 Found 484 hits with Last Name = 'herman' and Initial = 'd'
Found 484 hits with Last Name = 'herman' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377324

(CHEMBL257162)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCCNC(=O)C(F)(F)F)nn1)c1ccccc1 |w:8.28| Show InChI InChI=1S/C20H22F3N5O4S/c1-2-32-18(30)15-12-33-17(13-7-4-3-5-8-13)28(15)16(29)14-11-27(26-25-14)10-6-9-24-19(31)20(21,22)23/h3-5,7-8,11,15,17H,2,6,9-10,12H2,1H3,(H,24,31)/t15-,17?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377323
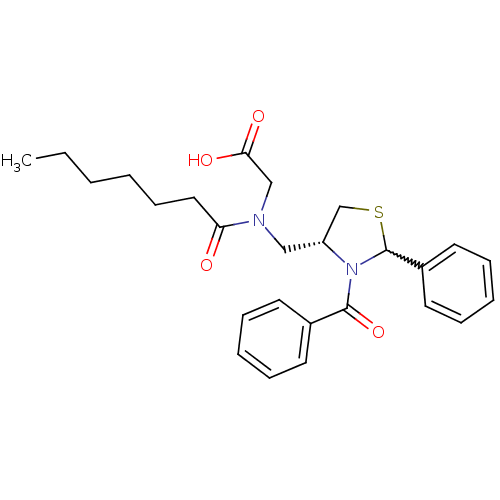
(CHEMBL255717)Show SMILES CCCCCCC(=O)N(C[C@@H]1CSC(N1C(=O)c1ccccc1)c1ccccc1)CC(O)=O |w:13.24| Show InChI InChI=1S/C26H32N2O4S/c1-2-3-4-11-16-23(29)27(18-24(30)31)17-22-19-33-26(21-14-9-6-10-15-21)28(22)25(32)20-12-7-5-8-13-20/h5-10,12-15,22,26H,2-4,11,16-19H2,1H3,(H,30,31)/t22-,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged Bcl-XL expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polarization as... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108987
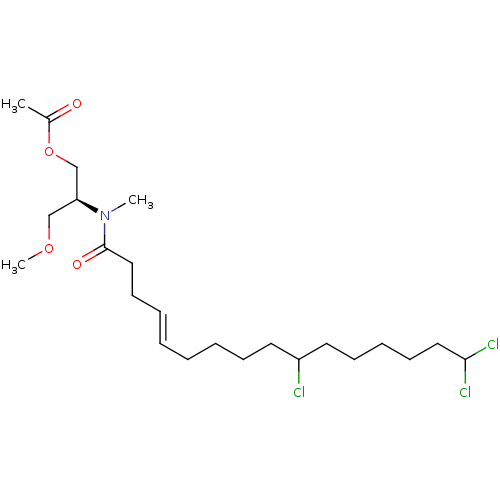
(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108989
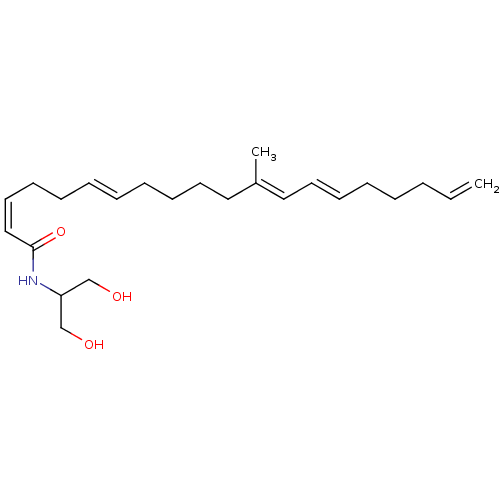
(CHEMBL3597332)Show SMILES C\C(CCCC\C=C\CC\C=C/C(=O)NC(CO)CO)=C/C=C/CCCC=C Show InChI InChI=1S/C24H39NO3/c1-3-4-5-6-11-14-17-22(2)18-15-12-9-7-8-10-13-16-19-24(28)25-23(20-26)21-27/h3,7-8,11,14,16-17,19,23,26-27H,1,4-6,9-10,12-13,15,18,20-21H2,2H3,(H,25,28)/b8-7+,14-11+,19-16-,22-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108988
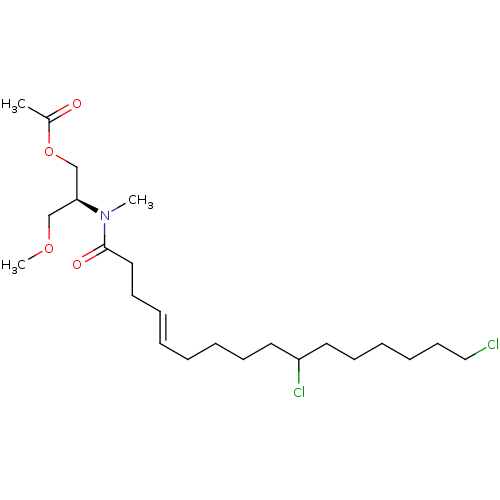
(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108987

(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377326
(CHEMBL402780)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCc2ccsc2)nn1)c1ccccc1 |w:8.26| Show InChI InChI=1S/C21H22N4O3S2/c1-2-28-21(27)18-14-30-20(16-6-4-3-5-7-16)25(18)19(26)17-12-24(23-22-17)10-8-15-9-11-29-13-15/h3-7,9,11-13,18,20H,2,8,10,14H2,1H3/t18-,20?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108988

(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377327
(CHEMBL402148)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCc2ccc(F)cc2)nn1)c1ccccc1 |w:8.28| Show InChI InChI=1S/C23H23FN4O3S/c1-2-31-23(30)20-15-32-22(17-6-4-3-5-7-17)28(20)21(29)19-14-27(26-25-19)13-12-16-8-10-18(24)11-9-16/h3-11,14,20,22H,2,12-13,15H2,1H3/t20-,22?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Probable flavin-dependent thymidylate synthase
(Paramecium bursaria Chlorella virus 1) | BDBM50377325

(CHEMBL257375)Show SMILES CCOC(=O)[C@@H]1CSC(N1C(=O)c1cn(CCCCCCCO)nn1)c1ccccc1 |w:8.26| Show InChI InChI=1S/C22H30N4O4S/c1-2-30-22(29)19-16-31-21(17-11-7-6-8-12-17)26(19)20(28)18-15-25(24-23-18)13-9-4-3-5-10-14-27/h6-8,11-12,15,19,21,27H,2-5,9-10,13-14,16H2,1H3/t19-,21?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ecole Nationale Sup£rieure de Chimie de Paris
Curated by ChEMBL
| Assay Description
Inhibition of PBCV1 Thymidylate synthase X |
Bioorg Med Chem Lett 18: 3628-31 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.080
BindingDB Entry DOI: 10.7270/Q2N87BNK |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM23223
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged MCL1 (171-326) expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polariz... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50297392

(CHEMBL560141 | ethyl 2-amino-4-(2-ethoxy-2-oxoethy...)Show SMILES CCOC(=O)CC1C(C(=O)OCC)C(=N)Oc2ccc(cc12)-c1ccccc1 Show InChI InChI=1S/C22H23NO5/c1-3-26-19(24)13-17-16-12-15(14-8-6-5-7-9-14)10-11-18(16)28-21(23)20(17)22(25)27-4-2/h5-12,17,20,23H,3-4,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged Bcl-XL expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polarization as... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50200965

(2-Amino-6-bromo-4-(cyano-ethoxycarbonyl-methyl)-4H...)Show SMILES CCOC(=O)C(C#N)C1C(C(=O)OCC)C(=N)Oc2ccc(Br)cc12 Show InChI InChI=1S/C17H17BrN2O5/c1-3-23-16(21)11(8-19)13-10-7-9(18)5-6-12(10)25-15(20)14(13)17(22)24-4-2/h5-7,11,13-14,20H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged Bcl-XL expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polarization as... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50297393
(CHEMBL561602 | Ethyl 2-Amino-6-(3',5'-dimethoxyphe...)Show SMILES CCOC(=O)CC1C(C(=O)OCC)C(=N)Oc2ccc(cc12)-c1cc(OC)cc(OC)c1 Show InChI InChI=1S/C24H27NO7/c1-5-30-21(26)13-19-18-11-14(15-9-16(28-3)12-17(10-15)29-4)7-8-20(18)32-23(25)22(19)24(27)31-6-2/h7-12,19,22,25H,5-6,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.24E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged Bcl-XL expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polarization as... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Induced myeloid leukemia cell differentiation protein Mcl-1
(Homo sapiens (Human)) | BDBM50297393

(CHEMBL561602 | Ethyl 2-Amino-6-(3',5'-dimethoxyphe...)Show SMILES CCOC(=O)CC1C(C(=O)OCC)C(=N)Oc2ccc(cc12)-c1cc(OC)cc(OC)c1 Show InChI InChI=1S/C24H27NO7/c1-5-30-21(26)13-19-18-11-14(15-9-16(28-3)12-17(10-15)29-4)7-8-20(18)32-23(25)22(19)24(27)31-6-2/h7-12,19,22,25H,5-6,13H2,1-4H3 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged MCL1 (171-326) expressed in Escherichia coli BL21 (DE3) and Flu-Bak peptide interaction by fluorescence polariz... |
J Med Chem 52: 5937-49 (2009)
Article DOI: 10.1021/jm9005059
BindingDB Entry DOI: 10.7270/Q28053JD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302052
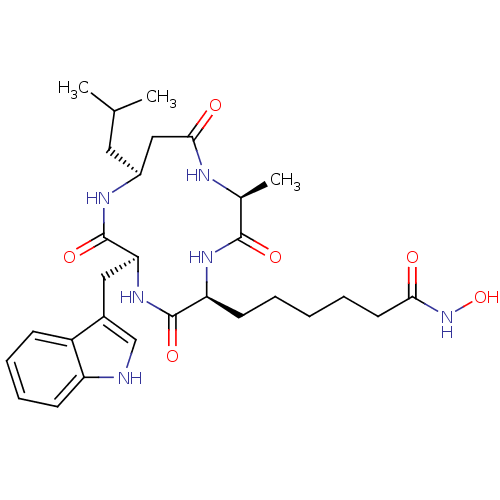
(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1803
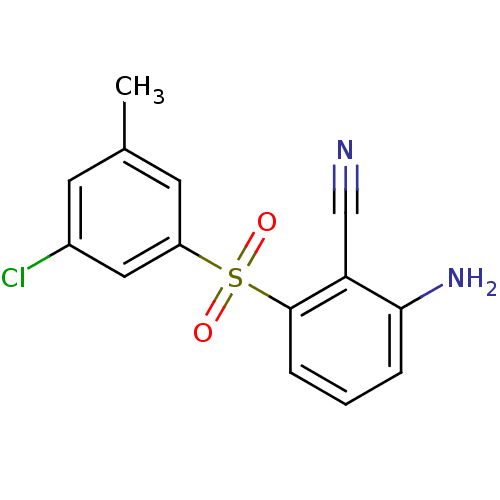
(2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...)Show InChI InChI=1S/C14H11BrN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50284330
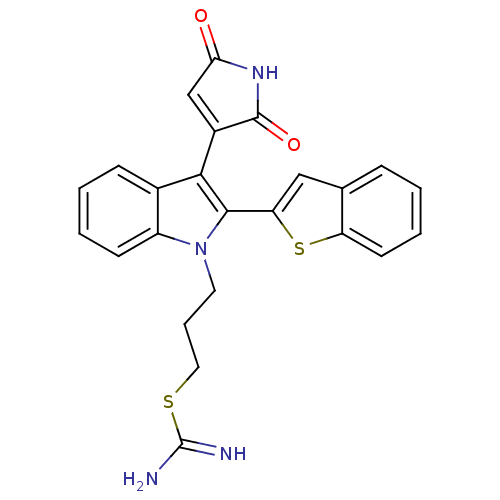
(2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...)Show SMILES NC(=N)SCCCn1c(-c2cc3ccccc3s2)c(C2=CC(=O)NC2=O)c2ccccc12 |t:21| Show InChI InChI=1S/C24H20N4O2S2/c25-24(26)31-11-5-10-28-17-8-3-2-7-15(17)21(16-13-20(29)27-23(16)30)22(28)19-12-14-6-1-4-9-18(14)32-19/h1-4,6-9,12-13H,5,10-11H2,(H3,25,26)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C beta |
Bioorg Med Chem Lett 5: 67-72 (1995)
Article DOI: 10.1016/0960-894X(94)00460-W
BindingDB Entry DOI: 10.7270/Q2ZG6S72 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50058202

((S)-2-[(2-{(S)-2-[((R)-2-Amino-3-mercapto-propiony...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@H](C(C)C)N(C)C(=O)[C@@H](N)CS)C(O)=O Show InChI InChI=1S/C24H36N4O5S2/c1-14(2)20(27(3)22(30)17(25)13-34)23(31)28-12-16-8-6-5-7-15(16)11-19(28)21(29)26-18(24(32)33)9-10-35-4/h5-8,14,17-20,34H,9-13,25H2,1-4H3,(H,26,29)(H,32,33)/t17-,18-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of Human farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1804
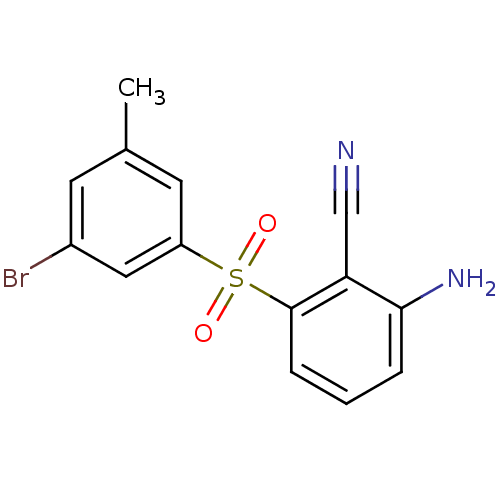
(2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...)Show InChI InChI=1S/C14H11ClN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379130
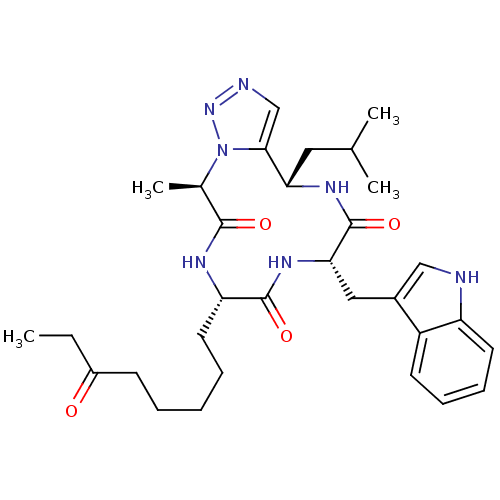
(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1802
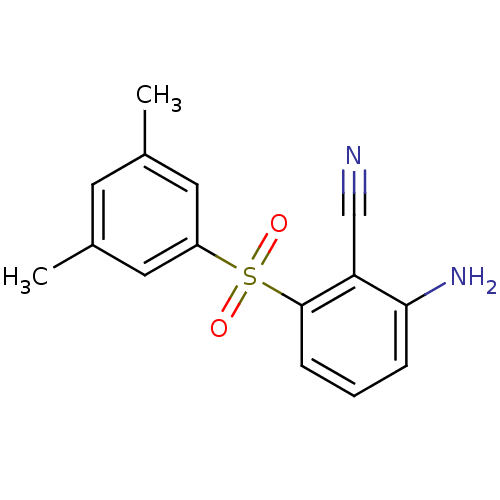
(2-Amino-6-arylthiobenzonitrile deriv. 3v, 739W94 |...)Show InChI InChI=1S/C15H14N2O2S/c1-10-6-11(2)8-12(7-10)20(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302073
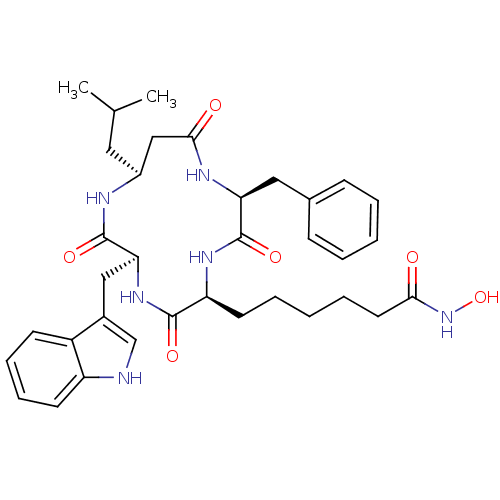
(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-2-benz...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C35H46N6O6/c1-22(2)17-25-20-32(43)38-29(18-23-11-5-3-6-12-23)35(46)39-28(15-7-4-8-16-31(42)41-47)33(44)40-30(34(45)37-25)19-24-21-36-27-14-10-9-13-26(24)27/h3,5-6,9-14,21-22,25,28-30,36,47H,4,7-8,15-20H2,1-2H3,(H,37,45)(H,38,43)(H,39,46)(H,40,44)(H,41,42)/t25-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379131
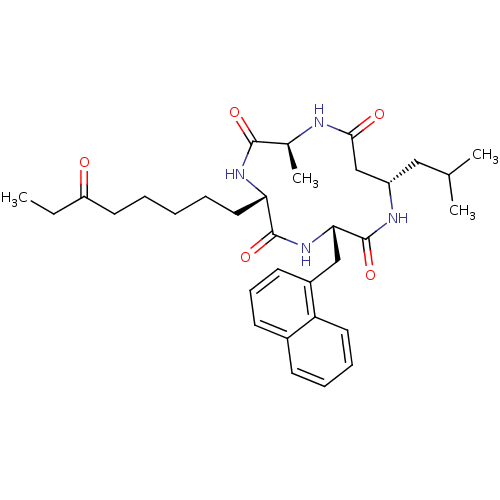
(CHEMBL2012815)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H46N4O5/c1-5-26(38)15-7-6-8-17-28-32(41)37-29(19-24-14-11-13-23-12-9-10-16-27(23)24)33(42)35-25(18-21(2)3)20-30(39)34-22(4)31(40)36-28/h9-14,16,21-22,25,28-29H,5-8,15,17-20H2,1-4H3,(H,34,39)(H,35,42)(H,36,40)(H,37,41)/t22-,25-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50379130

(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50284330

(2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...)Show SMILES NC(=N)SCCCn1c(-c2cc3ccccc3s2)c(C2=CC(=O)NC2=O)c2ccccc12 |t:21| Show InChI InChI=1S/C24H20N4O2S2/c25-24(26)31-11-5-10-28-17-8-3-2-7-15(17)21(16-13-20(29)27-23(16)30)22(28)19-12-14-6-1-4-9-18(14)32-19/h1-4,6-9,12-13H,5,10-11H2,(H3,25,26)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 5: 67-72 (1995)
Article DOI: 10.1016/0960-894X(94)00460-W
BindingDB Entry DOI: 10.7270/Q2ZG6S72 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50328678
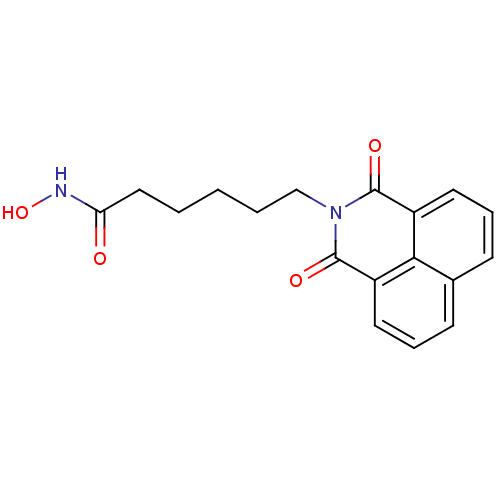
(6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexa...)Show InChI InChI=1S/C18H18N2O4/c21-15(19-24)10-2-1-3-11-20-17(22)13-8-4-6-12-7-5-9-14(16(12)13)18(20)23/h4-9,24H,1-3,10-11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1805
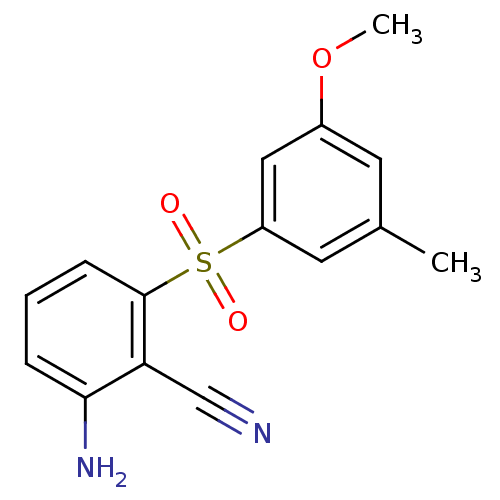
(2-Amino-6-arylthiobenzonitrile deriv. 3y | 2-amino...)Show InChI InChI=1S/C15H14N2O3S/c1-10-6-11(20-2)8-12(7-10)21(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379129
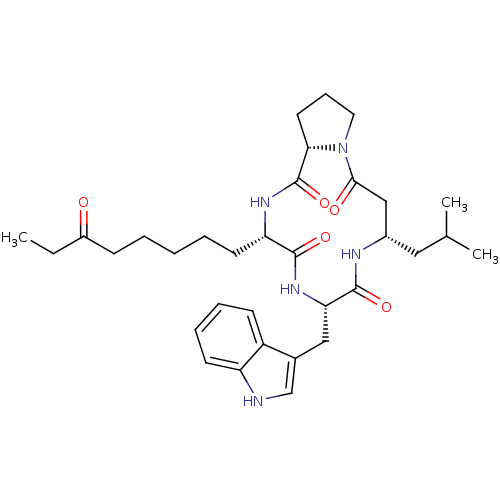
(CHEMBL2012814)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H47N5O5/c1-4-24(39)11-6-5-7-14-27-31(41)37-28(18-22-20-34-26-13-9-8-12-25(22)26)32(42)35-23(17-21(2)3)19-30(40)38-16-10-15-29(38)33(43)36-27/h8-9,12-13,20-21,23,27-29,34H,4-7,10-11,14-19H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t23-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50379129

(CHEMBL2012814)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H47N5O5/c1-4-24(39)11-6-5-7-14-27-31(41)37-28(18-22-20-34-26-13-9-8-12-25(22)26)32(42)35-23(17-21(2)3)19-30(40)38-16-10-15-29(38)33(43)36-27/h8-9,12-13,20-21,23,27-29,34H,4-7,10-11,14-19H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t23-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 in human HeLa cells nuclear extract using Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50379135
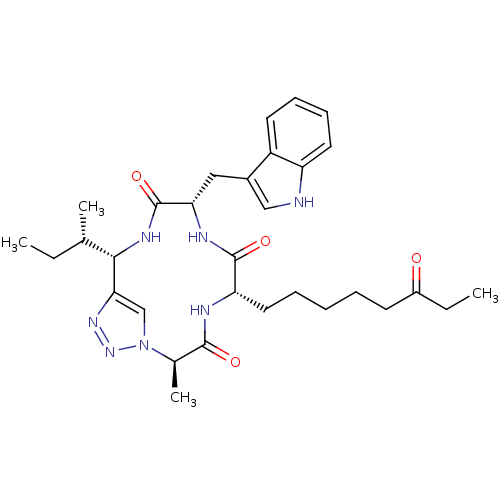
(CHEMBL2012818)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@@H](C)n2cc1nn2 |r| Show InChI InChI=1S/C31H43N7O4/c1-5-19(3)28-27-18-38(37-36-27)20(4)29(40)33-25(15-9-7-8-12-22(39)6-2)30(41)34-26(31(42)35-28)16-21-17-32-24-14-11-10-13-23(21)24/h10-11,13-14,17-20,25-26,28,32H,5-9,12,15-16H2,1-4H3,(H,33,40)(H,34,41)(H,35,42)/t19-,20+,25-,26-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179883

((4-Isopropylphenyl)-(4-isoquinolin-5-yl-phthalazin...)Show SMILES CC(C)c1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1 Show InChI InChI=1S/C26H22N4/c1-17(2)18-10-12-20(13-11-18)28-26-24-8-4-3-7-23(24)25(29-30-26)22-9-5-6-19-16-27-15-14-21(19)22/h3-17H,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302053
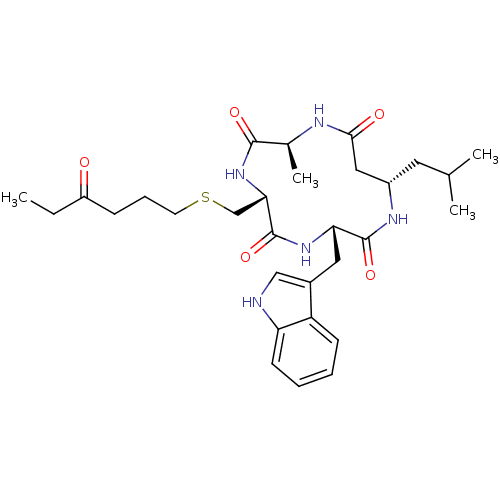
((3S,6R,9S,13S)-3-((1H-indol-3-yl)methyl)-13-isobut...)Show SMILES CCC(=O)CCCSC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C30H43N5O5S/c1-5-22(36)9-8-12-41-17-26-30(40)34-25(14-20-16-31-24-11-7-6-10-23(20)24)29(39)33-21(13-18(2)3)15-27(37)32-19(4)28(38)35-26/h6-7,10-11,16,18-19,21,25-26,31H,5,8-9,12-15,17H2,1-4H3,(H,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,21-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC9 by in vitro deacetylation assay |
Nat Chem Biol 6: 25-33 (2009)
Article DOI: 10.1038/nchembio.275
BindingDB Entry DOI: 10.7270/Q2FF3SKD |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50059852

((S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropyla...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C22H38N4O3S2/c1-15(2)20(24-12-17(23)14-30)13-25-19(11-16-7-5-4-6-8-16)21(27)26-18(22(28)29)9-10-31-3/h4-8,15,17-20,24-25,30H,9-14,23H2,1-3H3,(H,26,27)(H,28,29)/t17-,18+,19+,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibiion of bovine farnesyl transferase |
Bioorg Med Chem Lett 5: 2677-2682 (1995)
Article DOI: 10.1016/0960-894X(95)00482-9
BindingDB Entry DOI: 10.7270/Q2QC03GF |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632
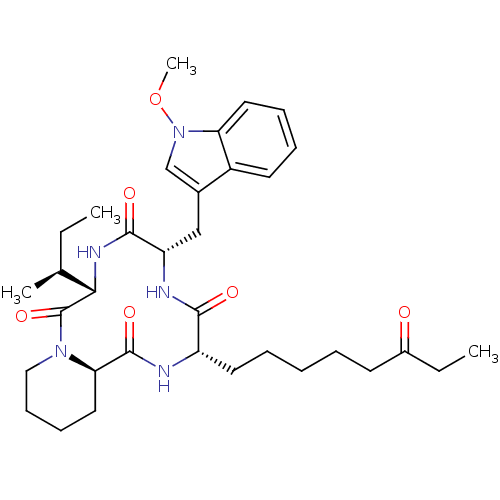
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data