 Found 1643 hits with Last Name = 'carr' and Initial = 'd'
Found 1643 hits with Last Name = 'carr' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438628
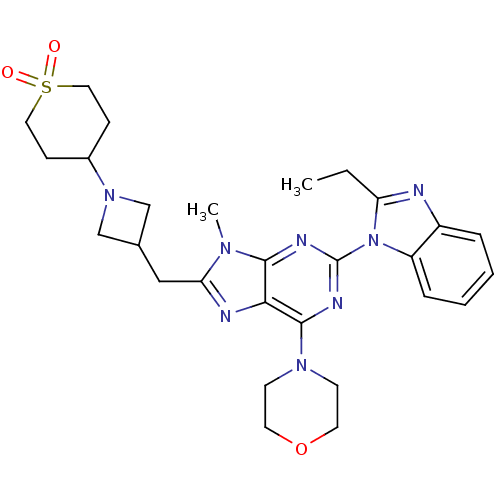
(CHEMBL2414299)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3S/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)34-10-12-39-13-11-34)30-24(33(26)2)16-19-17-35(18-19)20-8-14-40(37,38)15-9-20/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438634
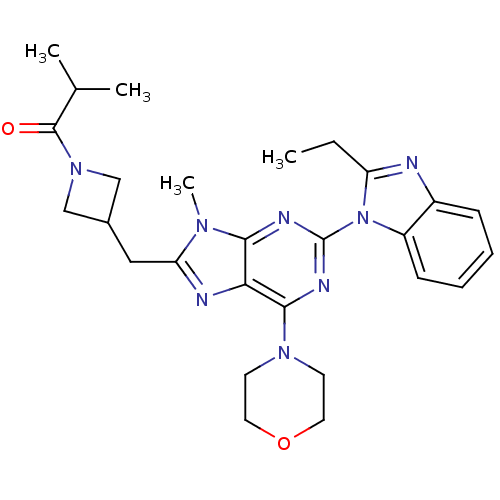
(CHEMBL2414301)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C27H34N8O2/c1-5-21-28-19-8-6-7-9-20(19)35(21)27-30-24-23(25(31-27)33-10-12-37-13-11-33)29-22(32(24)4)14-18-15-34(16-18)26(36)17(2)3/h6-9,17-18H,5,10-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403075
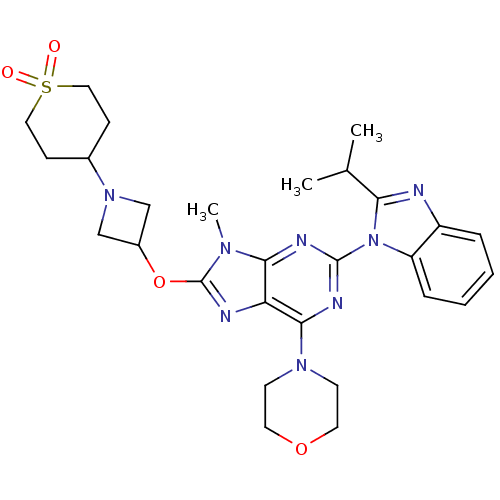
(CHEMBL2216902)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O4S/c1-18(2)24-29-21-6-4-5-7-22(21)36(24)27-31-25-23(26(32-27)34-10-12-39-13-11-34)30-28(33(25)3)40-20-16-35(17-20)19-8-14-41(37,38)15-9-19/h4-7,18-20H,8-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403074
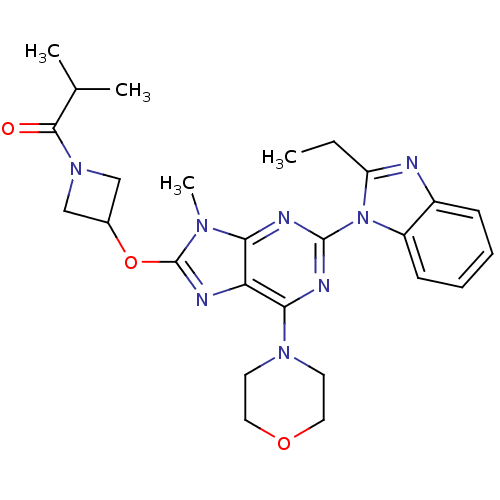
(CHEMBL2216903)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H32N8O3/c1-5-20-27-18-8-6-7-9-19(18)34(20)25-29-22-21(23(30-25)32-10-12-36-13-11-32)28-26(31(22)4)37-17-14-33(15-17)24(35)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438630
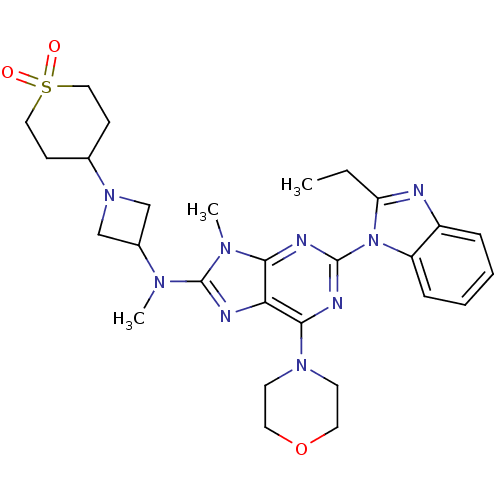
(CHEMBL2414297)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(N(C)C3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H37N9O3S/c1-4-23-29-21-7-5-6-8-22(21)37(23)27-31-25-24(26(32-27)35-11-13-40-14-12-35)30-28(34(25)3)33(2)20-17-36(18-20)19-9-15-41(38,39)16-10-19/h5-8,19-20H,4,9-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001468
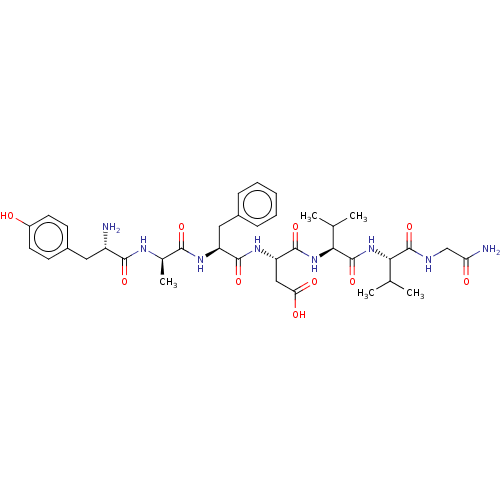
((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C37H52N8O10/c1-19(2)30(36(54)40-18-28(39)47)45-37(55)31(20(3)4)44-35(53)27(17-29(48)49)43-34(52)26(16-22-9-7-6-8-10-22)42-32(50)21(5)41-33(51)25(38)15-23-11-13-24(46)14-12-23/h6-14,19-21,25-27,30-31,46H,15-18,38H2,1-5H3,(H2,39,47)(H,40,54)(H,41,51)(H,42,50)(H,43,52)(H,44,53)(H,45,55)(H,48,49)/t21-,25+,26+,27+,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor delta |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438633
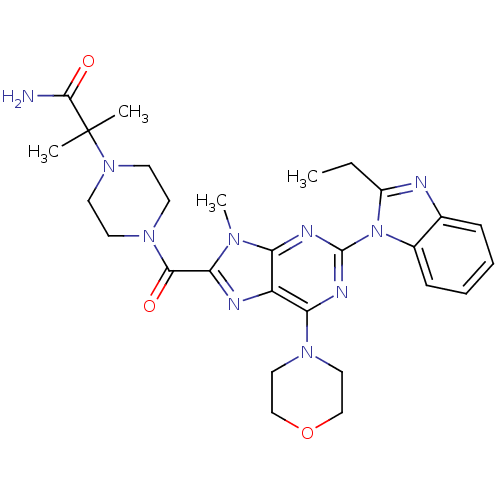
(CHEMBL2414294)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H36N10O3/c1-5-20-30-18-8-6-7-9-19(18)38(20)27-32-22-21(23(33-27)35-14-16-41-17-15-35)31-24(34(22)4)25(39)36-10-12-37(13-11-36)28(2,3)26(29)40/h6-9H,5,10-17H2,1-4H3,(H2,29,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438635
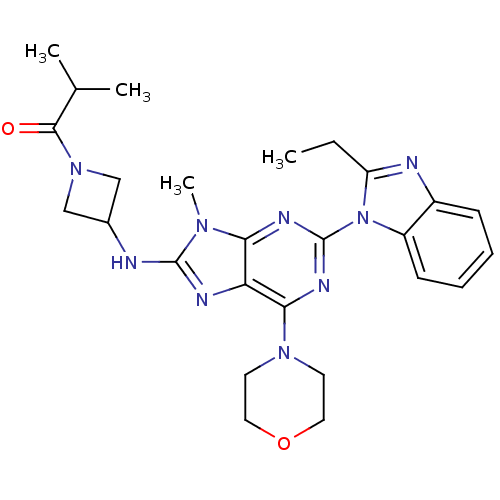
(CHEMBL2414300)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H33N9O2/c1-5-20-28-18-8-6-7-9-19(18)35(20)26-30-22-21(23(31-26)33-10-12-37-13-11-33)29-25(32(22)4)27-17-14-34(15-17)24(36)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438629
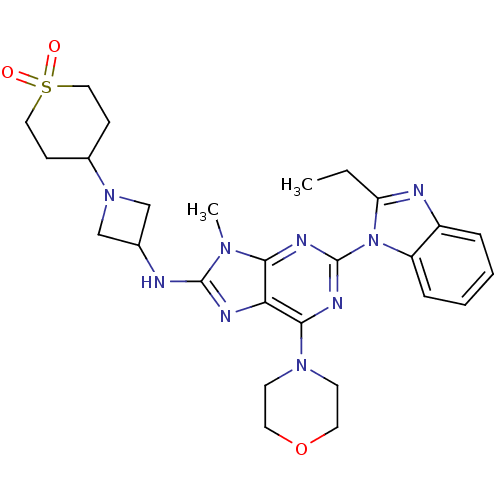
(CHEMBL2414298)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C27H35N9O3S/c1-3-22-29-20-6-4-5-7-21(20)36(22)27-31-24-23(25(32-27)34-10-12-39-13-11-34)30-26(33(24)2)28-18-16-35(17-18)19-8-14-40(37,38)15-9-19/h4-7,18-19H,3,8-17H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438637
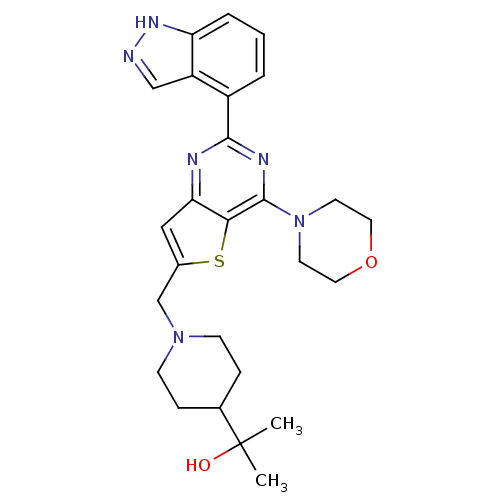
(CHEMBL2414238)Show SMILES CC(C)(O)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H32N6O2S/c1-26(2,33)17-6-8-31(9-7-17)16-18-14-22-23(35-18)25(32-10-12-34-13-11-32)29-24(28-22)19-4-3-5-21-20(19)15-27-30-21/h3-5,14-15,17,33H,6-13,16H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396642
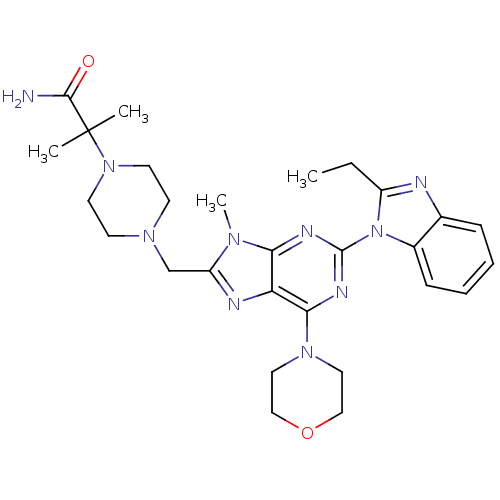
(CHEMBL2171939)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H38N10O2/c1-5-21-30-19-8-6-7-9-20(19)38(21)27-32-24-23(25(33-27)36-14-16-40-17-15-36)31-22(34(24)4)18-35-10-12-37(13-11-35)28(2,3)26(29)39/h6-9H,5,10-18H2,1-4H3,(H2,29,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396628
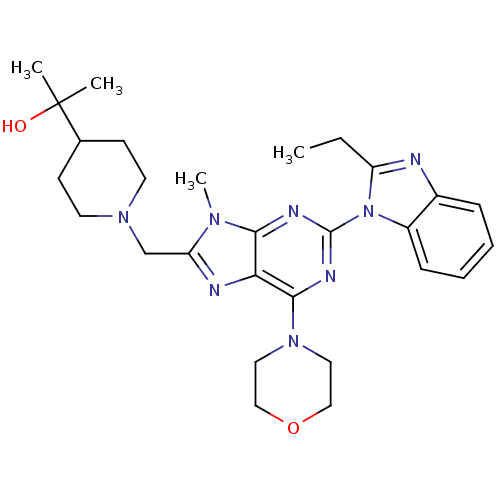
(CHEMBL2171944)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H38N8O2/c1-5-22-29-20-8-6-7-9-21(20)36(22)27-31-25-24(26(32-27)35-14-16-38-17-15-35)30-23(33(25)4)18-34-12-10-19(11-13-34)28(2,3)37/h6-9,19,37H,5,10-18H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396633
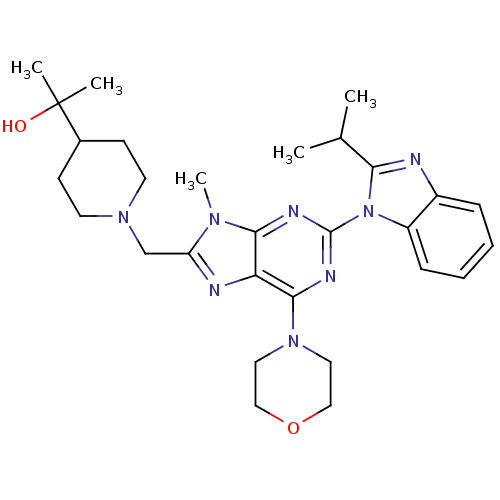
(CHEMBL2171952)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C29H40N8O2/c1-19(2)25-30-21-8-6-7-9-22(21)37(25)28-32-26-24(27(33-28)36-14-16-39-17-15-36)31-23(34(26)5)18-35-12-10-20(11-13-35)29(3,4)38/h6-9,19-20,38H,10-18H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438636
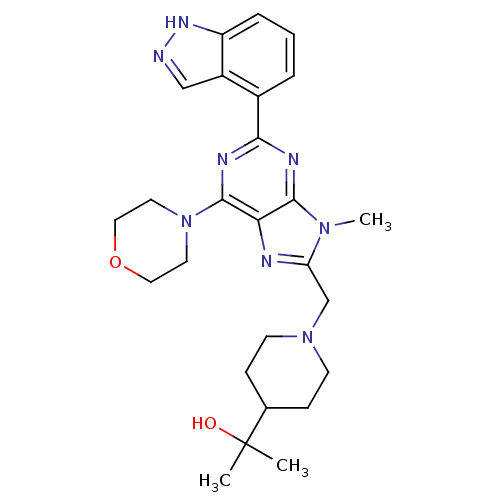
(CHEMBL2414239)Show SMILES Cn1c(CN2CCC(CC2)C(C)(C)O)nc2c(nc(nc12)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C26H34N8O2/c1-26(2,35)17-7-9-33(10-8-17)16-21-28-22-24(32(21)3)29-23(30-25(22)34-11-13-36-14-12-34)18-5-4-6-20-19(18)15-27-31-20/h4-6,15,17,35H,7-14,16H2,1-3H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50059841

((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C40H50N8O10/c1-23(44-37(55)29(41)18-25-9-13-27(50)14-10-25)36(54)46-30(19-24-6-3-2-4-7-24)38(56)43-21-34(52)45-31(20-26-11-15-28(51)16-12-26)40(58)48-17-5-8-33(48)39(57)47-32(22-49)35(42)53/h2-4,6-7,9-16,23,29-33,49-51H,5,8,17-22,41H2,1H3,(H2,42,53)(H,43,56)(H,44,55)(H,45,52)(H,46,54)(H,47,57)/t23-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor mu |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438631
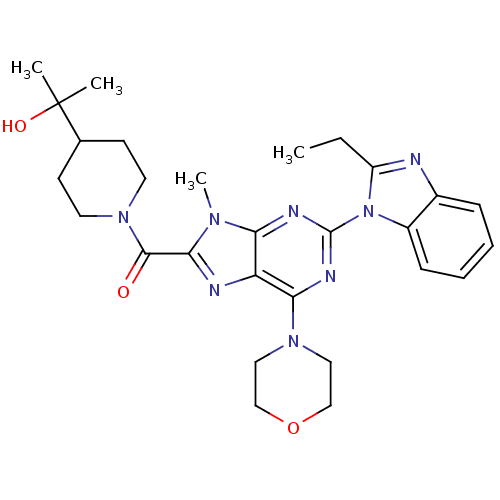
(CHEMBL2414296)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3/c1-5-21-29-19-8-6-7-9-20(19)36(21)27-31-23-22(24(32-27)34-14-16-39-17-15-34)30-25(33(23)4)26(37)35-12-10-18(11-13-35)28(2,3)38/h6-9,18,38H,5,10-17H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50139013

((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C32H37N5O5/c33-25(18-23-13-15-24(38)16-14-23)32(42)37-17-7-12-28(37)31(41)36-27(20-22-10-5-2-6-11-22)30(40)35-26(29(34)39)19-21-8-3-1-4-9-21/h1-6,8-11,13-16,25-28,38H,7,12,17-20,33H2,(H2,34,39)(H,35,40)(H,36,41)/t25-,26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor mu |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50060070
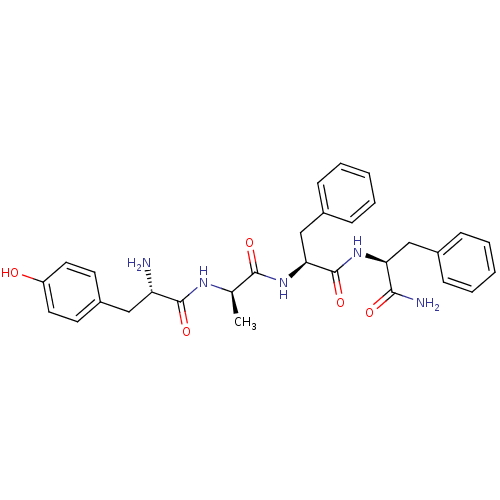
((S)-2-Amino-N-{(R)-1-[(S)-1-((S)-1-carbamoyl-2-phe...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C30H35N5O5/c1-19(33-29(39)24(31)16-22-12-14-23(36)15-13-22)28(38)35-26(18-21-10-6-3-7-11-21)30(40)34-25(27(32)37)17-20-8-4-2-5-9-20/h2-15,19,24-26,36H,16-18,31H2,1H3,(H2,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,24+,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor mu |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394894
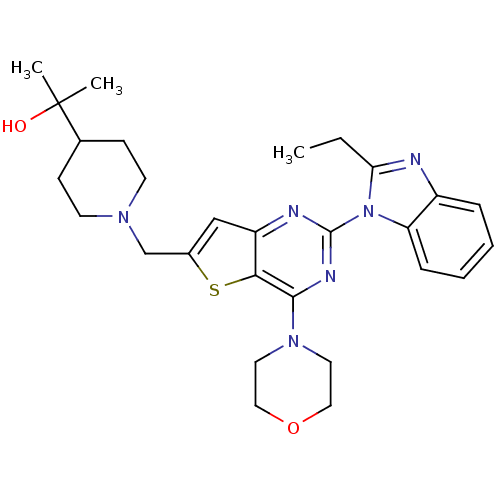
(CHEMBL2165501)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)C(C)(C)O)cc2n1 Show InChI InChI=1S/C28H36N6O2S/c1-4-24-29-21-7-5-6-8-23(21)34(24)27-30-22-17-20(18-32-11-9-19(10-12-32)28(2,3)35)37-25(22)26(31-27)33-13-15-36-16-14-33/h5-8,17,19,35H,4,9-16,18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166347
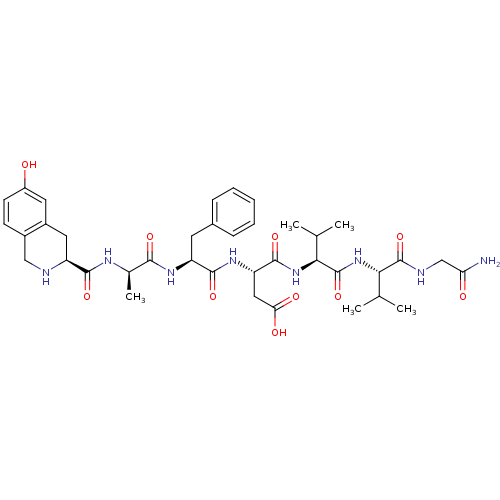
((S)-N-{(S)-1-[(S)-1-(Carbamoylmethyl-carbamoyl)-2-...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](C)NC(=O)[C@@H]1Cc2cc(O)ccc2CN1)C(C)C)C(=O)NCC(N)=O Show InChI InChI=1S/C38H52N8O10/c1-19(2)31(37(55)41-18-29(39)48)46-38(56)32(20(3)4)45-36(54)28(16-30(49)50)44-35(53)27(13-22-9-7-6-8-10-22)43-33(51)21(5)42-34(52)26-15-24-14-25(47)12-11-23(24)17-40-26/h6-12,14,19-21,26-28,31-32,40,47H,13,15-18H2,1-5H3,(H2,39,48)(H,41,55)(H,42,52)(H,43,51)(H,44,53)(H,45,54)(H,46,56)(H,49,50)/t21-,26+,27+,28+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor delta |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS-21680 from human adenosine A2A receptor expressed in CHO cells |
J Med Chem 50: 374-80 (2007)
Article DOI: 10.1021/jm061170a
BindingDB Entry DOI: 10.7270/Q2F76DDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438632
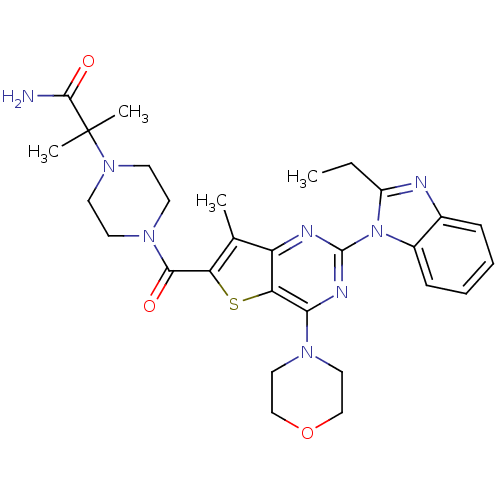
(CHEMBL2414295)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)c(C)c2n1 Show InChI InChI=1S/C29H36N8O3S/c1-5-21-31-19-8-6-7-9-20(19)37(21)28-32-22-18(2)23(41-24(22)25(33-28)34-14-16-40-17-15-34)26(38)35-10-12-36(13-11-35)29(3,4)27(30)39/h6-9H,5,10-17H2,1-4H3,(H2,30,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dipartimento di Scienze Farmaceutiche
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor expressed in CHO cells |
J Med Chem 51: 5875-9 (2008)
Article DOI: 10.1021/jm800586p
BindingDB Entry DOI: 10.7270/Q2PG1RJ9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50085658

((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NC3CCCC3)nc(Cl)nc12 |r| Show InChI InChI=1S/C15H20ClN5O4/c16-15-19-12(18-7-3-1-2-4-7)9-13(20-15)21(6-17-9)14-11(24)10(23)8(5-22)25-14/h6-8,10-11,14,22-24H,1-5H2,(H,18,19,20)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cells after 90 mins |
J Med Chem 55: 7719-35 (2012)
Article DOI: 10.1021/jm3007504
BindingDB Entry DOI: 10.7270/Q2H41SK5 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301329
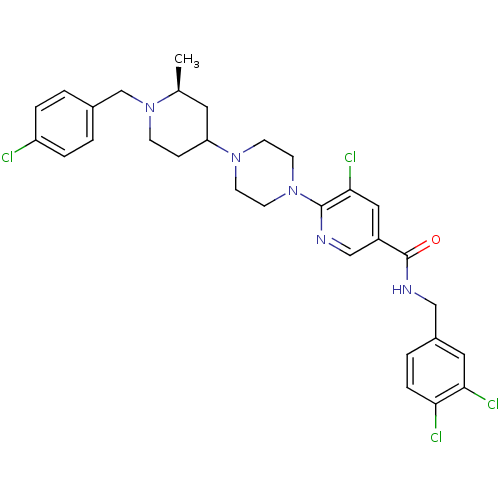
(5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...)Show SMILES C[C@H]1CC(CCN1Cc1ccc(Cl)cc1)N1CCN(CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-14-25(8-9-39(20)19-21-2-5-24(31)6-3-21)37-10-12-38(13-11-37)29-28(34)16-23(18-35-29)30(40)36-17-22-4-7-26(32)27(33)15-22/h2-7,15-16,18,20,25H,8-14,17,19H2,1H3,(H,36,40)/t20-,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]CHA from human adenosine A1 receptor expressed in CHO cells |
J Med Chem 50: 374-80 (2007)
Article DOI: 10.1021/jm061170a
BindingDB Entry DOI: 10.7270/Q2F76DDP |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50202575
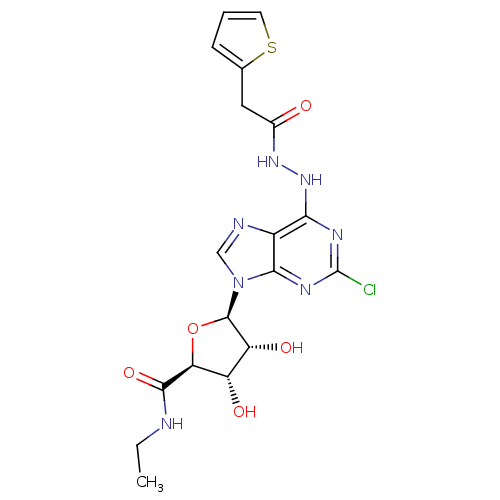
(1-deoxy-1-{2-chloro-6-[N'-(2-thiophen-2-yl-acetyl)...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NNC(=O)Cc3cccs3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H20ClN7O5S/c1-2-20-16(30)13-11(28)12(29)17(31-13)26-7-21-10-14(22-18(19)23-15(10)26)25-24-9(27)6-8-4-3-5-32-8/h3-5,7,11-13,17,28-29H,2,6H2,1H3,(H,20,30)(H,24,27)(H,22,23,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 50: 374-80 (2007)
Article DOI: 10.1021/jm061170a
BindingDB Entry DOI: 10.7270/Q2F76DDP |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
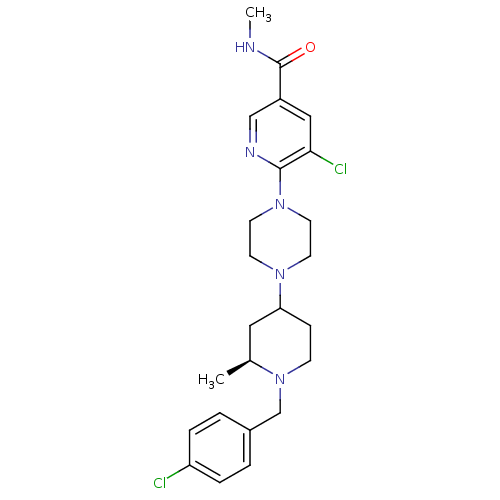
(Homo sapiens (Human)) | BDBM50301330
(5-chloro-6-(4-((2S)-1-(4-chlorobenzyl)-2-methylpip...)Show SMILES CNC(=O)c1cnc(N2CCN(CC2)C2CCN(Cc3ccc(Cl)cc3)[C@@H](C)C2)c(Cl)c1 |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-13-21(7-8-31(17)16-18-3-5-20(25)6-4-18)29-9-11-30(12-10-29)23-22(26)14-19(15-28-23)24(32)27-2/h3-6,14-15,17,21H,7-13,16H2,1-2H3,(H,27,32)/t17-,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM21220

((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C12H16N6O4/c1-2-14-11(21)8-6(19)7(20)12(22-8)18-4-17-5-9(13)15-3-16-10(5)18/h3-4,6-8,12,19-20H,2H2,1H3,(H,14,21)(H2,13,15,16)/t6-,7+,8-,12+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 34.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells |
J Med Chem 50: 374-80 (2007)
Article DOI: 10.1021/jm061170a
BindingDB Entry DOI: 10.7270/Q2F76DDP |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
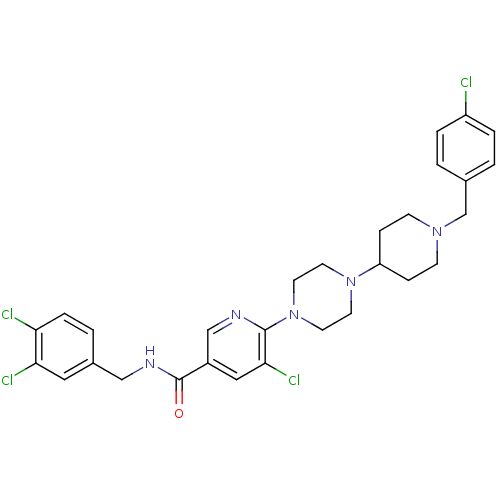
(Homo sapiens (Human)) | BDBM50301351
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Clc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C29H31Cl4N5O/c30-23-4-1-20(2-5-23)19-36-9-7-24(8-10-36)37-11-13-38(14-12-37)28-27(33)16-22(18-34-28)29(39)35-17-21-3-6-25(31)26(32)15-21/h1-6,15-16,18,24H,7-14,17,19H2,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
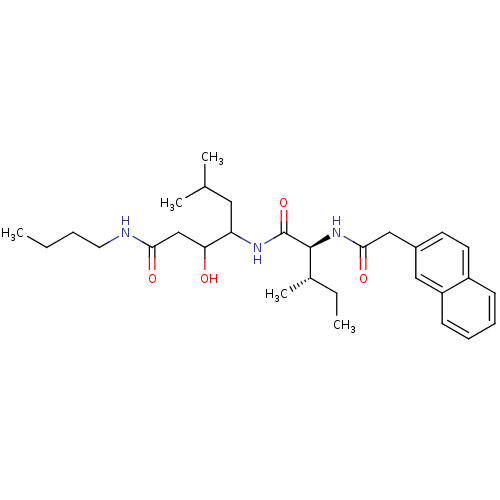
(Plasmodium falciparum) | BDBM50071558
(3-Hydroxy-6-methyl-4-[(2S,3S)-3-methyl-2-(2-naphth...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H45N3O4/c1-6-8-15-31-27(35)19-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)18-22-13-14-23-11-9-10-12-24(23)17-22/h9-14,17,20-21,25-26,29,34H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,32,37)(H,33,36)/t21-,25?,26?,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against plasmepsin-2 from Plasmodium falciparum |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071554

(3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O4/c1-4-6-18-34-30(38)22-29(37)28(20-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)21-25-16-17-26-14-10-11-15-27(26)19-25/h7-17,19,23,28-29,32,37H,4-6,18,20-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50166345

(6-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...)Show SMILES C[C@@H](NC(=O)C1Cc2cc(O)ccc2CN1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C31H35N5O5/c1-19(34-30(40)26-17-23-16-24(37)13-12-22(23)18-33-26)29(39)36-27(15-21-10-6-3-7-11-21)31(41)35-25(28(32)38)14-20-8-4-2-5-9-20/h2-13,16,19,25-27,33,37H,14-15,17-18H2,1H3,(H2,32,38)(H,34,40)(H,35,41)(H,36,39)/t19-,25+,26?,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-naloxone binding to rat brain homogenate Opioid receptor mu |
Bioorg Med Chem Lett 15: 2467-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.075
BindingDB Entry DOI: 10.7270/Q2057FF6 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50202575

(1-deoxy-1-{2-chloro-6-[N'-(2-thiophen-2-yl-acetyl)...)Show SMILES CCNC(=O)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NNC(=O)Cc3cccs3)nc(Cl)nc12 |r| Show InChI InChI=1S/C18H20ClN7O5S/c1-2-20-16(30)13-11(28)12(29)17(31-13)26-7-21-10-14(22-18(19)23-15(10)26)25-24-9(27)6-8-4-3-5-32-8/h3-5,7,11-13,17,28-29H,2,6H2,1H3,(H,20,30)(H,24,27)(H,22,23,25)/t11-,12+,13-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]CHA from human adenosine A1 receptor expressed in CHO cells |
J Med Chem 50: 374-80 (2007)
Article DOI: 10.1021/jm061170a
BindingDB Entry DOI: 10.7270/Q2F76DDP |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301340
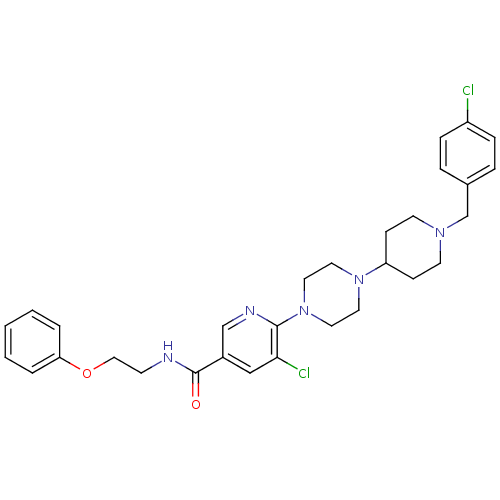
(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...)Show SMILES Clc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCCOc2ccccc2)cc1 Show InChI InChI=1S/C30H35Cl2N5O2/c31-25-8-6-23(7-9-25)22-35-13-10-26(11-14-35)36-15-17-37(18-16-36)29-28(32)20-24(21-34-29)30(38)33-12-19-39-27-4-2-1-3-5-27/h1-9,20-21,26H,10-19,22H2,(H,33,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301341
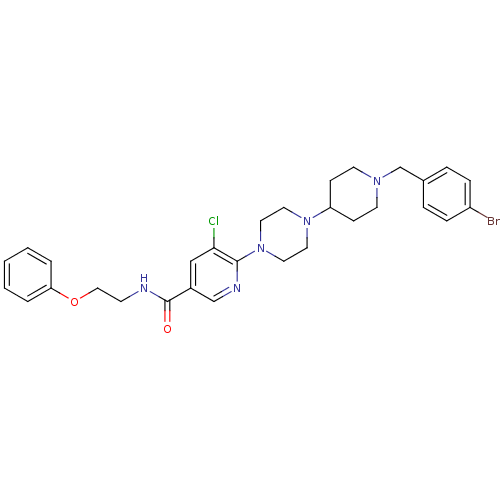
(6-(4-(1-(4-bromobenzyl)piperidin-4-yl)piperazin-1-...)Show SMILES Clc1cc(cnc1N1CCN(CC1)C1CCN(Cc2ccc(Br)cc2)CC1)C(=O)NCCOc1ccccc1 Show InChI InChI=1S/C30H35BrClN5O2/c31-25-8-6-23(7-9-25)22-35-13-10-26(11-14-35)36-15-17-37(18-16-36)29-28(32)20-24(21-34-29)30(38)33-12-19-39-27-4-2-1-3-5-27/h1-9,20-21,26H,10-19,22H2,(H,33,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301328
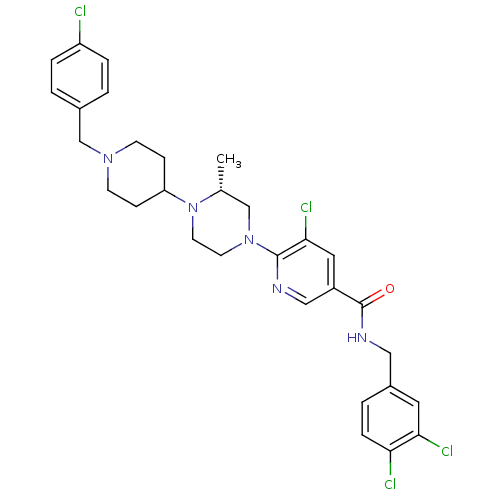
((R)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-28(34)15-23(17-35-29)30(40)36-16-22-4-7-26(32)27(33)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301326
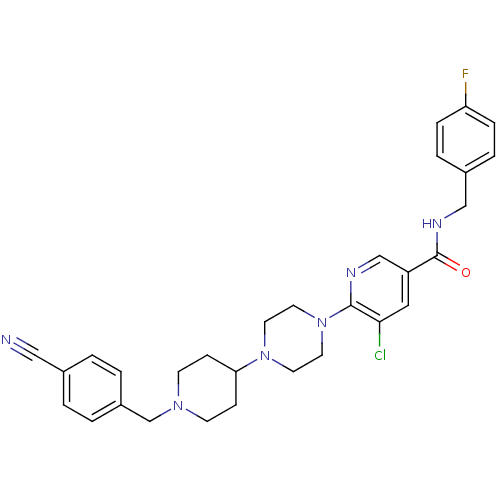
(5-chloro-6-(4-(1-(4-cyanobenzyl)piperidin-4-yl)pip...)Show SMILES Fc1ccc(CNC(=O)c2cnc(N3CCN(CC3)C3CCN(Cc4ccc(cc4)C#N)CC3)c(Cl)c2)cc1 Show InChI InChI=1S/C30H32ClFN6O/c31-28-17-25(30(39)35-19-23-5-7-26(32)8-6-23)20-34-29(28)38-15-13-37(14-16-38)27-9-11-36(12-10-27)21-24-3-1-22(18-33)2-4-24/h1-8,17,20,27H,9-16,19,21H2,(H,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071554

(3-Hydroxy-4-[(2S,3S)-3-methyl-2-(2-naphthalen-2-yl...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O4/c1-4-6-18-34-30(38)22-29(37)28(20-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)21-25-16-17-26-14-10-11-15-27(26)19-25/h7-17,19,23,28-29,32,37H,4-6,18,20-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50249090
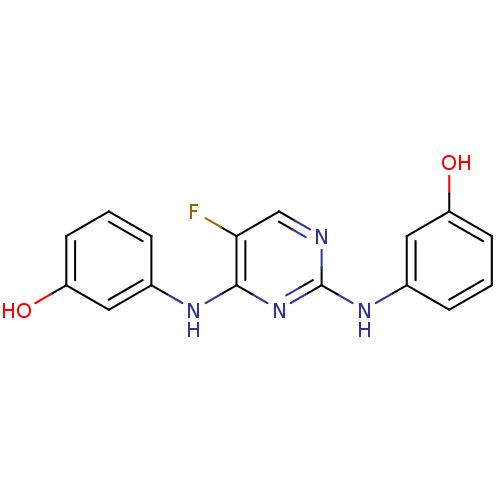
(3,3'-(5-fluoropyrimidine-2,4-diyl)bis(azanediyl)di...)Show InChI InChI=1S/C16H13FN4O2/c17-14-9-18-16(20-11-4-2-6-13(23)8-11)21-15(14)19-10-3-1-5-12(22)7-10/h1-9,22-23H,(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amarit Bioscience, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SYK (unknown origin) |
Bioorg Med Chem Lett 25: 2117-21 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.075
BindingDB Entry DOI: 10.7270/Q2R49SFK |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071546
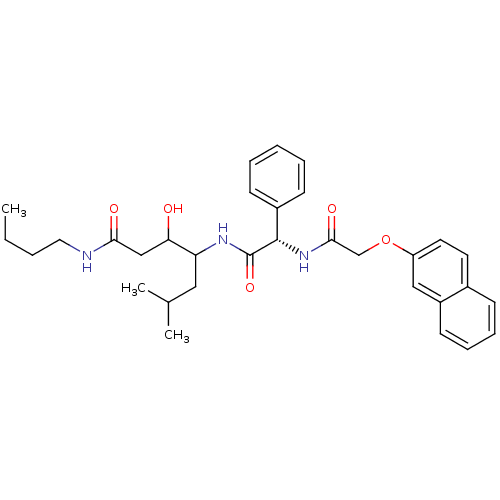
(3-Hydroxy-6-methyl-4-{(S)-2-[2-(naphthalen-2-yloxy...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)c1ccccc1 Show InChI InChI=1S/C32H41N3O5/c1-4-5-17-33-29(37)20-28(36)27(18-22(2)3)34-32(39)31(24-12-7-6-8-13-24)35-30(38)21-40-26-16-15-23-11-9-10-14-25(23)19-26/h6-16,19,22,27-28,31,36H,4-5,17-18,20-21H2,1-3H3,(H,33,37)(H,34,39)(H,35,38)/t27?,28?,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301335
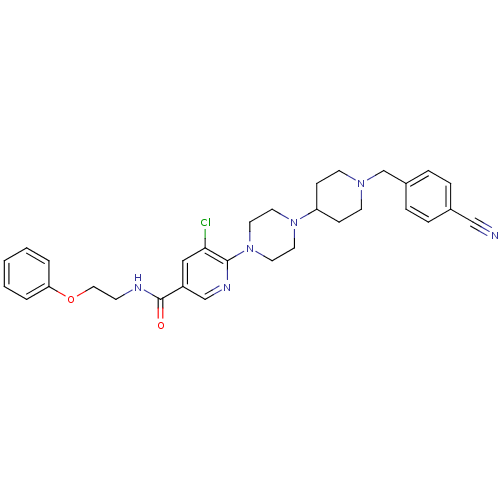
(5-chloro-6-(4-(1-(4-cyanobenzyl)piperidin-4-yl)pip...)Show SMILES Clc1cc(cnc1N1CCN(CC1)C1CCN(Cc2ccc(cc2)C#N)CC1)C(=O)NCCOc1ccccc1 Show InChI InChI=1S/C31H35ClN6O2/c32-29-20-26(31(39)34-12-19-40-28-4-2-1-3-5-28)22-35-30(29)38-17-15-37(16-18-38)27-10-13-36(14-11-27)23-25-8-6-24(21-33)7-9-25/h1-9,20,22,27H,10-19,23H2,(H,34,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50396642

(CHEMBL2171939)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H38N10O2/c1-5-21-30-19-8-6-7-9-20(19)38(21)27-32-24-23(25(33-27)36-14-16-40-17-15-36)31-22(34(24)4)18-35-10-12-37(13-11-35)28(2,3)26(29)39/h6-9H,5,10-18H2,1-4H3,(H2,29,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071561
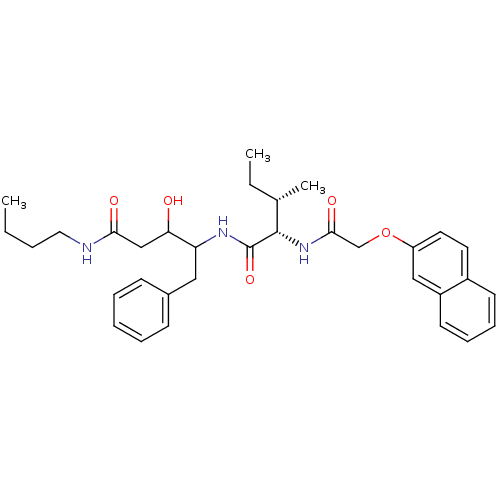
(3-Hydroxy-4-{(2S,3S)-3-methyl-2-[2-(naphthalen-2-y...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C33H43N3O5/c1-4-6-18-34-30(38)21-29(37)28(19-24-12-8-7-9-13-24)35-33(40)32(23(3)5-2)36-31(39)22-41-27-17-16-25-14-10-11-15-26(25)20-27/h7-17,20,23,28-29,32,37H,4-6,18-19,21-22H2,1-3H3,(H,34,38)(H,35,40)(H,36,39)/t23-,28?,29?,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301344
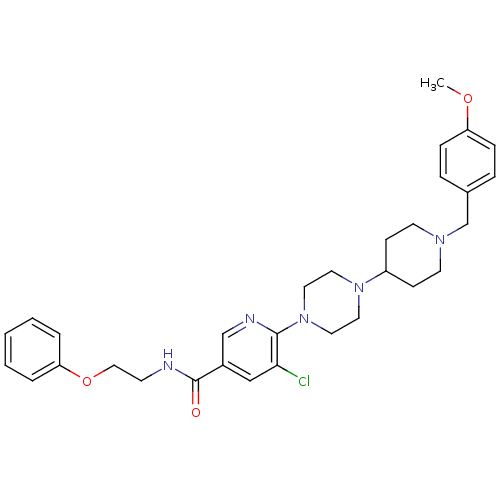
(5-chloro-6-(4-(1-(4-methoxybenzyl)piperidin-4-yl)p...)Show SMILES COc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCCOc2ccccc2)cc1 Show InChI InChI=1S/C31H38ClN5O3/c1-39-27-9-7-24(8-10-27)23-35-14-11-26(12-15-35)36-16-18-37(19-17-36)30-29(32)21-25(22-34-30)31(38)33-13-20-40-28-5-3-2-4-6-28/h2-10,21-22,26H,11-20,23H2,1H3,(H,33,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301348
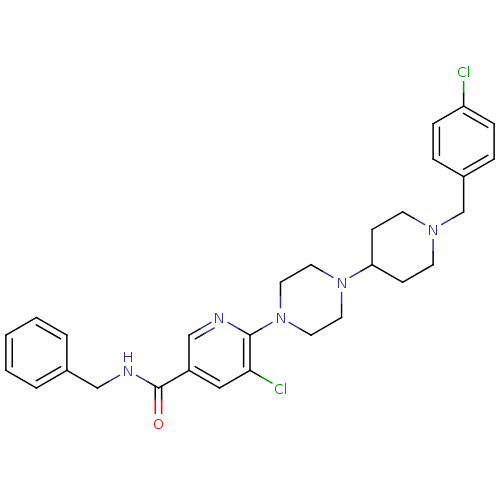
(CHEMBL577409 | N-benzyl-5-chloro-6-(4-(1-(4-chloro...)Show SMILES Clc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCc2ccccc2)cc1 Show InChI InChI=1S/C29H33Cl2N5O/c30-25-8-6-23(7-9-25)21-34-12-10-26(11-13-34)35-14-16-36(17-15-35)28-27(31)18-24(20-32-28)29(37)33-19-22-4-2-1-3-5-22/h1-9,18,20,26H,10-17,19,21H2,(H,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50438632

(CHEMBL2414295)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)c(C)c2n1 Show InChI InChI=1S/C29H36N8O3S/c1-5-21-31-19-8-6-7-9-20(19)37(21)28-32-22-18(2)23(41-24(22)25(33-28)34-14-16-40-17-15-34)26(38)35-10-12-36(13-11-35)29(3,4)27(30)39/h6-9H,5,10-17H2,1-4H3,(H2,30,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50301342
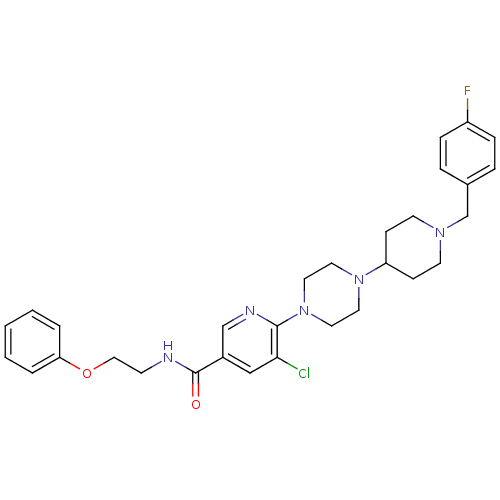
(5-chloro-6-(4-(1-(4-fluorobenzyl)piperidin-4-yl)pi...)Show SMILES Fc1ccc(CN2CCC(CC2)N2CCN(CC2)c2ncc(cc2Cl)C(=O)NCCOc2ccccc2)cc1 Show InChI InChI=1S/C30H35ClFN5O2/c31-28-20-24(30(38)33-12-19-39-27-4-2-1-3-5-27)21-34-29(28)37-17-15-36(16-18-37)26-10-13-35(14-11-26)22-23-6-8-25(32)9-7-23/h1-9,20-21,26H,10-19,22H2,(H,33,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of 125I-IP10 from recombinant human CXCR3 receptor expressed in Ba/F3 cell membrane after 1 to 4 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 5205-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.020
BindingDB Entry DOI: 10.7270/Q21V5F18 |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50071555
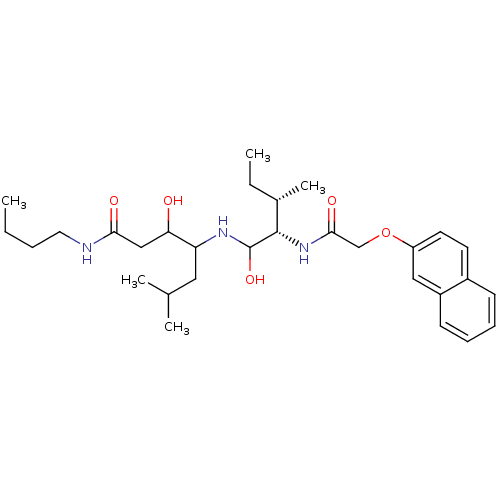
(3-Hydroxy-4-{(2S,3S)-1-hydroxy-3-methyl-2-[2-(naph...)Show SMILES CCCCNC(=O)CC(O)C(CC(C)C)NC(O)[C@@H](NC(=O)COc1ccc2ccccc2c1)[C@@H](C)CC Show InChI InChI=1S/C30H47N3O5/c1-6-8-15-31-27(35)18-26(34)25(16-20(3)4)32-30(37)29(21(5)7-2)33-28(36)19-38-24-14-13-22-11-9-10-12-23(22)17-24/h9-14,17,20-21,25-26,29-30,32,34,37H,6-8,15-16,18-19H2,1-5H3,(H,31,35)(H,33,36)/t21-,25?,26?,29-,30?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of plasmepsin-2 from Plasmodium falciparum. |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50071559
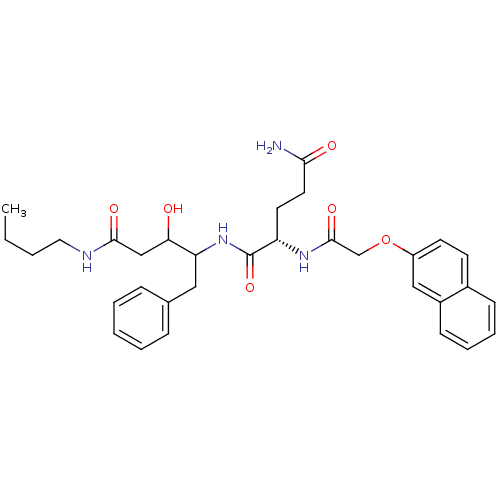
((S)-2-[2-(Naphthalen-2-yloxy)-acetylamino]-pentane...)Show SMILES CCCCNC(=O)CC(O)C(Cc1ccccc1)NC(=O)[C@H](CCC(N)=O)NC(=O)COc1ccc2ccccc2c1 Show InChI InChI=1S/C32H40N4O6/c1-2-3-17-34-30(39)20-28(37)27(18-22-9-5-4-6-10-22)36-32(41)26(15-16-29(33)38)35-31(40)21-42-25-14-13-23-11-7-8-12-24(23)19-25/h4-14,19,26-28,37H,2-3,15-18,20-21H2,1H3,(H2,33,38)(H,34,39)(H,35,40)(H,36,41)/t26-,27?,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against cathepsin D |
Bioorg Med Chem Lett 8: 2315-20 (1999)
BindingDB Entry DOI: 10.7270/Q2SN09G9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data