

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Bos taurus (bovine)) | BDBM16499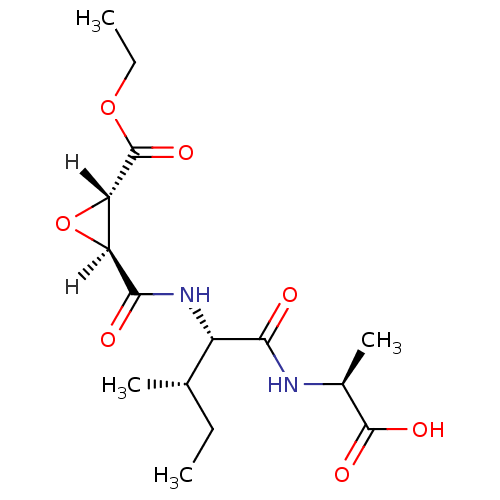 ((2S)-2-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16500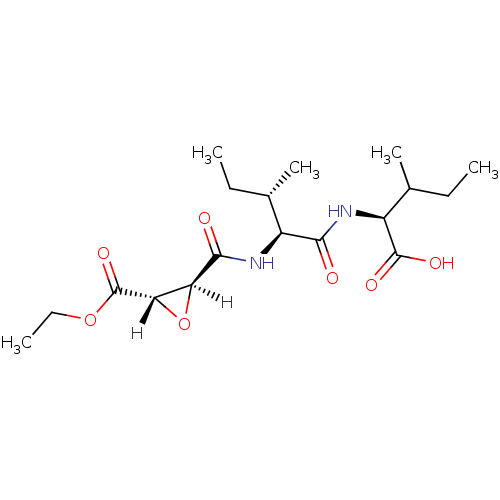 ((2S)-2-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16502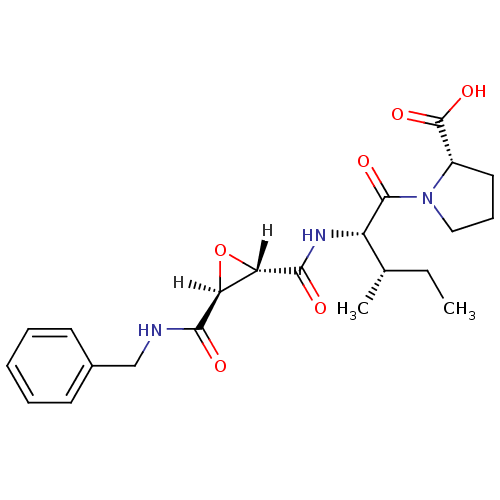 ((2S)-1-[(2S,3S)-2-{[(2S,3S)-3-(benzylcarbamoyl)oxi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16509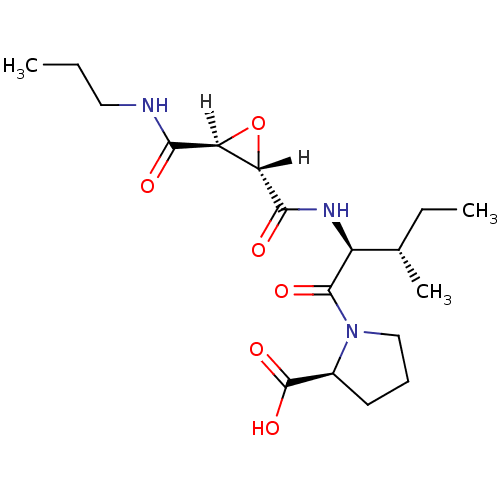 ((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16510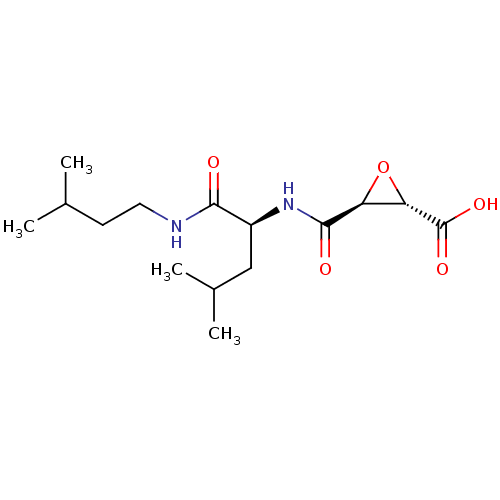 ((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16508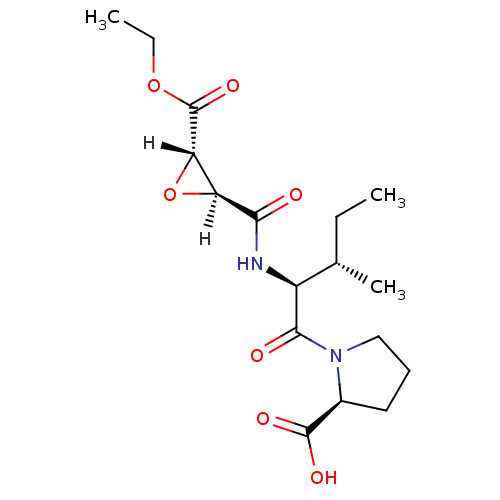 ((2S)-1-[(2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16501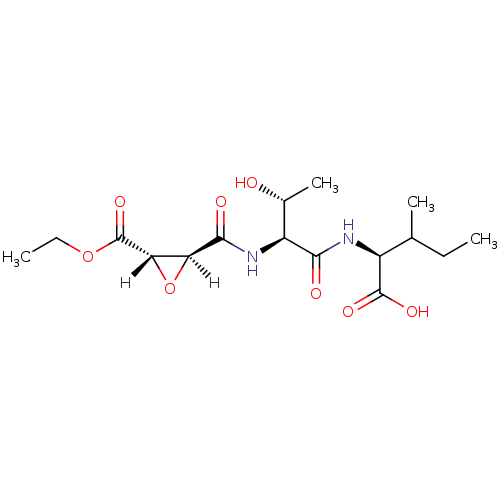 ((2S)-2-[(2S,3R)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16504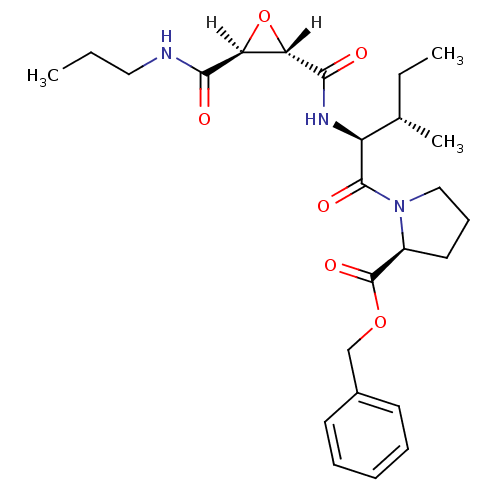 (CA inhibitor 7 | CA073 | PrNH-tES-Ile-Pro-OBzl | b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16506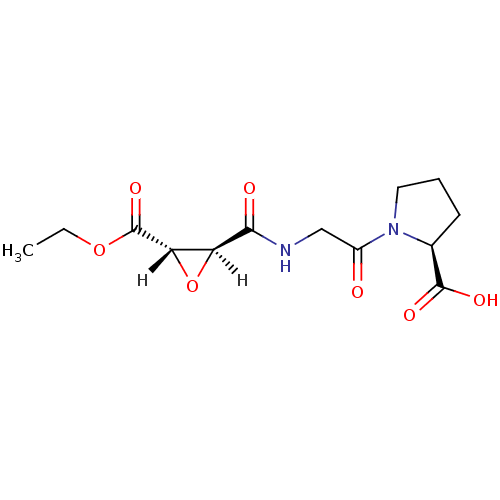 ((2S)-1-(2-{[(2S,3S)-3-(ethoxycarbonyl)oxiran-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16497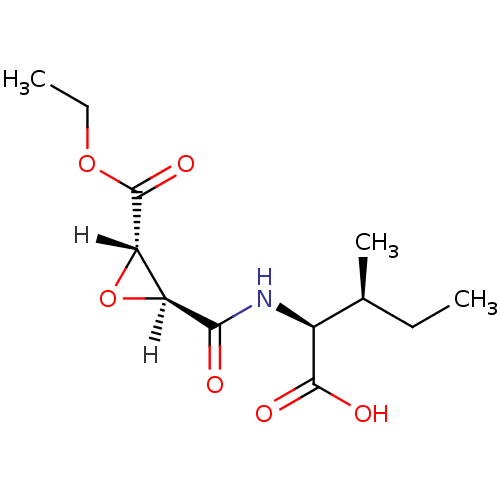 ((2S,3S)-2-{[(2S,3S)-3-(ethoxycarbonyl)oxiran-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16505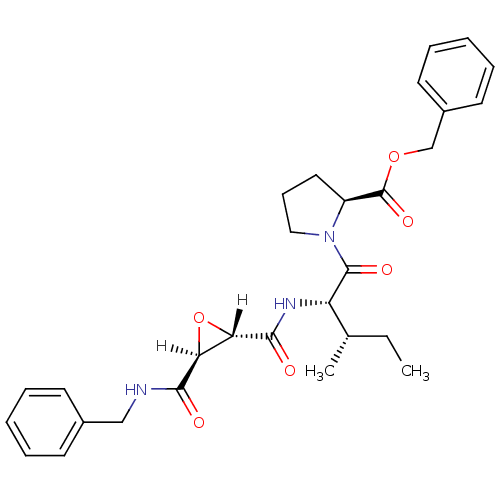 (BzlNH-tES-Ile-Pro-OBzl | CA inhibitor 8 | CA077 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16503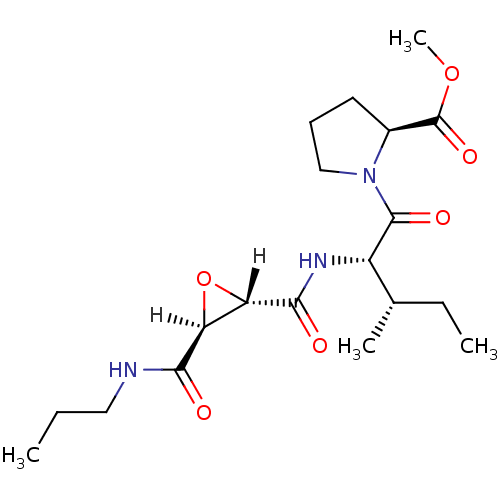 (CA inhibitor 6 | CA074Me | PrNH-tES-Ile-Pro-OMe | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.80E+4 | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Bos taurus (bovine)) | BDBM16511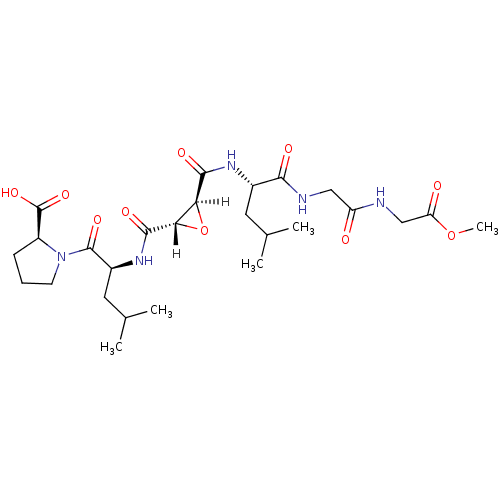 ((2S)-1-[(2S)-2-{[(2S,3S)-3-{[(1S)-1-({[(2-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | 40 |
Osaka University of Pharmaceutical Sciences | Assay Description Inhibitory activities (IC50, concentration of 50% inhibition) of compounds for bovine CB in vitro were determined by monitoring the cleavage of fluor... | J Mol Biol 362: 979-93 (2006) Article DOI: 10.1016/j.jmb.2006.07.070 BindingDB Entry DOI: 10.7270/Q2DF6PGN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50296967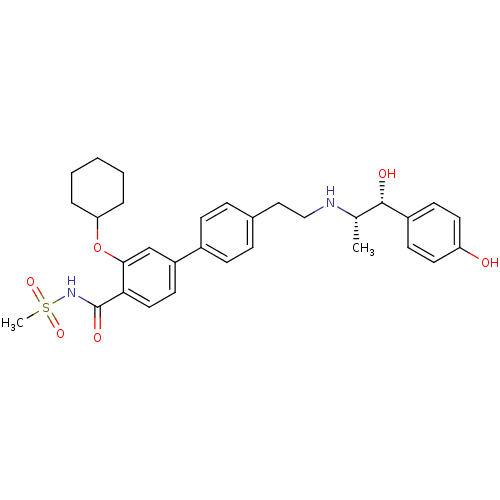 (3-(cyclohexyloxy)-4'-(2-((1R,2S)-1-hydroxy-1-(4-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta2 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta2 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50296957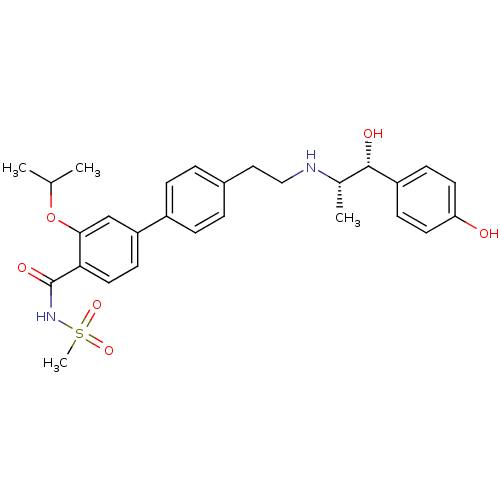 (4'-(2-((1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta2 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296958 (4'-((R)-2-((R)-2-hydroxy-2-phenylethylamino)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296959 (3-(cyclohexyloxy)-4'-((S)-3-hydroxy-2-((R)-2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296960 ((R)-4'-(2-(2-(6-acetamidopyridin-3-yl)-2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296961 ((R)-4'-(2-(2-(3-aminophenyl)-2-hydroxyethylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296962 ((R)-4'-(2-(2-(3-aminophenyl)-2-hydroxyethylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50249637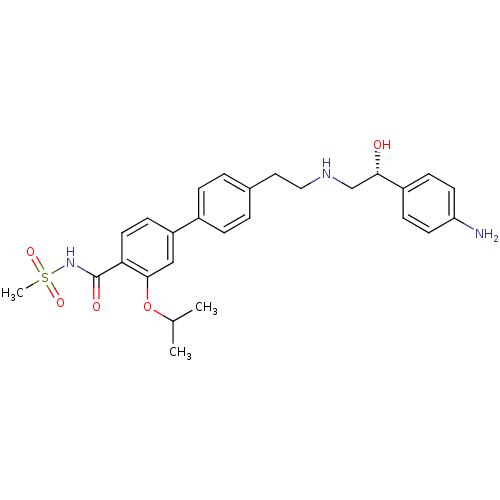 (4'-(2-{[(2R)-2-(4-Aminophenyl)-2-hydroxyethyl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50249638 (4'-(2-{[(2R)-2-(4-Aminophenyl)-2-hydroxyethyl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296965 ((R)-4'-(2-(2-hydroxy-2-(4-hydroxy-3-(methylsulfona...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296966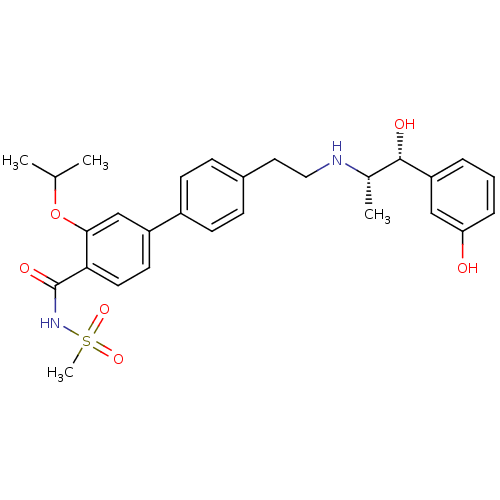 (4'-(2-((1R,2S)-1-hydroxy-1-(3-hydroxyphenyl)propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296957 (4'-(2-((1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296967 (3-(cyclohexyloxy)-4'-(2-((1R,2S)-1-hydroxy-1-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50296968 (3-(cyclohexyloxy)-4'-(2-((1R,2S)-1-hydroxy-1-(4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme imm... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296958 (4'-((R)-2-((R)-2-hydroxy-2-phenylethylamino)propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296959 (3-(cyclohexyloxy)-4'-((S)-3-hydroxy-2-((R)-2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296960 ((R)-4'-(2-(2-(6-acetamidopyridin-3-yl)-2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296961 ((R)-4'-(2-(2-(3-aminophenyl)-2-hydroxyethylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296962 ((R)-4'-(2-(2-(3-aminophenyl)-2-hydroxyethylamino)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50249637 (4'-(2-{[(2R)-2-(4-Aminophenyl)-2-hydroxyethyl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50249638 (4'-(2-{[(2R)-2-(4-Aminophenyl)-2-hydroxyethyl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296965 ((R)-4'-(2-(2-hydroxy-2-(4-hydroxy-3-(methylsulfona...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296966 (4'-(2-((1R,2S)-1-hydroxy-1-(3-hydroxyphenyl)propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296957 (4'-(2-((1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296967 (3-(cyclohexyloxy)-4'-(2-((1R,2S)-1-hydroxy-1-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50296968 (3-(cyclohexyloxy)-4'-(2-((1R,2S)-1-hydroxy-1-(4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at human recombinant adrenergic beta-1 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme im... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Canis familiaris) | BDBM50296957 (4'-(2-((1R,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Agonist activity at dog recombinant adrenergic beta3 receptor expressed in CHO cells assessed as stimulation of cAMP level after 1 hr by enzyme immun... | Bioorg Med Chem Lett 19: 4679-83 (2009) Article DOI: 10.1016/j.bmcl.2009.06.083 BindingDB Entry DOI: 10.7270/Q2S183DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM392659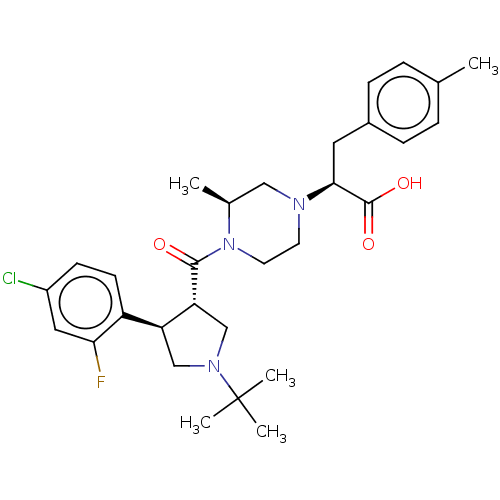 (US10301286, Example 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM392659 (US10301286, Example 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM392660 (US10301286, Example 76) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM392660 (US10301286, Example 76) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM392660 (US10301286, Example 76) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM392661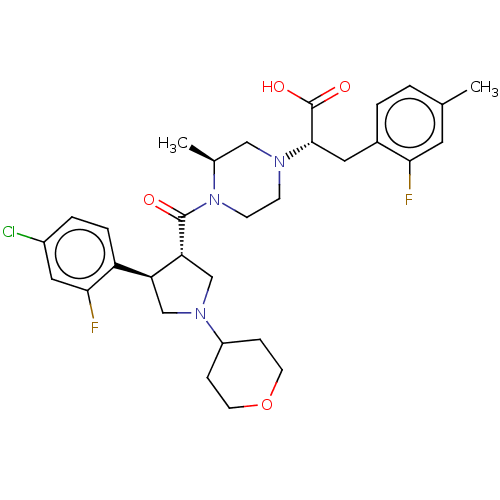 (US10301286, Example 87) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM392661 (US10301286, Example 87) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM392661 (US10301286, Example 87) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 920 | n/a | n/a | n/a | n/a |
GSK | Assay Description Experiment Method(1) Construction of Human MC Receptor-Expressing VectorA human MC4 receptor gene (GenBank Accession No.: NM_005912.2), a human MC1 r... | Bioorg Med Chem Lett 19: 1332-6 (2009) BindingDB Entry DOI: 10.7270/Q2833VCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 121 total ) | Next | Last >> |