

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000335 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at Opioid receptor delta 1 by displacing [3H]- DSLET | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at Opioid receptor delta 1 by displacing [3H]- DSLET | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000335 (13-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at opioid receptor mu by displacing [3H]- DAMGO | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50265896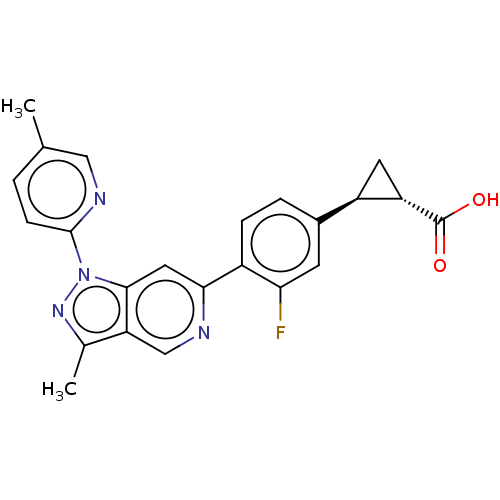 (CHEMBL4070359) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Adenosine A2A receptor (unknown origin) | J Med Chem 60: 3187-3197 (2017) Article DOI: 10.1021/acs.jmedchem.7b00210 BindingDB Entry DOI: 10.7270/Q2PZ5C9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description In Vitro evaluation for the binding affinity in homogenates of rat brain at opioid receptor mu by displacing [3H]- DAMGO | J Med Chem 35: 684-7 (1992) BindingDB Entry DOI: 10.7270/Q2FB53JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001852 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-1-{2-[(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453625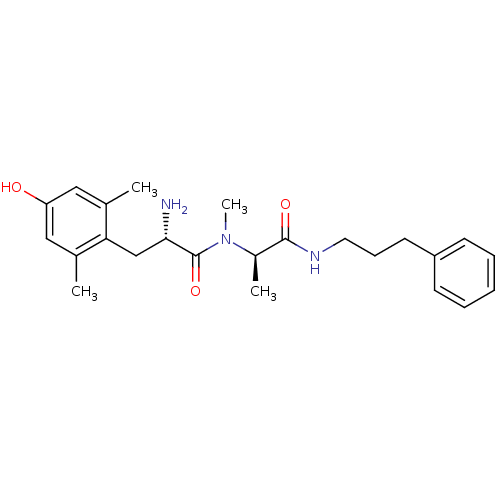 (CHEMBL2110229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036787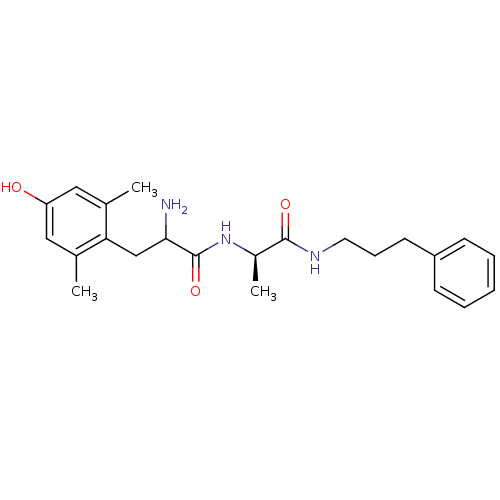 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036787 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001848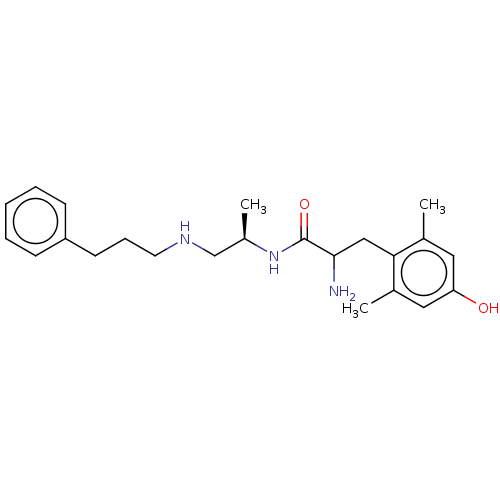 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[1-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001850 ((S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453625 (CHEMBL2110229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001846 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[1-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453616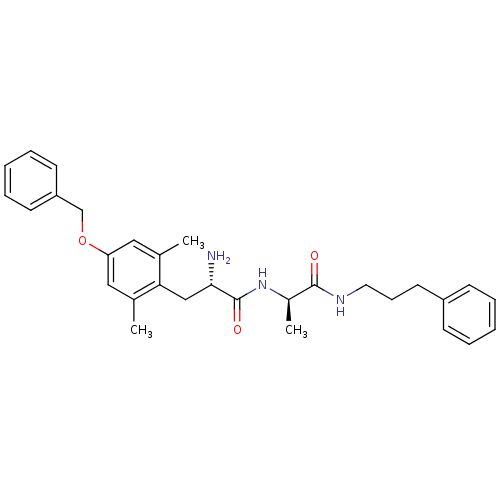 (CHEMBL2110337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036790 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036790 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001849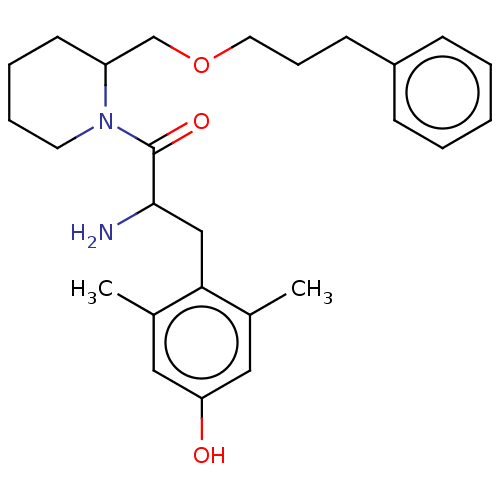 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-1-[2-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001845 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[2-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036806 (2-Amino-3-[2,6-dimethyl-4-(4-nitro-benzyloxy)-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453616 (CHEMBL2110337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001850 ((S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor delta expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453625 (CHEMBL2110229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Displacement of [3H]-DSLET from rat brain membrane delta opioid receptor | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001851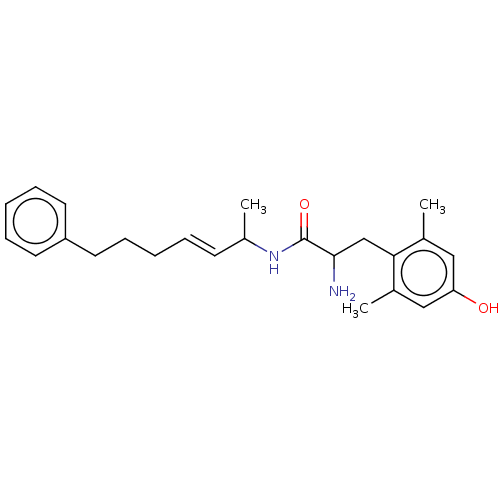 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-(1-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001853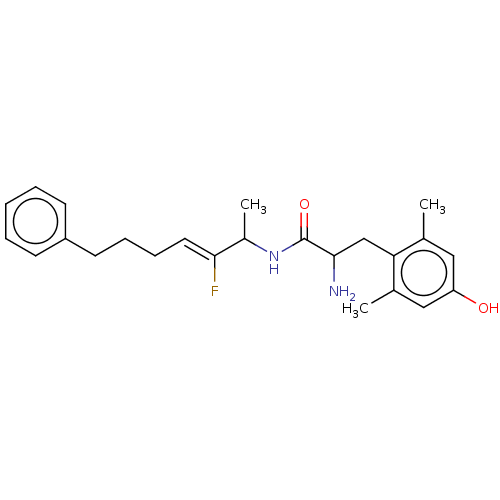 (2-Amino-N-(2-fluoro-1-methyl-6-phenyl-hex-2-enyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453625 (CHEMBL2110229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against delta opioid receptor by the displacement of tritiated DSLET from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036787 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Displacement of [3H]-DSLET from rat brain membrane delta opioid receptor | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036793 (2-Amino-3-[4-(4-fluoro-benzyloxy)-2,6-dimethyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036787 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against delta opioid receptor by the displacement of tritiated DSLET from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710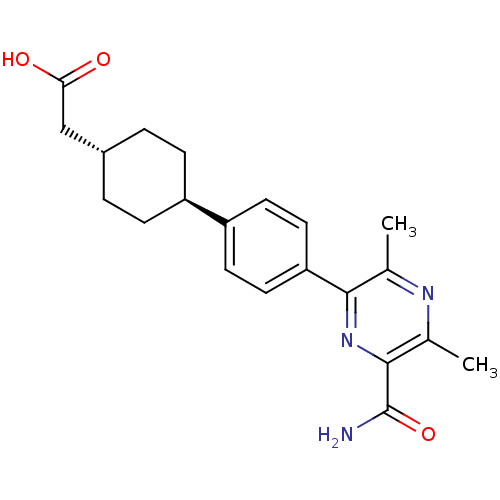 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in human HuTu80 cells | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001852 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-1-{2-[(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor kappa expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399710 (CHEMBL2178953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in human adipose tissue assessed as reduction in triacylglycerol synthesis | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001852 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-1-{2-[(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor delta expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036805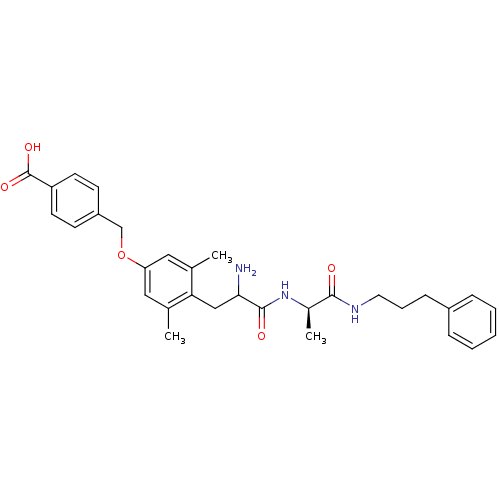 (4-(4-{2-Amino-2-[(R)-1-(3-phenyl-propylcarbamoyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036793 (2-Amino-3-[4-(4-fluoro-benzyloxy)-2,6-dimethyl-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001846 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[1-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor delta expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036802 ((S)-2-Amino-N-cyclopropylmethyl-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036802 ((S)-2-Amino-N-cyclopropylmethyl-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50036797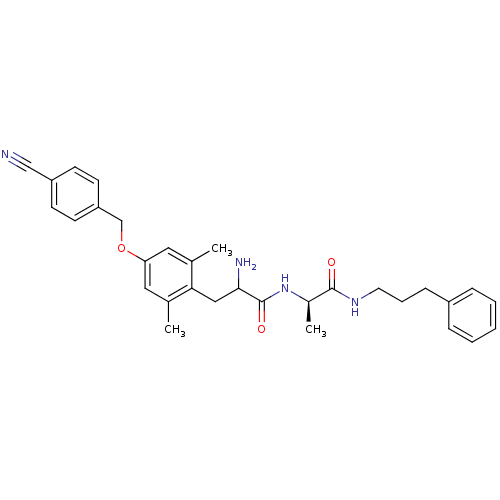 (2-Amino-3-[4-(4-cyano-benzyloxy)-2,6-dimethyl-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399678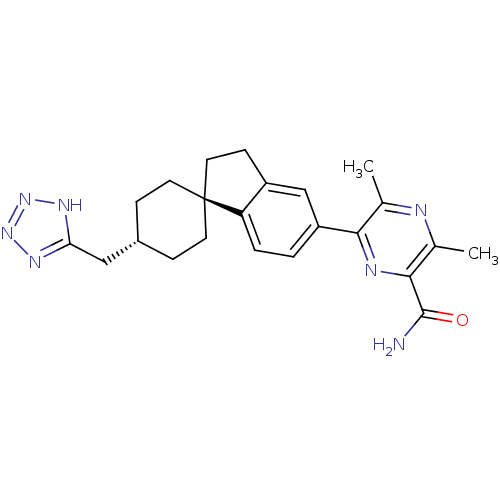 (CHEMBL2178369) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399681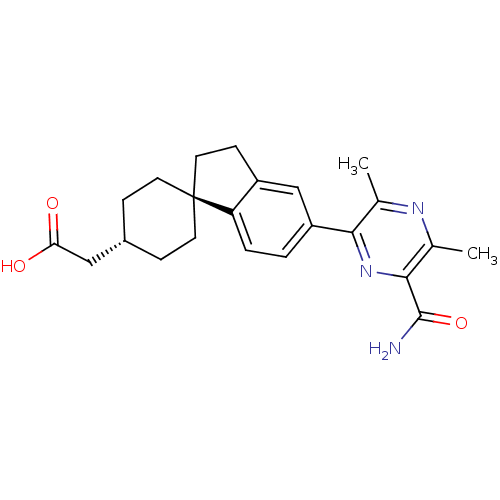 (CHEMBL2178947) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399685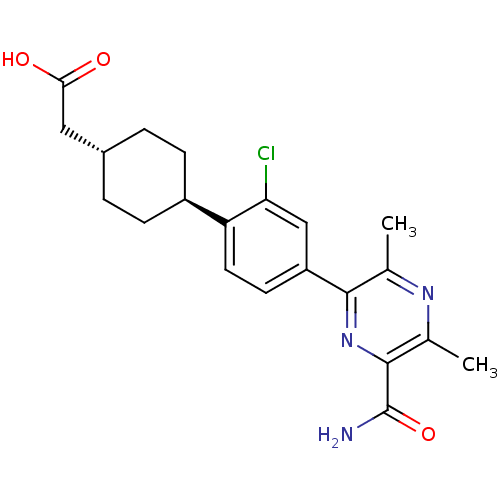 (CHEMBL2178943) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453629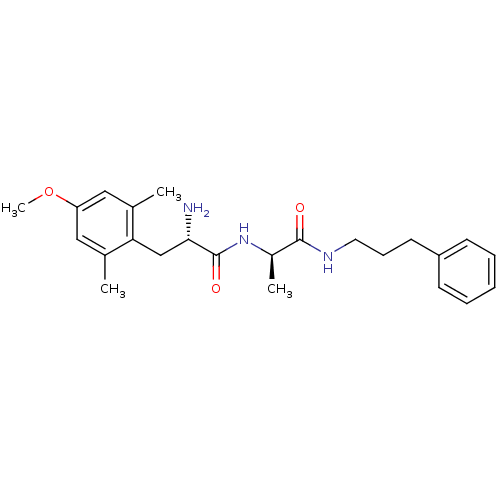 (CHEMBL170594) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001847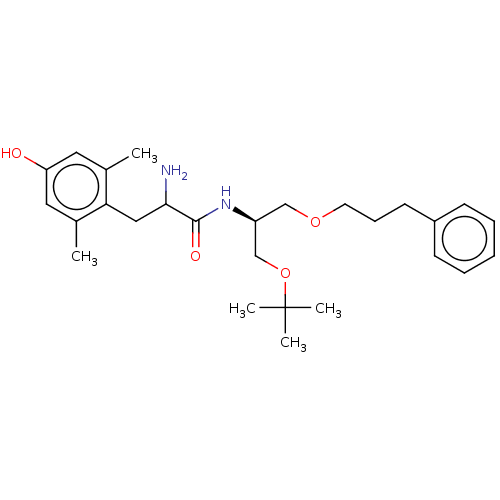 (2-Amino-N-[2-tert-butoxy-1-(3-phenyl-propoxymethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor mu expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399715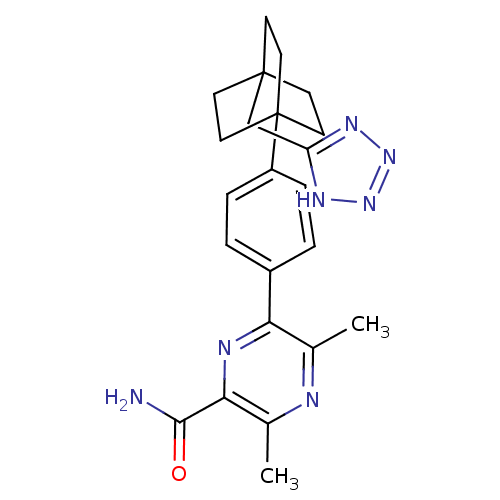 (CHEMBL2178373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453628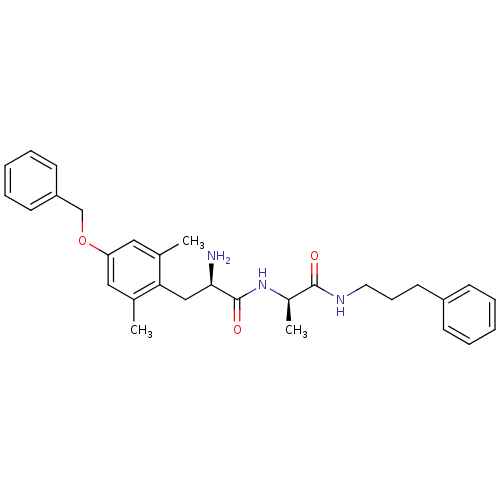 (CHEMBL2110227) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated against mu opioid receptor by the displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001848 (2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-N-[1-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company Curated by ChEMBL | Assay Description Binding ability towards opioid receptor delta expressed in homogenates of rat brain. | J Med Chem 35: 223-33 (1992) BindingDB Entry DOI: 10.7270/Q26T0KKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453616 (CHEMBL2110337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Displacement of [3H]-DSLET from rat brain membrane delta opioid receptor | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50453629 (CHEMBL170594) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Curated by ChEMBL | Assay Description Affinity for mu opioid receptor was measured by displacement of tritiated DAMGO from rat brain membranes | J Med Chem 37: 888-96 (1994) BindingDB Entry DOI: 10.7270/Q27H1HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 [148-636] (Canis familiaris) | BDBM50399710 (CHEMBL2178953) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of DGAT1 in dog liver microsomes | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Diacylglycerol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50399686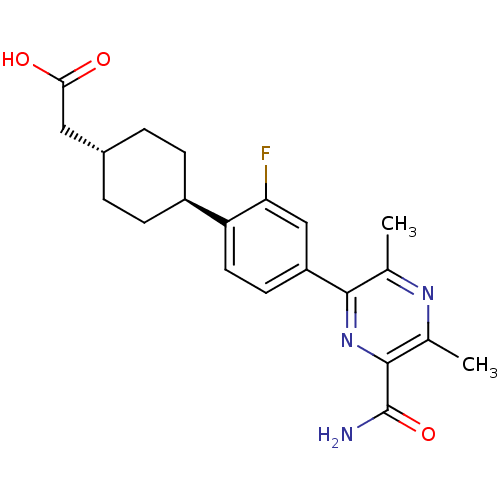 (CHEMBL2178942) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human recombinant DGAT1 expressed in Sf9 cells by liquid scintillography | J Med Chem 55: 10610-29 (2012) Article DOI: 10.1021/jm301296t BindingDB Entry DOI: 10.7270/Q2XD12T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |