-2-6-11-19)16-15-22-14-9-17(16)21/h16H,1-15,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081424

(2-((R)-3-{(R)-3-[(S)-2-((S)-Benzyloxycarbonylamino...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C37H40N4O7/c42-33(39-32(36(45)46)21-27-22-38-29-16-8-7-15-28(27)29)19-18-26-14-9-17-30(34(26)43)40-35(44)31(20-24-10-3-1-4-11-24)41-37(47)48-23-25-12-5-2-6-13-25/h1-8,10-13,15-16,22,26,30-32,38H,9,14,17-21,23H2,(H,39,42)(H,40,44)(H,41,47)(H,45,46)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50462600

(CHEMBL4238387)Show InChI InChI=1S/C11H13N3O4/c1-6(15)18-9(10(16)17)7-2-4-8(5-3-7)14-11(12)13/h2-5,9H,1H3,(H,16,17)(H4,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of human arginase 2 using thioarginine as substrate measured up to 360 mins by UV micro plate method |
Bioorg Med Chem 26: 3939-3946 (2018)
Article DOI: 10.1016/j.bmc.2018.06.015
BindingDB Entry DOI: 10.7270/Q2TX3J1K |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50081424

(2-((R)-3-{(R)-3-[(S)-2-((S)-Benzyloxycarbonylamino...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C37H40N4O7/c42-33(39-32(36(45)46)21-27-22-38-29-16-8-7-15-28(27)29)19-18-26-14-9-17-30(34(26)43)40-35(44)31(20-24-10-3-1-4-11-24)41-37(47)48-23-25-12-5-2-6-13-25/h1-8,10-13,15-16,22,26,30-32,38H,9,14,17-21,23H2,(H,39,42)(H,40,44)(H,41,47)(H,45,46)/t26-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against papain |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079383
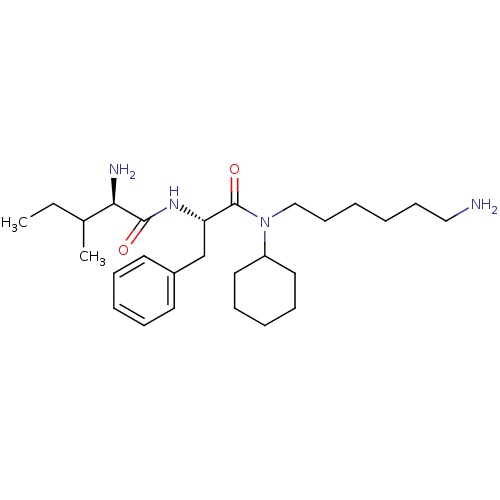
((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CCC(C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CCCCC1 Show InChI InChI=1S/C27H46N4O2/c1-3-21(2)25(29)26(32)30-24(20-22-14-8-6-9-15-22)27(33)31(19-13-5-4-12-18-28)23-16-10-7-11-17-23/h6,8-9,14-15,21,23-25H,3-5,7,10-13,16-20,28-29H2,1-2H3,(H,30,32)/t21?,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50079381

((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against kallikrein |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079381

((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against thrombin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079381

((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50079381

((R)-2-Amino-3-methyl-pentanoic acid {(S)-1-[(6-ami...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCCCCN)C1CSCCC1=O Show InChI InChI=1S/C26H42N4O3S/c1-3-19(2)24(28)25(32)29-21(17-20-11-7-6-8-12-20)26(33)30(15-10-5-4-9-14-27)22-18-34-16-13-23(22)31/h6-8,11-12,19,21-22,24H,3-5,9-10,13-18,27-28H2,1-2H3,(H,29,32)/t19?,21-,22?,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against trypsin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50081423
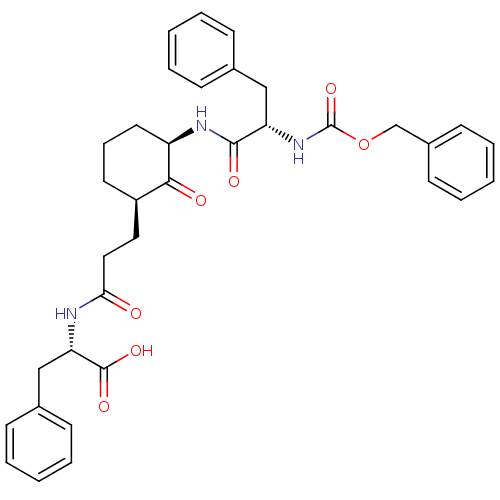
(2-{(R)-3-[(R)-3-((S)-2-Benzyloxycarbonylamino-3-ph...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C35H39N3O7/c39-31(36-30(34(42)43)22-25-13-6-2-7-14-25)20-19-27-17-10-18-28(32(27)40)37-33(41)29(21-24-11-4-1-5-12-24)38-35(44)45-23-26-15-8-3-9-16-26/h1-9,11-16,27-30H,10,17-23H2,(H,36,39)(H,37,41)(H,38,44)(H,42,43)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against papain |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50081423

(2-{(R)-3-[(R)-3-((S)-2-Benzyloxycarbonylamino-3-ph...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C35H39N3O7/c39-31(36-30(34(42)43)22-25-13-6-2-7-14-25)20-19-27-17-10-18-28(32(27)40)37-33(41)29(21-24-11-4-1-5-12-24)38-35(44)45-23-26-15-8-3-9-16-26/h1-9,11-16,27-30H,10,17-23H2,(H,36,39)(H,37,41)(H,38,44)(H,42,43)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Cathepsin B |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50079380

(3-[Bis-(6-amino-hexyl)-amino]-tetrahydro-thiopyran...)Show InChI InChI=1S/C17H35N3OS/c18-10-5-1-3-7-12-20(13-8-4-2-6-11-19)16-15-22-14-9-17(16)21/h16H,1-15,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against trypsin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50081423

(2-{(R)-3-[(R)-3-((S)-2-Benzyloxycarbonylamino-3-ph...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)CC[C@H]1CCC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)OCc2ccccc2)C1=O Show InChI InChI=1S/C35H39N3O7/c39-31(36-30(34(42)43)22-25-13-6-2-7-14-25)20-19-27-17-10-18-28(32(27)40)37-33(41)29(21-24-11-4-1-5-12-24)38-35(44)45-23-26-15-8-3-9-16-26/h1-9,11-16,27-30H,10,17-23H2,(H,36,39)(H,37,41)(H,38,44)(H,42,43)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
J Med Chem 42: 4001-9 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23MG |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50079380

(3-[Bis-(6-amino-hexyl)-amino]-tetrahydro-thiopyran...)Show InChI InChI=1S/C17H35N3OS/c18-10-5-1-3-7-12-20(13-8-4-2-6-11-19)16-15-22-14-9-17(16)21/h16H,1-15,18-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against kallikrein |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079380

(3-[Bis-(6-amino-hexyl)-amino]-tetrahydro-thiopyran...)Show InChI InChI=1S/C17H35N3OS/c18-10-5-1-3-7-12-20(13-8-4-2-6-11-19)16-15-22-14-9-17(16)21/h16H,1-15,18-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against thrombin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079382
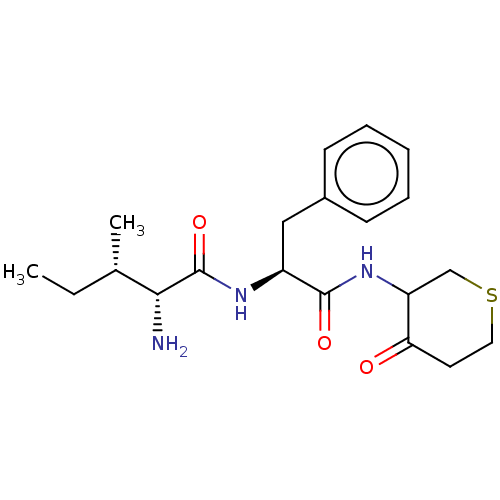
((R)-2-Amino-3-methyl-pentanoic acid [(S)-1-(4-oxo-...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC1CSCCC1=O Show InChI InChI=1S/C20H29N3O3S/c1-3-13(2)18(21)20(26)22-15(11-14-7-5-4-6-8-14)19(25)23-16-12-27-10-9-17(16)24/h4-8,13,15-16,18H,3,9-12,21H2,1-2H3,(H,22,26)(H,23,25)/t13?,15-,16?,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50079382

((R)-2-Amino-3-methyl-pentanoic acid [(S)-1-(4-oxo-...)Show SMILES CC[C@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC1CSCCC1=O Show InChI InChI=1S/C20H29N3O3S/c1-3-13(2)18(21)20(26)22-15(11-14-7-5-4-6-8-14)19(25)23-16-12-27-10-9-17(16)24/h4-8,13,15-16,18H,3,9-12,21H2,1-2H3,(H,22,26)(H,23,25)/t13?,15-,16?,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against plasmin |
J Med Chem 42: 2969-76 (1999)
Article DOI: 10.1021/jm990110k
BindingDB Entry DOI: 10.7270/Q2WM1CMW |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326496

(4, 4'-[[2-[[(4-(4-Trifluoromethylthio)phenoxy phen...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(cc3)-c3ccccc3)cc2)cc1 Show InChI InChI=1S/C43H31NO10/c45-39(42(48)49)30-10-16-34(17-11-30)52-25-27-6-7-32(26-53-35-18-12-31(13-19-35)40(46)43(50)51)38(24-27)41(47)44-33-14-22-37(23-15-33)54-36-20-8-29(9-21-36)28-4-2-1-3-5-28/h1-24H,25-26H2,(H,44,47)(H,48,49)(H,50,51) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326497
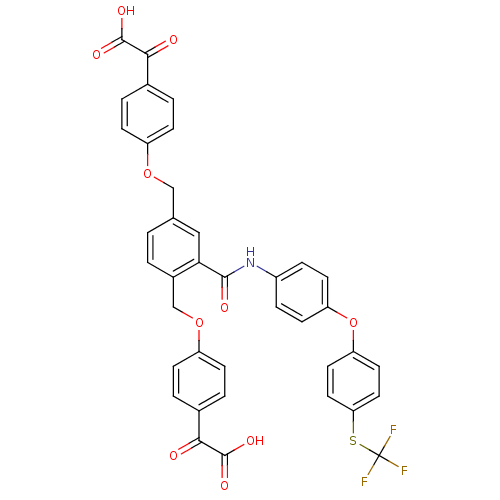
(4, 4'-[[2-[[(4-(4-Trifluoromethylthio)phenoxypheny...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(SC(F)(F)F)cc3)cc2)cc1 Show InChI InChI=1S/C38H26F3NO10S/c39-38(40,41)53-31-17-15-30(16-18-31)52-29-13-7-26(8-14-29)42-35(45)32-19-22(20-50-27-9-3-23(4-10-27)33(43)36(46)47)1-2-25(32)21-51-28-11-5-24(6-12-28)34(44)37(48)49/h1-19H,20-21H2,(H,42,45)(H,46,47)(H,48,49) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326498
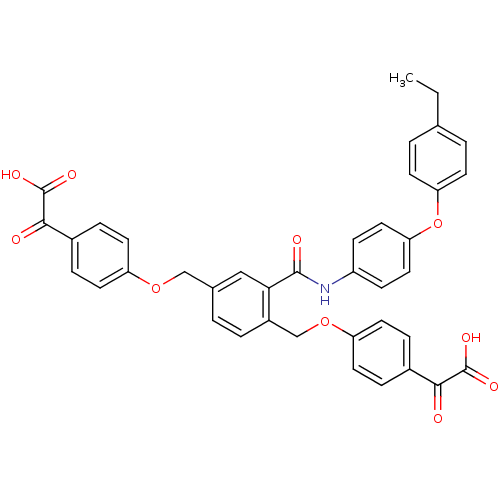
(4, 4'-[[2-[[(4-(4-Ethyl)phenoxyphenyl)amino]carbon...)Show SMILES CCc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C39H31NO10/c1-2-24-4-13-32(14-5-24)50-33-19-11-29(12-20-33)40-37(43)34-21-25(22-48-30-15-7-26(8-16-30)35(41)38(44)45)3-6-28(34)23-49-31-17-9-27(10-18-31)36(42)39(46)47/h3-21H,2,22-23H2,1H3,(H,40,43)(H,44,45)(H,46,47) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326499
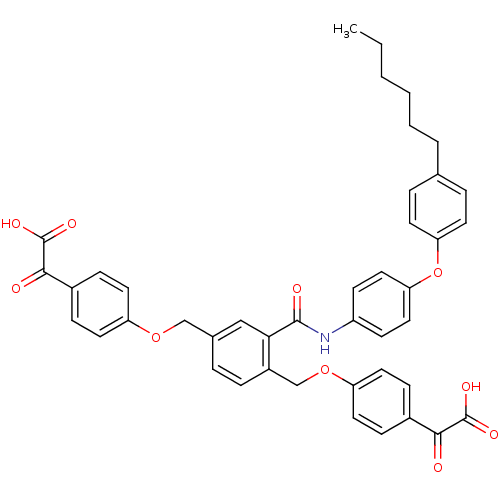
(4, 4'-[[2-[[(4-(4-n-Hexyl)phenoxyphenyl)amino]carb...)Show SMILES CCCCCCc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C43H39NO10/c1-2-3-4-5-6-28-8-17-36(18-9-28)54-37-23-15-33(16-24-37)44-41(47)38-25-29(26-52-34-19-11-30(12-20-34)39(45)42(48)49)7-10-32(38)27-53-35-21-13-31(14-22-35)40(46)43(50)51/h7-25H,2-6,26-27H2,1H3,(H,44,47)(H,48,49)(H,50,51) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326500

(2,2'-(4,4'-(2-(3-(benzyloxy)phenylcarbamoyl)-1,4-p...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2cccc(OCc3ccccc3)c2)cc1 Show InChI InChI=1S/C38H29NO10/c40-34(37(43)44)26-11-15-30(16-12-26)47-22-25-9-10-28(23-49-31-17-13-27(14-18-31)35(41)38(45)46)33(19-25)36(42)39-29-7-4-8-32(20-29)48-21-24-5-2-1-3-6-24/h1-20H,21-23H2,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326502

(2,2'-(4,4'-(2-(4-(4-fluorophenoxy)phenylcarbamoyl)...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(F)cc3)cc2)cc1 Show InChI InChI=1S/C37H26FNO10/c38-26-7-15-30(16-8-26)49-31-17-9-27(10-18-31)39-35(42)32-19-22(20-47-28-11-3-23(4-12-28)33(40)36(43)44)1-2-25(32)21-48-29-13-5-24(6-14-29)34(41)37(45)46/h1-19H,20-21H2,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326501
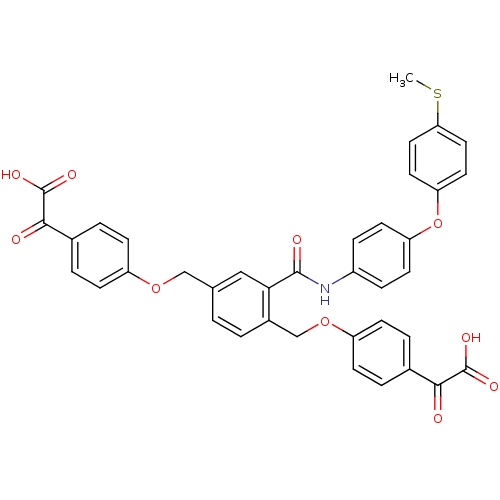
(2,2'-(4,4'-(2-(4-(4-(methylthio)phenoxy)phenylcarb...)Show SMILES CSc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C38H29NO10S/c1-50-32-18-16-31(17-19-32)49-30-14-8-27(9-15-30)39-36(42)33-20-23(21-47-28-10-4-24(5-11-28)34(40)37(43)44)2-3-26(33)22-48-29-12-6-25(7-13-29)35(41)38(45)46/h2-20H,21-22H2,1H3,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326503

(2,2'-(4,4'-(2-(4-(p-tolyloxy)phenylcarbamoyl)-1,4-...)Show SMILES Cc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C38H29NO10/c1-23-2-12-31(13-3-23)49-32-18-10-28(11-19-32)39-36(42)33-20-24(21-47-29-14-6-25(7-15-29)34(40)37(43)44)4-5-27(33)22-48-30-16-8-26(9-17-30)35(41)38(45)46/h2-20H,21-22H2,1H3,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326504
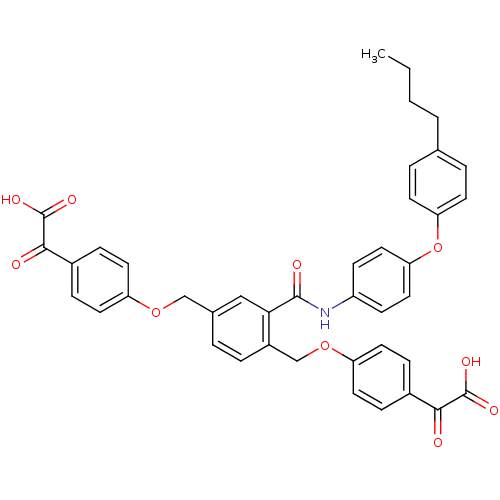
(2,2'-(4,4'-(2-(4-(4-butylphenoxy)phenylcarbamoyl)-...)Show SMILES CCCCc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C41H35NO10/c1-2-3-4-26-6-15-34(16-7-26)52-35-21-13-31(14-22-35)42-39(45)36-23-27(24-50-32-17-9-28(10-18-32)37(43)40(46)47)5-8-30(36)25-51-33-19-11-29(12-20-33)38(44)41(48)49/h5-23H,2-4,24-25H2,1H3,(H,42,45)(H,46,47)(H,48,49) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326505
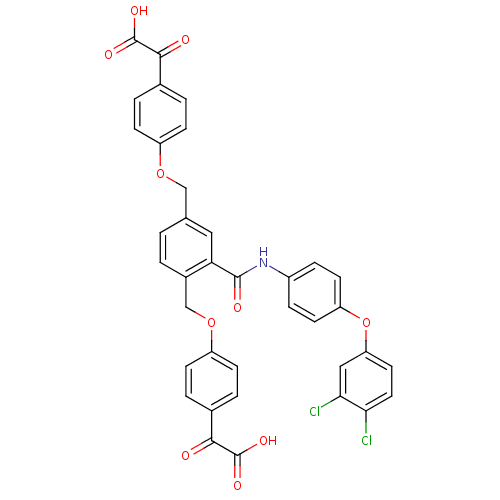
(2,2'-(4,4'-(2-(4-(3,4-dichlorophenoxy)phenylcarbam...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(Cl)c(Cl)c3)cc2)cc1 Show InChI InChI=1S/C37H25Cl2NO10/c38-31-16-15-29(18-32(31)39)50-28-13-7-25(8-14-28)40-35(43)30-17-21(19-48-26-9-3-22(4-10-26)33(41)36(44)45)1-2-24(30)20-49-27-11-5-23(6-12-27)34(42)37(46)47/h1-18H,19-20H2,(H,40,43)(H,44,45)(H,46,47) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326496

(4, 4'-[[2-[[(4-(4-Trifluoromethylthio)phenoxy phen...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(cc3)-c3ccccc3)cc2)cc1 Show InChI InChI=1S/C43H31NO10/c45-39(42(48)49)30-10-16-34(17-11-30)52-25-27-6-7-32(26-53-35-18-12-31(13-19-35)40(46)43(50)51)38(24-27)41(47)44-33-14-22-37(23-15-33)54-36-20-8-29(9-21-36)28-4-2-1-3-5-28/h1-24H,25-26H2,(H,44,47)(H,48,49)(H,50,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326506

(2,2'-(4,4'-(2-(4-(3-chlorophenoxy)phenylcarbamoyl)...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3cccc(F)c3)cc2)cc1 Show InChI InChI=1S/C37H26FNO10/c38-26-2-1-3-31(19-26)49-30-16-10-27(11-17-30)39-35(42)32-18-22(20-47-28-12-6-23(7-13-28)33(40)36(43)44)4-5-25(32)21-48-29-14-8-24(9-15-29)34(41)37(45)46/h1-19H,20-21H2,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326507
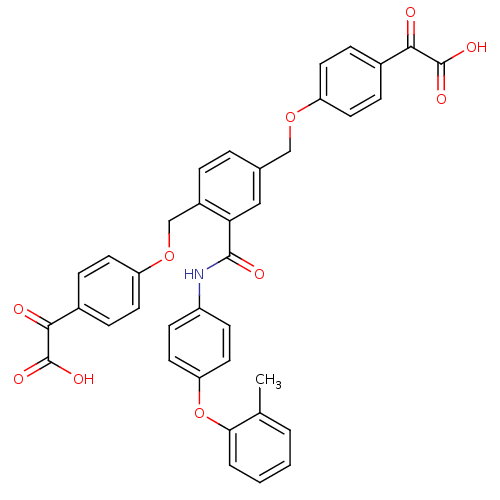
(2,2'-(4,4'-(2-(4-(o-tolyloxy)phenylcarbamoyl)-1,4-...)Show SMILES Cc1ccccc1Oc1ccc(NC(=O)c2cc(COc3ccc(cc3)C(=O)C(O)=O)ccc2COc2ccc(cc2)C(=O)C(O)=O)cc1 Show InChI InChI=1S/C38H29NO10/c1-23-4-2-3-5-33(23)49-31-18-12-28(13-19-31)39-36(42)32-20-24(21-47-29-14-8-25(9-15-29)34(40)37(43)44)6-7-27(32)22-48-30-16-10-26(11-17-30)35(41)38(45)46/h2-20H,21-22H2,1H3,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326508
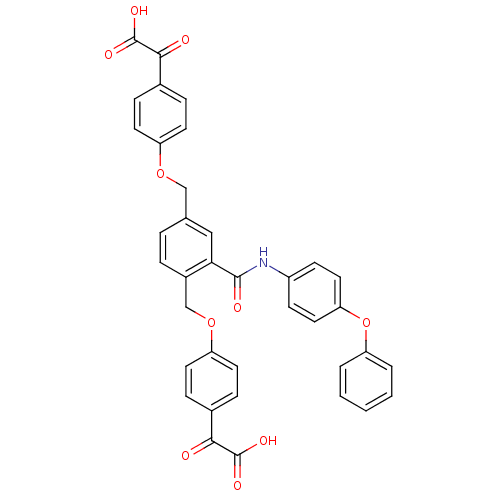
(2,2'-(4,4'-(2-(4-phenoxyphenylcarbamoyl)-1,4-pheny...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccccc3)cc2)cc1 Show InChI InChI=1S/C37H27NO10/c39-33(36(42)43)24-8-14-28(15-9-24)46-21-23-6-7-26(22-47-29-16-10-25(11-17-29)34(40)37(44)45)32(20-23)35(41)38-27-12-18-31(19-13-27)48-30-4-2-1-3-5-30/h1-20H,21-22H2,(H,38,41)(H,42,43)(H,44,45) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326498

(4, 4'-[[2-[[(4-(4-Ethyl)phenoxyphenyl)amino]carbon...)Show SMILES CCc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C39H31NO10/c1-2-24-4-13-32(14-5-24)50-33-19-11-29(12-20-33)40-37(43)34-21-25(22-48-30-15-7-26(8-16-30)35(41)38(44)45)3-6-28(34)23-49-31-17-9-27(10-18-31)36(42)39(46)47/h3-21H,2,22-23H2,1H3,(H,40,43)(H,44,45)(H,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50118096
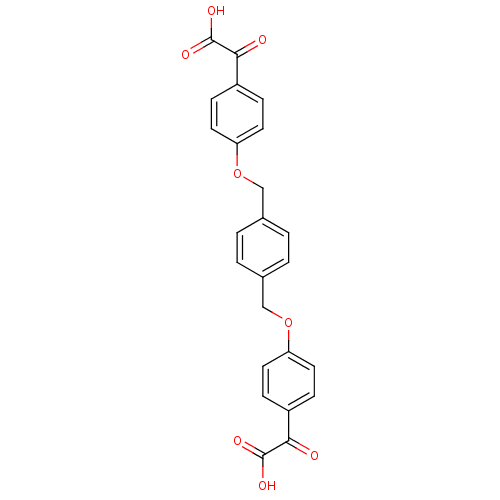
(2,2'-(4,4'-(1,4-phenylenebis(methylene))bis(oxy)bi...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C24H18O8/c25-21(23(27)28)17-5-9-19(10-6-17)31-13-15-1-2-16(4-3-15)14-32-20-11-7-18(8-12-20)22(26)24(29)30/h1-12H,13-14H2,(H,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Concentration required for 50% Inhibition of activity against Yersinia Protein-tyrosine phosphatase |
J Med Chem 45: 3946-52 (2002)
BindingDB Entry DOI: 10.7270/Q2F76BW4 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326509

(2,2'-(4,4'-(2-(4-(4-isopropylphenoxy)phenylcarbamo...)Show SMILES CC(C)c1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C40H33NO10/c1-24(2)26-5-17-33(18-6-26)51-34-19-11-30(12-20-34)41-38(44)35-21-25(22-49-31-13-7-27(8-14-31)36(42)39(45)46)3-4-29(35)23-50-32-15-9-28(10-16-32)37(43)40(47)48/h3-21,24H,22-23H2,1-2H3,(H,41,44)(H,45,46)(H,47,48) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326510
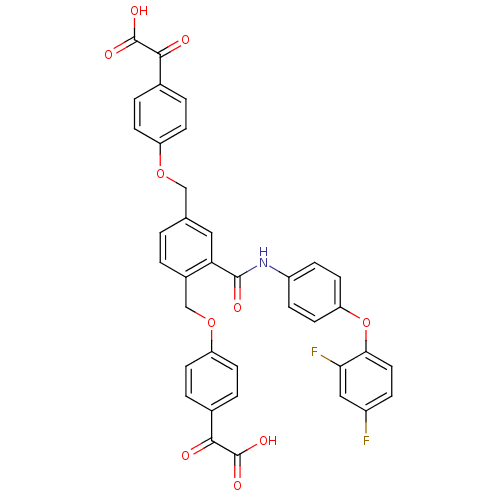
(2,2'-(4,4'-(2-(4-(2,4-difluorophenoxy)phenylcarbam...)Show SMILES OC(=O)C(=O)c1ccc(OCc2ccc(COc3ccc(cc3)C(=O)C(O)=O)c(c2)C(=O)Nc2ccc(Oc3ccc(F)cc3F)cc2)cc1 Show InChI InChI=1S/C37H25F2NO10/c38-25-7-16-32(31(39)18-25)50-29-14-8-26(9-15-29)40-35(43)30-17-21(19-48-27-10-3-22(4-11-27)33(41)36(44)45)1-2-24(30)20-49-28-12-5-23(6-13-28)34(42)37(46)47/h1-18H,19-20H2,(H,40,43)(H,44,45)(H,46,47) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine-phosphatase
(Yersinia pestis) | BDBM50326511

(2,2'-(4,4'-(2-(4-(4-methoxyphenoxy)phenylcarbamoyl...)Show SMILES COc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C38H29NO11/c1-47-28-16-18-32(19-17-28)50-31-14-8-27(9-15-31)39-36(42)33-20-23(21-48-29-10-4-24(5-11-29)34(40)37(43)44)2-3-26(33)22-49-30-12-6-25(7-13-30)35(41)38(45)46/h2-20H,21-22H2,1H3,(H,39,42)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of Yersinia pestis YOpH |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50326499

(4, 4'-[[2-[[(4-(4-n-Hexyl)phenoxyphenyl)amino]carb...)Show SMILES CCCCCCc1ccc(Oc2ccc(NC(=O)c3cc(COc4ccc(cc4)C(=O)C(O)=O)ccc3COc3ccc(cc3)C(=O)C(O)=O)cc2)cc1 Show InChI InChI=1S/C43H39NO10/c1-2-3-4-5-6-28-8-17-36(18-9-28)54-37-23-15-33(16-24-37)44-41(47)38-25-29(26-52-34-19-11-30(12-20-34)39(45)42(48)49)7-10-32(38)27-53-35-21-13-31(14-22-35)40(46)43(50)51/h7-25H,2-6,26-27H2,1H3,(H,44,47)(H,48,49)(H,50,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Brown University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 6768-72 (2010)
Article DOI: 10.1021/jm100528p
BindingDB Entry DOI: 10.7270/Q2988777 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data