

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441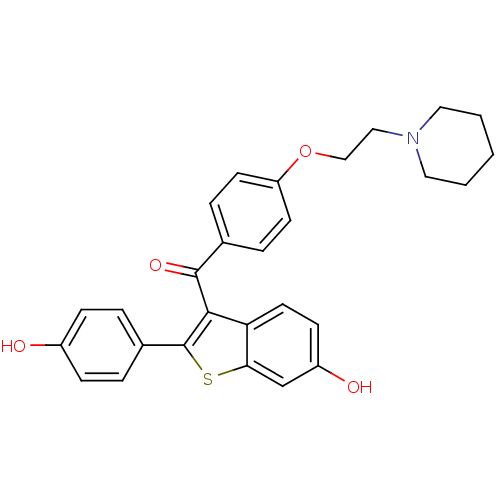 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using vanillin as substrate by HPLC-MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using phthalazine as substrate preincubated for 30 mins followed by substr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldehyde oxidase (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Uncompetitive type inhibition of human AOX assessed as inhibition constant using nicotine-1(S)-iminium ion as substrate incubated for 2 mins by HPLC-... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01125 BindingDB Entry DOI: 10.7270/Q2C82F55 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50009859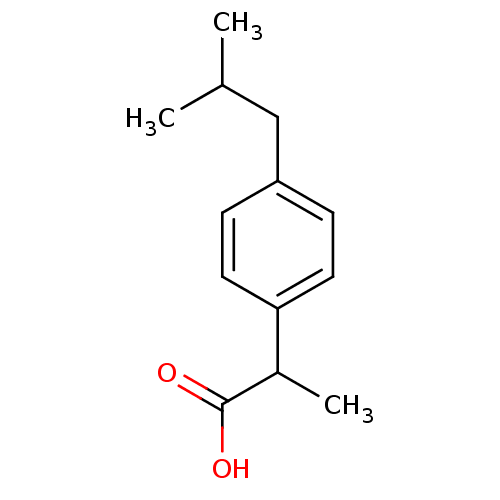 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Inhibition of sheep placental cotyledons COX1 | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Inhibition of sheep placental cotyledons COX2 | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM50445691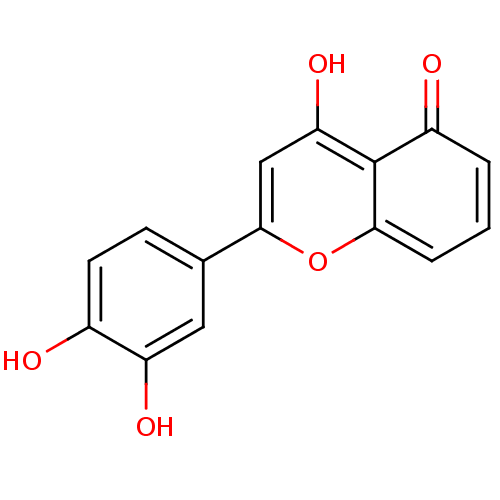 (CHEMBL243677) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM7461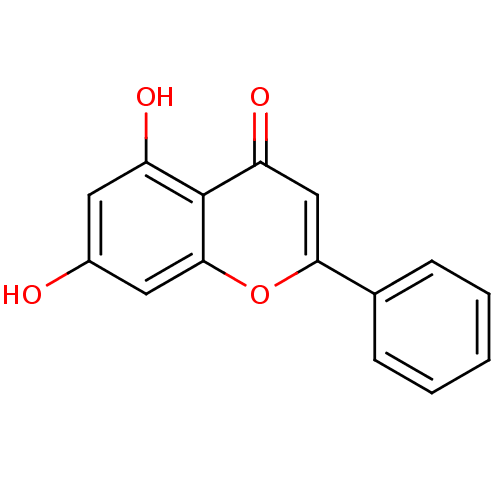 (5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM50157555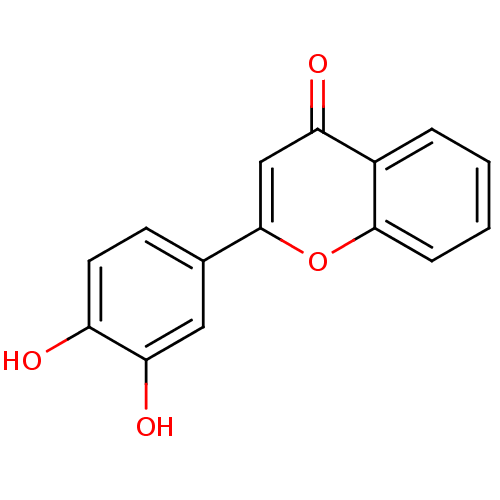 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM50445690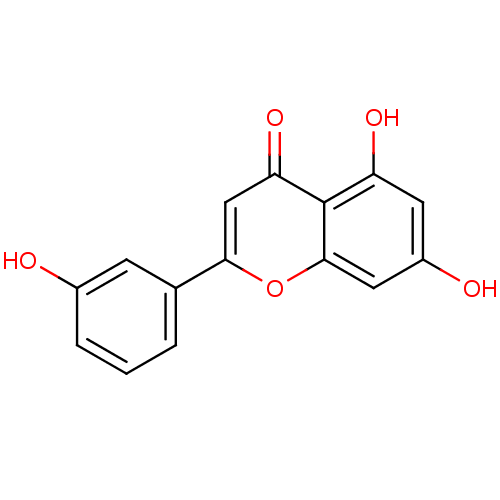 (CHEMBL457821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM50312650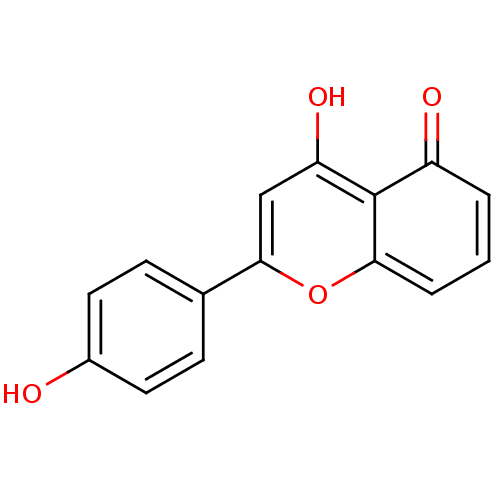 (5,4'-dihydroxyflavone | 5-hydroxy-2-(4-hydroxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM7459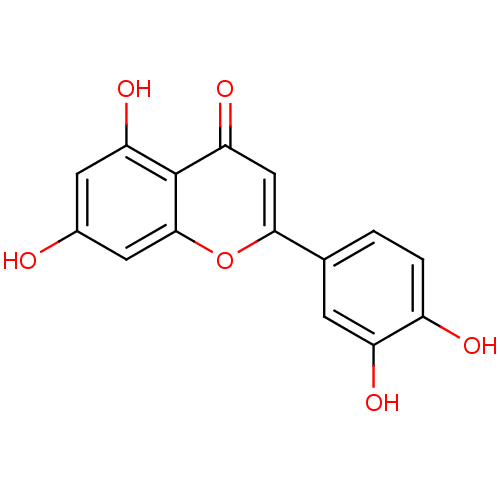 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Uncompetitive inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Lineweaver-Burk ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Seed linoleate 13S-lipoxygenase-1 (Glycine max (soybean)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Mixed noncompetitive type inhibition of soybean LOX-1 using linoleic acid as substrate preincubated for 5 mins followed by substrate addition by Line... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Ovis aries (Sheep)) | BDBM13066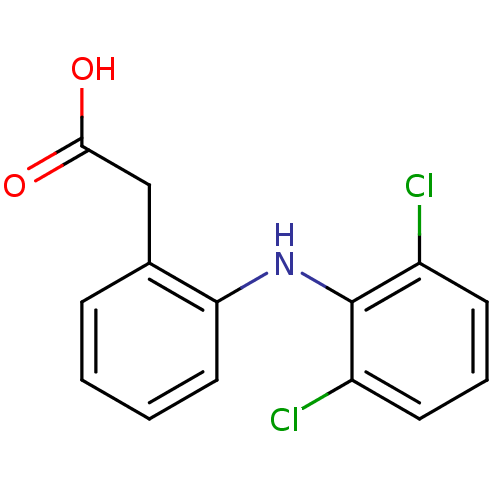 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Inhibition of sheep placental cotyledons COX2 | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM85511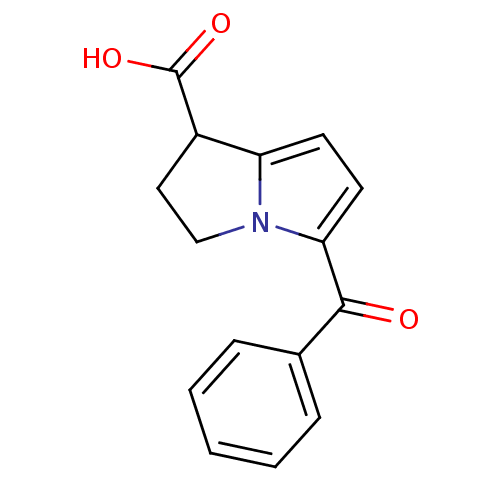 (CAS_74103-07-4 | KETOROLAC | Ketorolac tris salt |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Inhibition of human COX2 measured after pre-incubation of enzyme with compound | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50009859 ((+-)-2-(p-isobutylphenyl)propionic acid | (+-)-alp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in baculovirus system | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50157555 (2-(3,4-dihydroxyphenyl)-4H-chromen-4-one | 3',4'-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50077325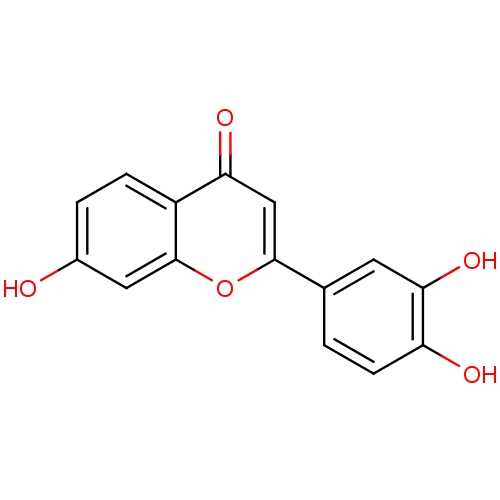 (2-(3,4-Dihydroxy-phenyl)-7-hydroxy-chromen-4-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50445691 (CHEMBL243677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto Curated by ChEMBL | Assay Description Inhibition of 5-LOX-mediated LTB4 production in human neutrophils using arachidonic acid as substrate preincubated for 10 mins followed by substrate ... | Eur J Med Chem 72: 137-45 (2014) Article DOI: 10.1016/j.ejmech.2013.11.030 BindingDB Entry DOI: 10.7270/Q27H1M2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM85511 (CAS_74103-07-4 | KETOROLAC | Ketorolac tris salt |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | 6.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Faculdade de Ci�ncias e Tecnologia-UNL Curated by ChEMBL | Assay Description Instantaneous inhibition of human COX2 measured immediately after incubation of enzyme with compound | J Med Chem 54: 8555-62 (2011) Article DOI: 10.1021/jm201090k BindingDB Entry DOI: 10.7270/Q2W95B6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||