 Found 221 hits with Last Name = 'krosky' and Initial = 'd'
Found 221 hits with Last Name = 'krosky' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50258579
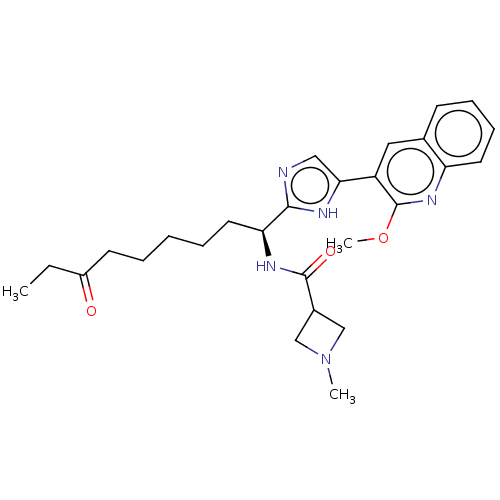
((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435757
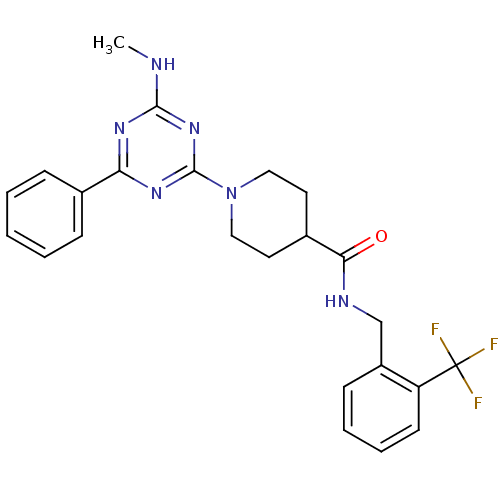
(CHEMBL2392694)Show SMILES CNc1nc(nc(n1)-c1ccccc1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C24H25F3N6O/c1-28-22-30-20(16-7-3-2-4-8-16)31-23(32-22)33-13-11-17(12-14-33)21(34)29-15-18-9-5-6-10-19(18)24(25,26)27/h2-10,17H,11-15H2,1H3,(H,29,34)(H,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435765
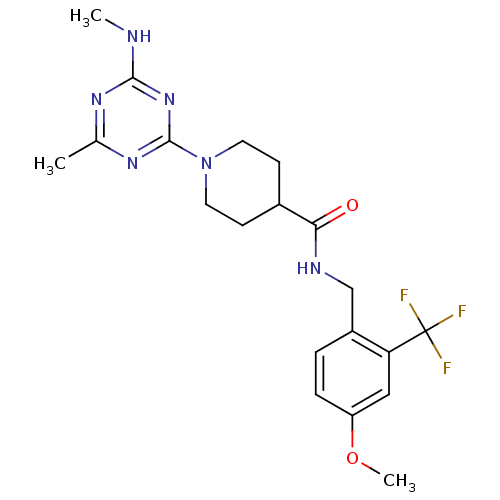
(CHEMBL2392691)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(OC)cc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-12-26-18(24-2)28-19(27-12)29-8-6-13(7-9-29)17(30)25-11-14-4-5-15(31-3)10-16(14)20(21,22)23/h4-5,10,13H,6-9,11H2,1-3H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435745
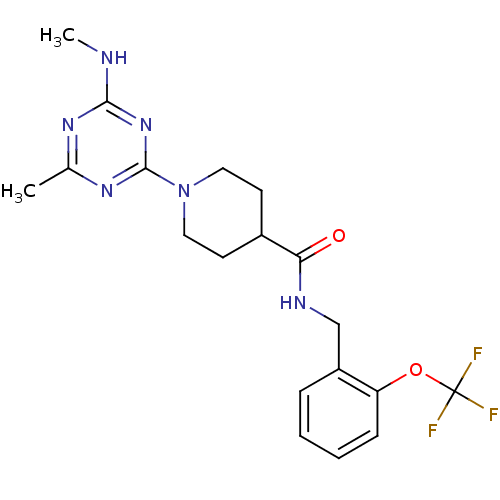
(CHEMBL2392706)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1OC(F)(F)F Show InChI InChI=1S/C19H23F3N6O2/c1-12-25-17(23-2)27-18(26-12)28-9-7-13(8-10-28)16(29)24-11-14-5-3-4-6-15(14)30-19(20,21)22/h3-6,13H,7-11H2,1-2H3,(H,24,29)(H,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435753

(CHEMBL2392698)Show SMILES COCCNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-14-27-19(25-9-12-32-2)29-20(28-14)30-10-7-15(8-11-30)18(31)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,15H,7-13H2,1-2H3,(H,26,31)(H,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435755

(CHEMBL2392696)Show SMILES CC(C)Nc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O/c1-13(2)26-19-27-14(3)28-20(29-19)30-10-8-15(9-11-30)18(31)25-12-16-6-4-5-7-17(16)21(22,23)24/h4-7,13,15H,8-12H2,1-3H3,(H,25,31)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435756

(CHEMBL2392695)Show SMILES CN(C)c1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O/c1-13-25-18(28(2)3)27-19(26-13)29-10-8-14(9-11-29)17(30)24-12-15-6-4-5-7-16(15)20(21,22)23/h4-7,14H,8-12H2,1-3H3,(H,24,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435758

(CHEMBL2392693)Show SMILES CNc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)C1CCCCC1 Show InChI InChI=1S/C24H31F3N6O/c1-28-22-30-20(16-7-3-2-4-8-16)31-23(32-22)33-13-11-17(12-14-33)21(34)29-15-18-9-5-6-10-19(18)24(25,26)27/h5-6,9-10,16-17H,2-4,7-8,11-15H2,1H3,(H,29,34)(H,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435753

(CHEMBL2392698)Show SMILES COCCNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O2/c1-14-27-19(25-9-12-32-2)29-20(28-14)30-10-7-15(8-11-30)18(31)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,15H,7-13H2,1-2H3,(H,26,31)(H,25,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435754
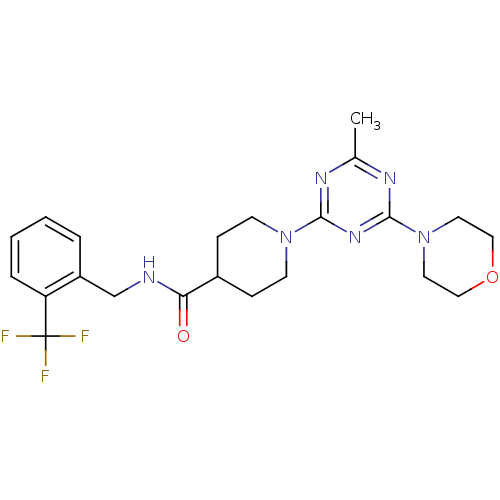
(CHEMBL2392697)Show SMILES Cc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C22H27F3N6O2/c1-15-27-20(29-21(28-15)31-10-12-33-13-11-31)30-8-6-16(7-9-30)19(32)26-14-17-4-2-3-5-18(17)22(23,24)25/h2-5,16H,6-14H2,1H3,(H,26,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569630
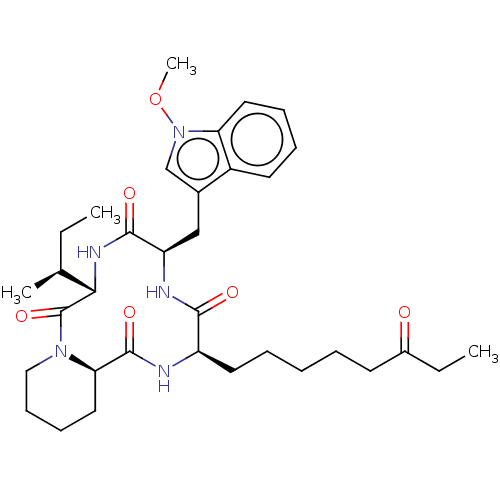
(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435765

(CHEMBL2392691)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(OC)cc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O2/c1-12-26-18(24-2)28-19(27-12)29-8-6-13(7-9-29)17(30)25-11-14-4-5-15(31-3)10-16(14)20(21,22)23/h4-5,10,13H,6-9,11H2,1-3H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prior ... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435764

(CHEMBL2392692)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(Br)cc1OC(F)(F)F Show InChI InChI=1S/C19H22BrF3N6O2/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)31-19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435760

(CHEMBL2392714)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C19H23F3N6O/c1-12-25-17(23-2)27-18(26-12)28-9-7-13(8-10-28)16(29)24-11-14-5-3-4-6-15(14)19(20,21)22/h3-6,13H,7-11H2,1-2H3,(H,24,29)(H,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435745

(CHEMBL2392706)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1OC(F)(F)F Show InChI InChI=1S/C19H23F3N6O2/c1-12-25-17(23-2)27-18(26-12)28-9-7-13(8-10-28)16(29)24-11-14-5-3-4-6-15(14)30-19(20,21)22/h3-6,13H,7-11H2,1-2H3,(H,24,29)(H,23,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435754

(CHEMBL2392697)Show SMILES Cc1nc(nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C22H27F3N6O2/c1-15-27-20(29-21(28-15)31-10-12-33-13-11-31)30-8-6-16(7-9-30)19(32)26-14-17-4-2-3-5-18(17)22(23,24)25/h2-5,16H,6-14H2,1H3,(H,26,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435738

(CHEMBL2392690)Show SMILES CNc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccc(F)cc1C(F)(F)F Show InChI InChI=1S/C19H22F4N6O/c1-11-26-17(24-2)28-18(27-11)29-7-5-12(6-8-29)16(30)25-10-13-3-4-14(20)9-15(13)19(21,22)23/h3-4,9,12H,5-8,10H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435759

(CHEMBL2392715)Show SMILES CCc1nc(NC)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O/c1-3-16-26-18(24-2)28-19(27-16)29-10-8-13(9-11-29)17(30)25-12-14-6-4-5-7-15(14)20(21,22)23/h4-7,13H,3,8-12H2,1-2H3,(H,25,30)(H,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435750

(CHEMBL2392701)Show SMILES CNc1cc(nc(C)n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H24F3N5O/c1-13-26-17(24-2)11-18(27-13)28-9-7-14(8-10-28)19(29)25-12-15-5-3-4-6-16(15)20(21,22)23/h3-6,11,14H,7-10,12H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569643

(CHEMBL4867665)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554257
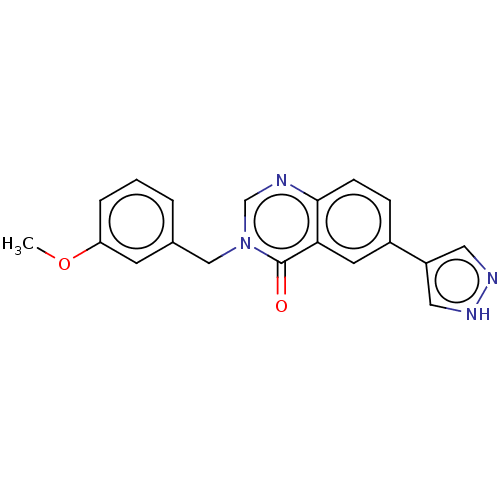
(CHEMBL4742990) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by LANCE assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50258579

((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C1CN(C)C1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C26H33N5O3/c1-17(32)9-5-4-6-12-22(29-25(33)19-15-31(2)16-19)24-27-14-23(28-24)20-13-18-10-7-8-11-21(18)30-26(20)34-3/h7-8,10-11,13-14,19,22H,4-6,9,12,15-16H2,1-3H3,(H,27,28)(H,29,33)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25150
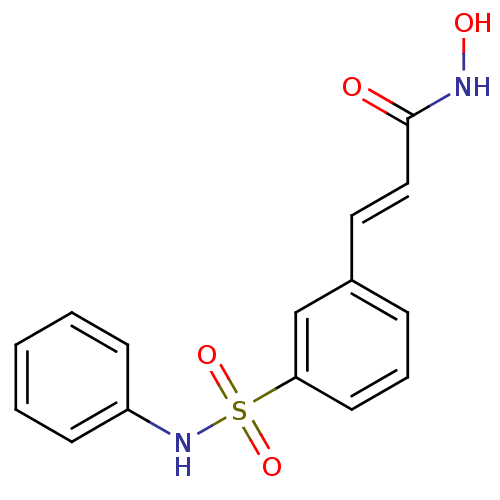
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569630

(CHEMBL4873847)Show SMILES [H][C@]12CCCCN1C(=O)[C@@H](NC(=O)[C@@H](Cc1cn(OC)c3ccccc13)NC(=O)[C@@H](CCCCCC(=O)CC)NC2=O)[C@@H](C)CC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554266
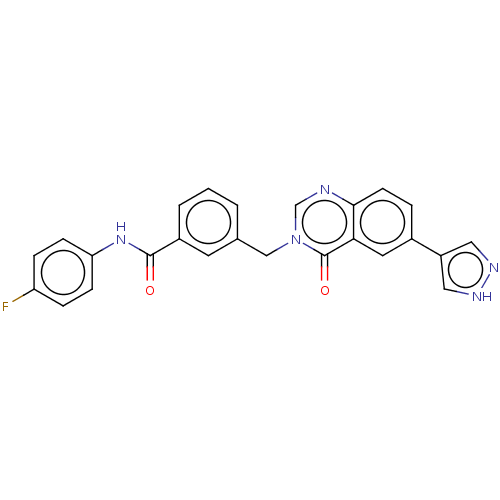
(CHEMBL4785990)Show SMILES Fc1ccc(NC(=O)c2cccc(Cn3cnc4ccc(cc4c3=O)-c3cn[nH]c3)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435755

(CHEMBL2392696)Show SMILES CC(C)Nc1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H27F3N6O/c1-13(2)26-19-27-14(3)28-20(29-19)30-10-8-15(9-11-30)18(31)25-12-16-6-4-5-7-17(16)21(22,23)24/h4-7,13,15H,8-12H2,1-3H3,(H,25,31)(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435747

(CHEMBL2392704)Show SMILES CNc1cc(C)cc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C21H25F3N4O/c1-14-11-18(25-2)27-19(12-14)28-9-7-15(8-10-28)20(29)26-13-16-5-3-4-6-17(16)21(22,23)24/h3-6,11-12,15H,7-10,13H2,1-2H3,(H,25,27)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554267
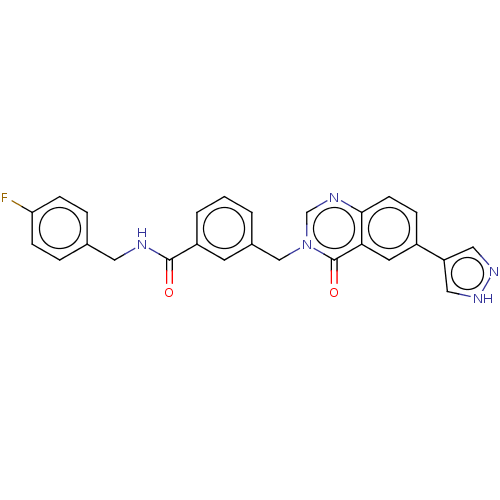
(CHEMBL4760734)Show SMILES Fc1ccc(CNC(=O)c2cccc(Cn3cnc4ccc(cc4c3=O)-c3cn[nH]c3)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554268
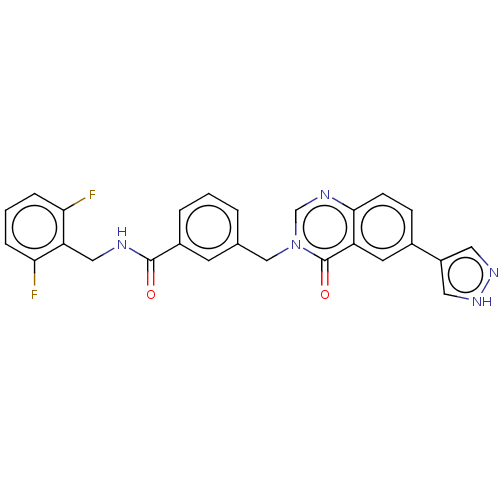
(CHEMBL4745381)Show SMILES Fc1cccc(F)c1CNC(=O)c1cccc(Cn2cnc3ccc(cc3c2=O)-c2cn[nH]c2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50554257

(CHEMBL4742990) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Aurora A |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554276
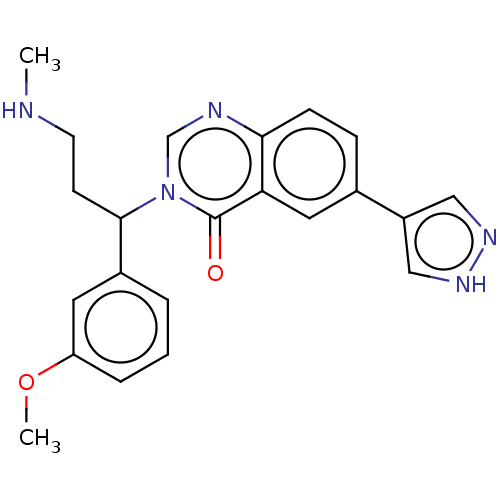
(CHEMBL4753687)Show SMILES CNCCC(c1cccc(OC)c1)n1cnc2ccc(cc2c1=O)-c1cn[nH]c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554274
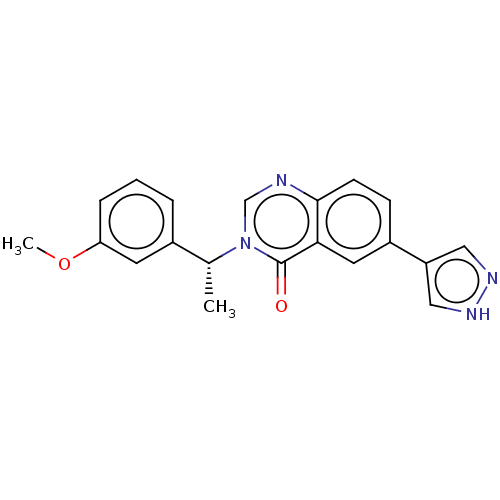
(CHEMBL4782910)Show SMILES COc1cccc(c1)[C@@H](C)n1cnc2ccc(cc2c1=O)-c1cn[nH]c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435756

(CHEMBL2392695)Show SMILES CN(C)c1nc(C)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H25F3N6O/c1-13-25-18(28(2)3)27-19(26-13)29-10-8-14(9-11-29)17(30)24-12-15-6-4-5-7-16(15)20(21,22)23/h4-7,14H,8-12H2,1-3H3,(H,24,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase overexpressed in HEK293F cells using EET as substrate assessed as formation of DHET incubated for 30 mi... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554272

(CHEMBL4751285)Show SMILES COc1cc(Cn2cnc3ccc(cc3c2=O)-c2cn[nH]c2)ccc1F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Beta-adrenergic receptor kinase 1
(Homo sapiens (Human)) | BDBM50554258
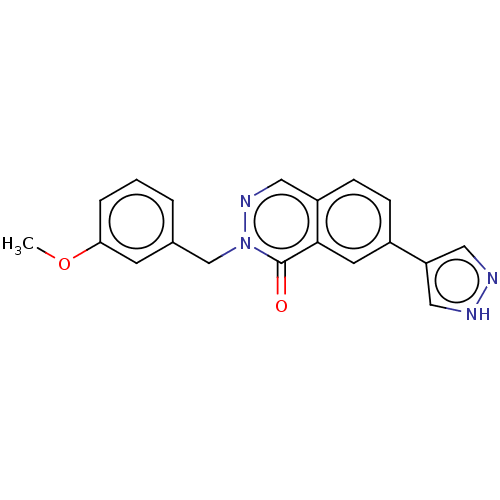
(CHEMBL4744858) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human GRK2 by transcreener assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435762

(CHEMBL2392712)Show SMILES CNc1nc(NC)nc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C19H24F3N7O/c1-23-16-26-17(24-2)28-18(27-16)29-9-7-12(8-10-29)15(30)25-11-13-5-3-4-6-14(13)19(20,21)22/h3-6,12H,7-11H2,1-2H3,(H,25,30)(H2,23,24,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435746
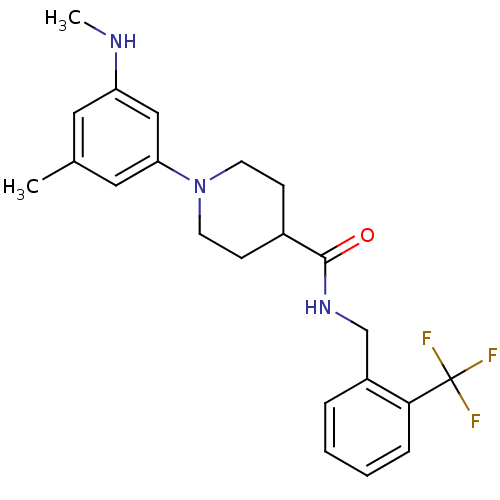
(CHEMBL2392705)Show SMILES CNc1cc(C)cc(c1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C22H26F3N3O/c1-15-11-18(26-2)13-19(12-15)28-9-7-16(8-10-28)21(29)27-14-17-5-3-4-6-20(17)22(23,24)25/h3-6,11-13,16,26H,7-10,14H2,1-2H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50569640

(CHEMBL4861270)Show SMILES CCCC[C@H](NC(=O)[C@H](C)c1c(C)[nH]c2ccc(O)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC1 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435752

(CHEMBL2392699)Show SMILES CNc1nc(C)cc(n1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C20H24F3N5O/c1-13-11-17(27-19(24-2)26-13)28-9-7-14(8-10-28)18(29)25-12-15-5-3-4-6-16(15)20(21,22)23/h3-6,11,14H,7-10,12H2,1-2H3,(H,25,29)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435763
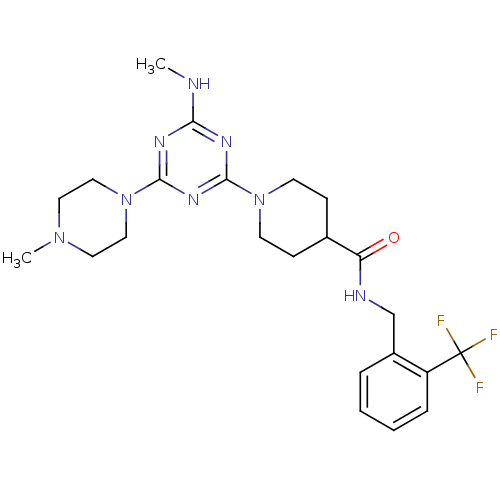
(CHEMBL2392711)Show SMILES CNc1nc(nc(n1)N1CCN(C)CC1)N1CCC(CC1)C(=O)NCc1ccccc1C(F)(F)F Show InChI InChI=1S/C23H31F3N8O/c1-27-20-29-21(31-22(30-20)34-13-11-32(2)12-14-34)33-9-7-16(8-10-33)19(35)28-15-17-5-3-4-6-18(17)23(24,25)26/h3-6,16H,7-15H2,1-2H3,(H,28,35)(H,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... |
Bioorg Med Chem Lett 23: 3584-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.019
BindingDB Entry DOI: 10.7270/Q2J967S5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50569642

(CHEMBL4879154)Show SMILES CCCC[C@H](NC(=O)[C@H](CN)c1c(C)[nH]c2ccc(OC)cc12)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-tagged HDAC2 expressed in human HEK293F cells using Fluor-de-lys substrate as substrate incubated for... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00074
BindingDB Entry DOI: 10.7270/Q2V41004 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data