

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202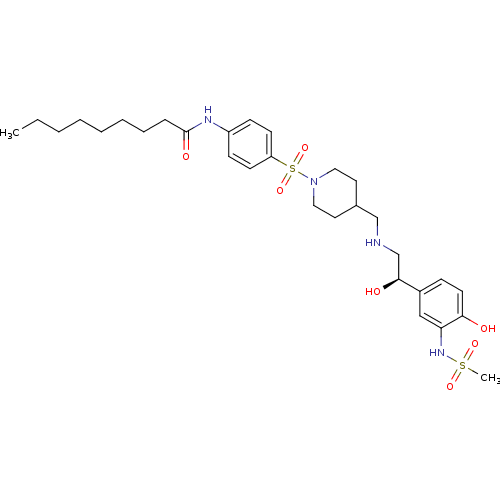 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378590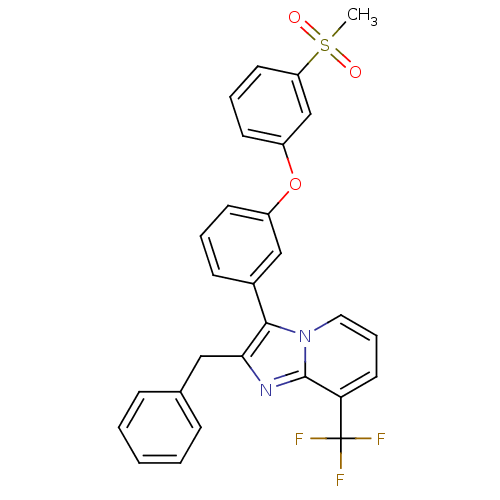 (CHEMBL611735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001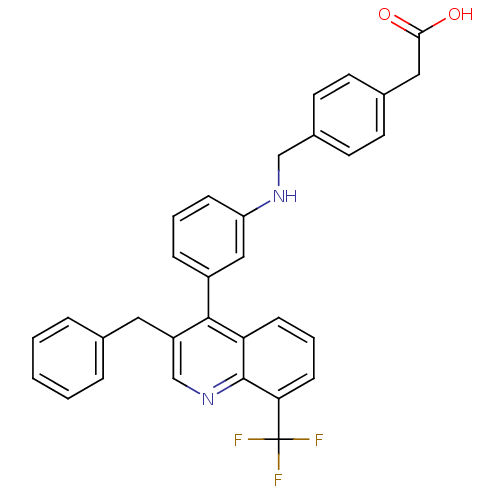 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000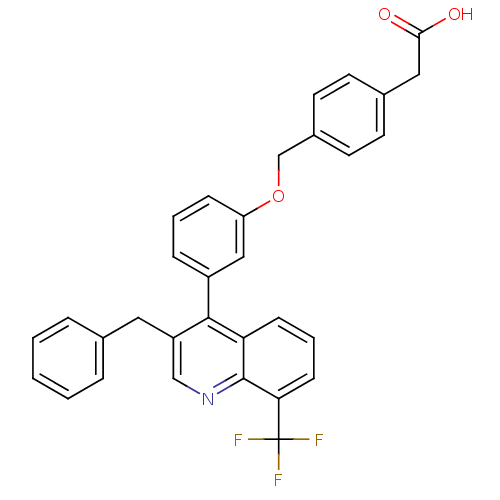 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378593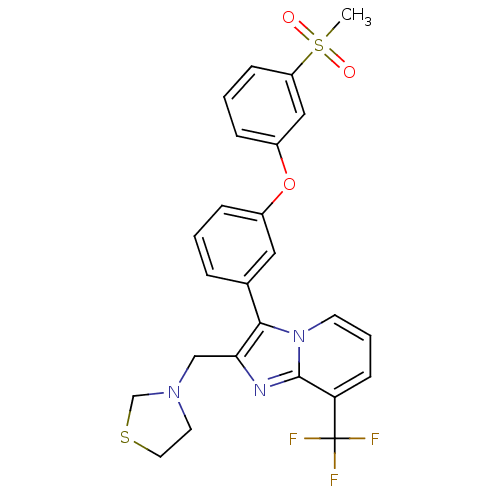 (CHEMBL612007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157485 (4-[1-isopropyl-7-(trifluoromethyl)-1H-indazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378587 (CHEMBL611466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-2 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50378590 (CHEMBL611735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378588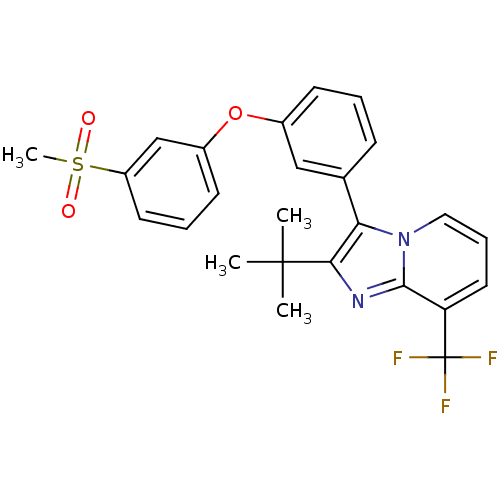 (CHEMBL611467) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306028 (2-methyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378589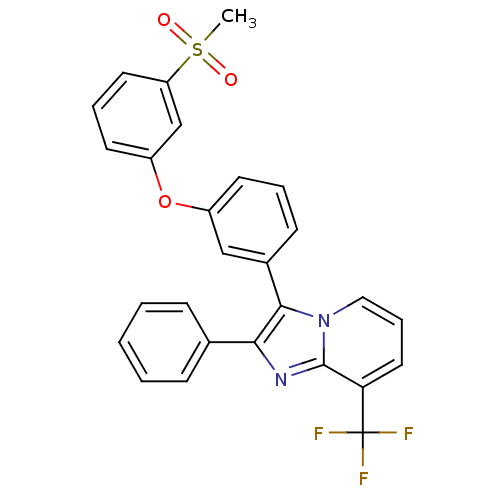 (CHEMBL611734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306020 (3-(3-(8-chloro-2-isopropylimidazo[1,2-a]pyridin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 160 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50378593 (CHEMBL612007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | 170 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26065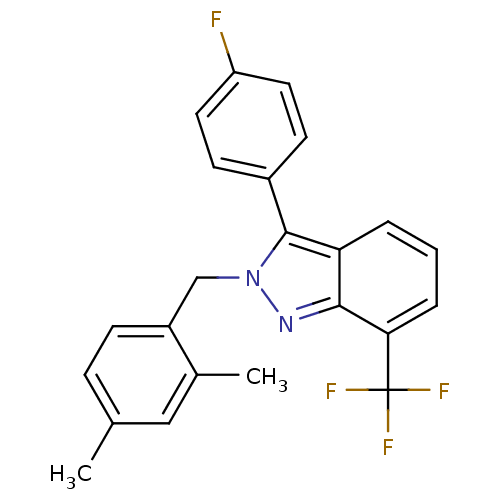 (2-[(2,4-dimethylphenyl)methyl]-3-(4-fluorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | 990 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306041 (8-chloro-2-isopropyl-3-(3-(3-(methylsulfonyl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | 240 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | 310 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 13 | n/a | 140 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378586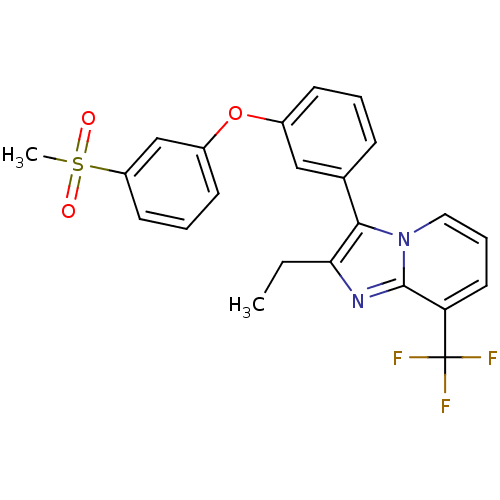 (CHEMBL611465) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157496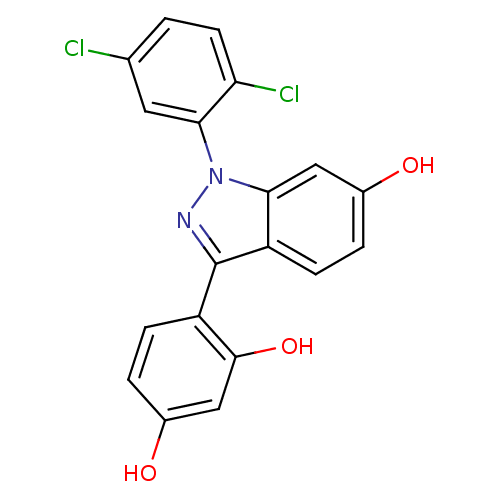 (4-[1-(2,5-dichlorophenyl)-6-hydroxy-1H-indazol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157488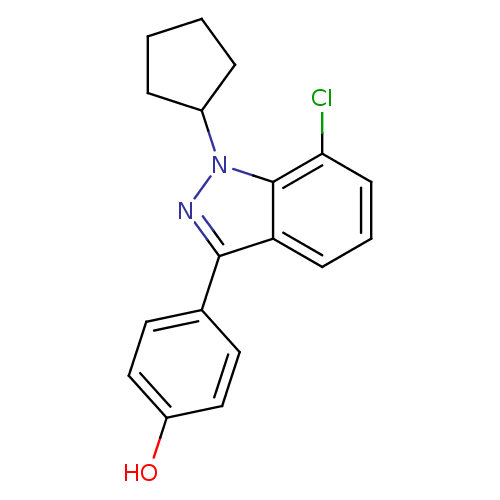 (4-(7-chloro-1-cyclopentyl-1H-indazol-3-yl)phenol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157483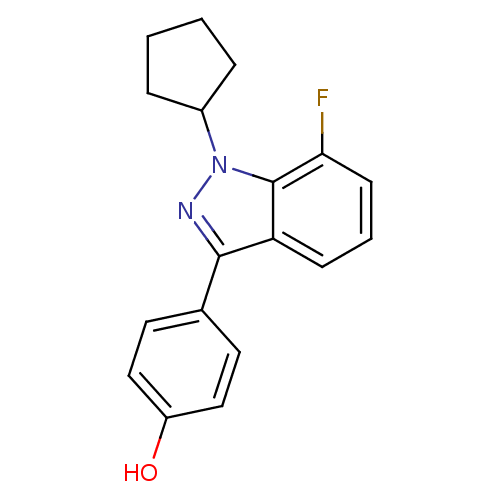 (4-(1-cyclopentyl-7-fluoro-1H-indazol-3-yl)phenol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157501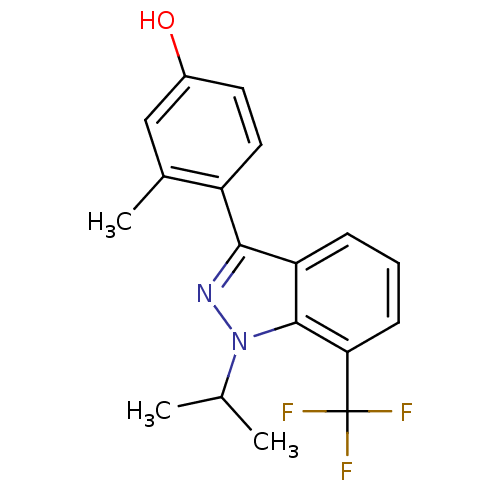 (4-[1-isopropyl-7-(trifluoromethyl)-1H-indazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26066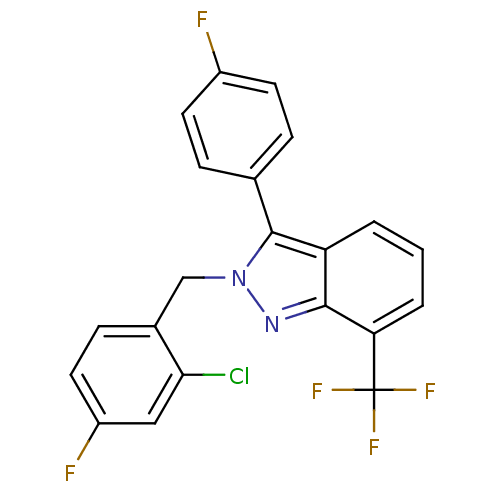 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 3.67E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306046 (3-(3-(8-chloro-2-isopropylimidazo[1,2-a]pyridin-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157498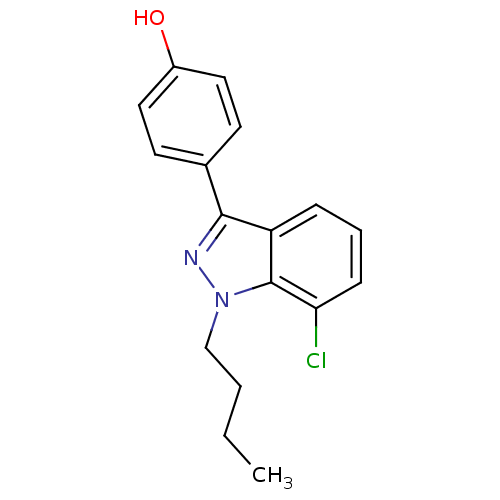 (4-(1-butyl-7-chloro-1H-indazol-3-yl)phenol | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-1 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26072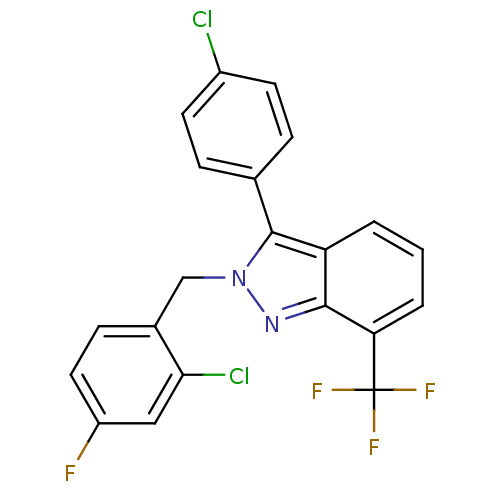 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | 1.12E+4 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306030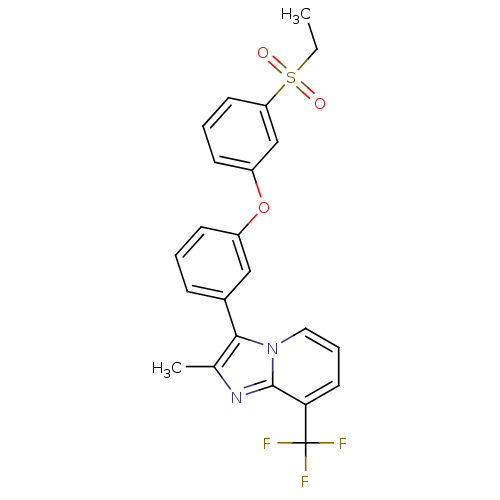 (3-(3-(3-(ethylsulfonyl)phenoxy)phenyl)-2-methyl-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378594 (CHEMBL608531) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157491 (4-[1-(2,5-difluorophenyl)-6-hydroxy-1H-indazol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26068 (2-[(2-chlorophenyl)methyl]-3-(4-fluorophenyl)-7-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | 2.89E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306031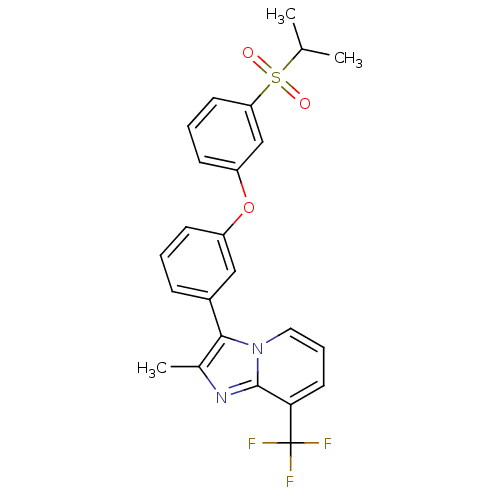 (3-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26067 (3-(4-fluorophenyl)-2-[(2-fluorophenyl)methyl]-7-(t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | 4.15E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157504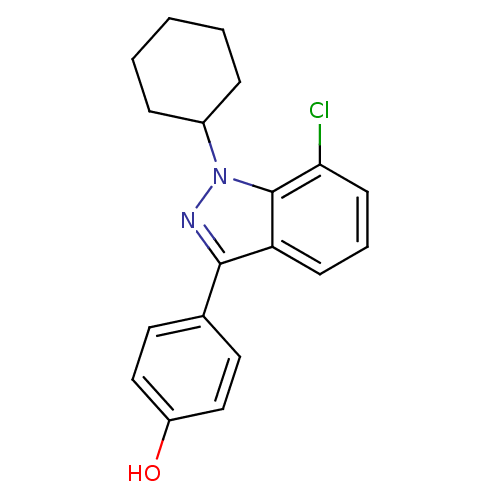 (4-(7-chloro-1-cyclohexyl-1H-indazol-3-yl)phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26071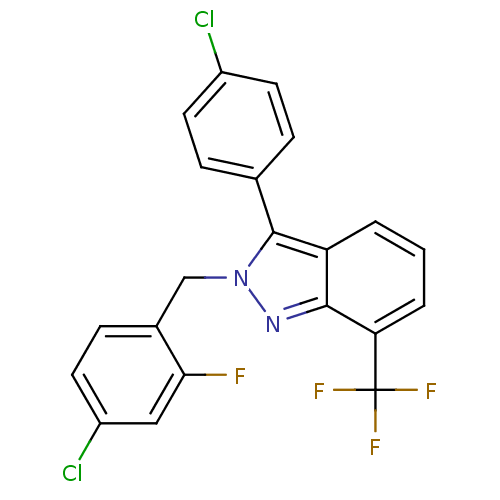 (2-[(4-chloro-2-fluorophenyl)methyl]-3-(4-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | 9.80E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26074 (2-benzyl-3-aryl-7-trifluoromethylindazole, 20 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | 7.44E+3 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157489 (4-[1-(3-fluorophenyl)-6-hydroxy-1H-indazol-3-yl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157503 (4-(7-methyl-1-propyl-1H-indazol-3-yl)phenol | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM26073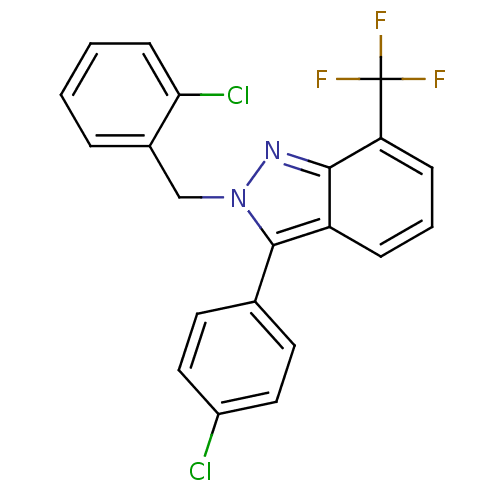 (2-benzyl-3-aryl-7-trifluoromethylindazole, 19 | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | 1.02E+4 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 258 total ) | Next | Last >> |