 Found 222 hits with Last Name = 'bassani' and Initial = 'f'
Found 222 hits with Last Name = 'bassani' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595424
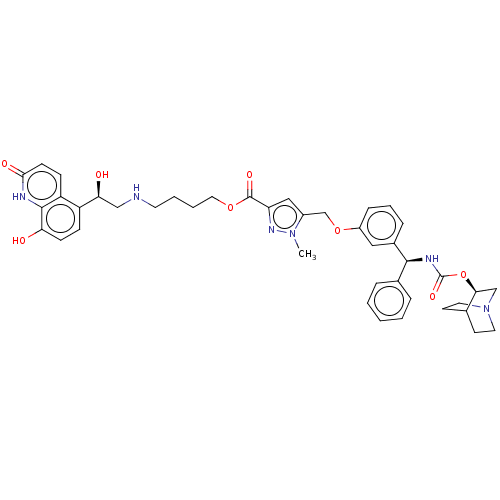
(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595404

(CHEMBL5184455)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8.01,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.55,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595423
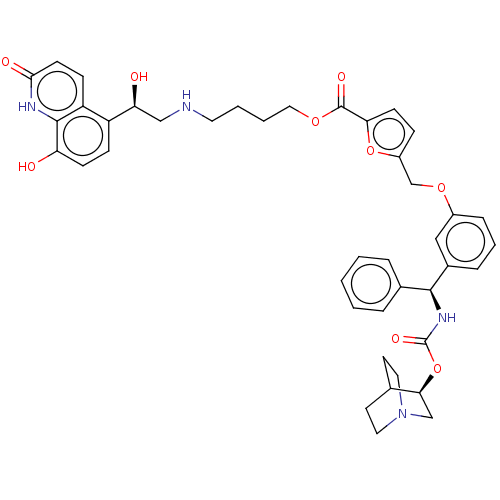
(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595426
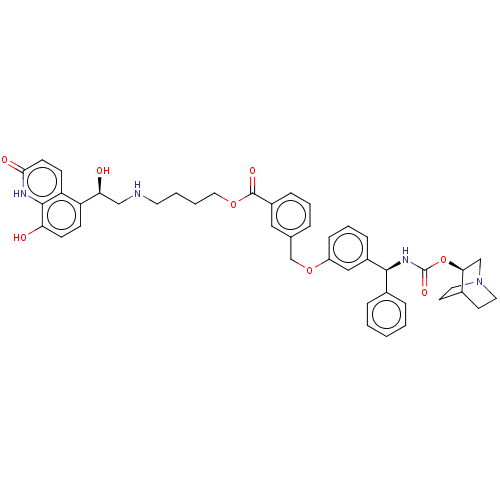
(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595427
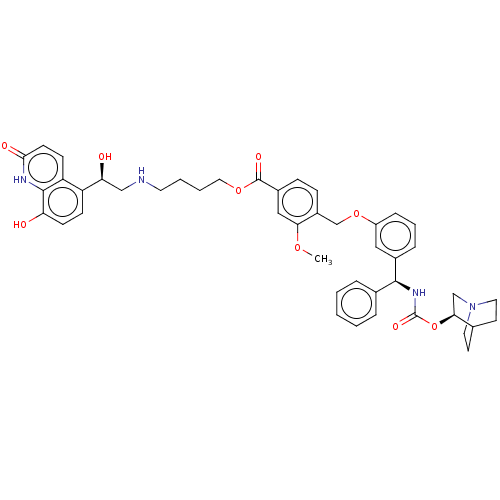
(CHEMBL5200887)Show SMILES COc1cc(ccc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:44.49,wD:16.18,21.22,(3.34,-1.15,;3.34,.39,;2,1.16,;.67,.39,;-.67,1.15,;-.67,2.69,;.67,3.47,;2,2.7,;3.33,3.47,;4.67,2.7,;6,3.47,;6,5.01,;7.33,5.78,;8.67,5.02,;8.67,3.48,;7.33,2.7,;10,2.71,;10,1.17,;8.67,.4,;7.34,1.16,;8.67,-1.14,;7.34,-1.92,;6.01,-1.15,;4.67,-1.92,;6.1,-1.92,;5.92,-3.46,;7.34,-3.46,;6.01,-4.23,;4.68,-3.46,;11.33,3.48,;11.33,5.02,;12.67,5.79,;14,5.02,;14,3.48,;12.67,2.71,;-2,.38,;-2,-1.16,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-7.33,.38,;-8.67,1.14,;-10,.37,;-11.33,1.14,;-12.67,.37,;-14,1.14,;-12.67,-1.17,;-14,-1.94,;-14,-3.48,;-12.66,-4.25,;-12.66,-5.79,;-11.33,-3.48,;-9.99,-4.25,;-8.66,-3.48,;-7.33,-4.24,;-8.66,-1.94,;-10,-1.17,;-11.33,-1.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595403
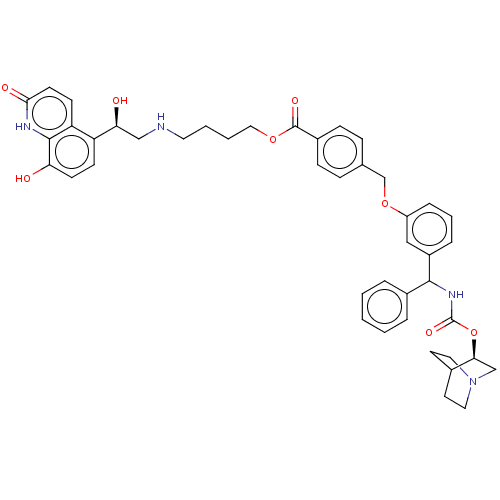
(CHEMBL5208201)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)C(NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.66,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-3.99,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;14.67,3.46,;14.67,5,;13.34,5.77,;14.05,4.54,;12.63,3.93,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.33,-6.54,;-13.33,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595405
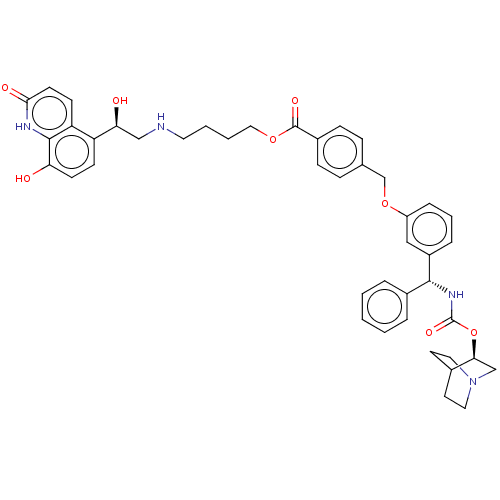
(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595425
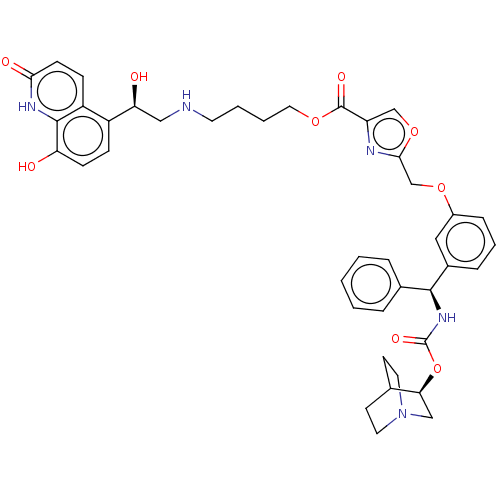
(CHEMBL5208957)Show SMILES O[C@@H](CNCCCCOC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595424

(CHEMBL5172865)Show SMILES Cn1nc(cc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:42.47,wD:14.16,19.20,(.73,4.28,;.18,2.84,;-1.31,2.44,;-1.39,.91,;.04,.35,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;-2.69,.07,;-2.61,-1.47,;-4.06,.77,;-5.35,-.07,;-6.72,.63,;-8.01,-.2,;-9.38,.5,;-10.68,-.34,;-12.05,.36,;-13.34,-.48,;-14.71,.22,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595423

(CHEMBL5190387)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)o1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595426

(CHEMBL5193853)Show SMILES O[C@@H](CNCCCCOC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:24.25,29.29,(-13.81,.82,;-12.39,.23,;-11.16,1.16,;-9.74,.56,;-8.52,1.49,;-7.1,.89,;-5.87,1.82,;-4.45,1.23,;-3.22,2.16,;-1.8,1.56,;-1.61,.03,;-.58,2.49,;-.77,4.02,;.46,4.95,;1.88,4.35,;2.07,2.82,;3.49,2.22,;3.68,.7,;5.1,.1,;5.29,-1.43,;6.71,-2.03,;7.94,-1.1,;7.75,.43,;6.33,1.03,;8.98,1.36,;10.4,.76,;10.59,-.76,;9.36,-1.69,;12.01,-1.36,;12.2,-2.89,;10.97,-3.82,;11.16,-5.35,;11.72,-4.04,;13.07,-4.8,;13.62,-3.49,;13.81,-5.02,;12.58,-5.95,;8.78,2.89,;7.36,3.49,;7.17,5.02,;8.4,5.95,;9.82,5.35,;10.01,3.82,;.84,1.89,;-12.39,-1.31,;-13.73,-2.09,;-13.72,-3.63,;-12.39,-4.39,;-12.39,-5.93,;-11.06,-3.62,;-9.72,-4.39,;-8.4,-3.62,;-7.06,-4.39,;-8.4,-2.09,;-9.73,-1.32,;-11.06,-2.08,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569301
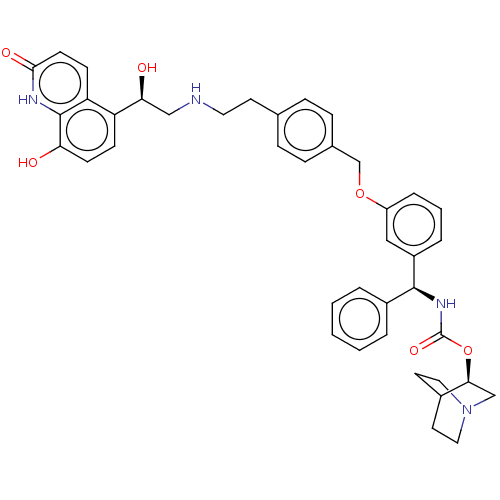
(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595404

(CHEMBL5184455)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8.01,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.55,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595425

(CHEMBL5208957)Show SMILES O[C@@H](CNCCCCOC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:23.24,28.28,(-14.71,.22,;-13.34,-.48,;-12.05,.36,;-10.68,-.34,;-9.38,.5,;-8.01,-.2,;-6.72,.63,;-5.35,-.07,;-4.06,.77,;-2.69,.07,;-2.61,-1.47,;-1.39,.91,;-1.31,2.44,;.18,2.84,;1.01,1.55,;2.55,1.47,;3.25,.1,;4.79,.01,;5.49,-1.36,;7.02,-1.44,;7.86,-.15,;7.16,1.22,;5.63,1.3,;8,2.51,;9.54,2.43,;10.24,1.06,;9.4,-.23,;11.78,.98,;12.48,-.4,;11.64,-1.69,;12.33,-3.06,;12.41,-1.64,;13.94,-1.9,;14.01,-.48,;14.71,-1.85,;13.87,-3.14,;7.31,3.89,;5.77,3.97,;5.07,5.34,;5.91,6.63,;7.45,6.55,;8.15,5.18,;.04,.35,;-13.26,-2.02,;-14.55,-2.86,;-14.47,-4.39,;-13.1,-5.09,;-13.02,-6.63,;-11.81,-4.26,;-10.44,-4.96,;-9.15,-4.12,;-7.77,-4.82,;-9.23,-2.58,;-10.6,-1.88,;-11.89,-2.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595427

(CHEMBL5200887)Show SMILES COc1cc(ccc1COc1cccc(c1)[C@@H](NC(=O)O[C@H]1CN2CCC1CC2)c1ccccc1)C(=O)OCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:44.49,wD:16.18,21.22,(3.34,-1.15,;3.34,.39,;2,1.16,;.67,.39,;-.67,1.15,;-.67,2.69,;.67,3.47,;2,2.7,;3.33,3.47,;4.67,2.7,;6,3.47,;6,5.01,;7.33,5.78,;8.67,5.02,;8.67,3.48,;7.33,2.7,;10,2.71,;10,1.17,;8.67,.4,;7.34,1.16,;8.67,-1.14,;7.34,-1.92,;6.01,-1.15,;4.67,-1.92,;6.1,-1.92,;5.92,-3.46,;7.34,-3.46,;6.01,-4.23,;4.68,-3.46,;11.33,3.48,;11.33,5.02,;12.67,5.79,;14,5.02,;14,3.48,;12.67,2.71,;-2,.38,;-2,-1.16,;-3.33,1.15,;-4.67,.38,;-6,1.15,;-7.33,.38,;-8.67,1.14,;-10,.37,;-11.33,1.14,;-12.67,.37,;-14,1.14,;-12.67,-1.17,;-14,-1.94,;-14,-3.48,;-12.66,-4.25,;-12.66,-5.79,;-11.33,-3.48,;-9.99,-4.25,;-8.66,-3.48,;-7.33,-4.24,;-8.66,-1.94,;-10,-1.17,;-11.33,-1.94,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569301

(CHEMBL4854091)Show SMILES O[C@@H](CNCCc1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,18.19,wD:23.23,(1.74,-37.97,;3.08,-38.73,;4.41,-37.96,;5.75,-38.72,;7.08,-37.95,;8.41,-38.72,;9.75,-37.94,;11.08,-38.71,;12.41,-37.94,;12.4,-36.4,;13.74,-35.62,;15.07,-36.39,;16.4,-35.61,;16.39,-34.08,;17.71,-33.3,;19.06,-34.06,;19.06,-35.6,;17.73,-36.38,;20.4,-36.37,;21.73,-35.59,;23.07,-36.36,;23.07,-37.9,;24.4,-35.58,;25.74,-36.35,;25.73,-37.9,;27.06,-38.66,;28.39,-37.89,;28.39,-36.35,;27.05,-35.58,;27.81,-36.91,;26.28,-37.32,;20.41,-37.91,;19.08,-38.68,;19.08,-40.22,;20.42,-40.98,;21.76,-40.2,;21.74,-38.66,;11.06,-35.63,;9.73,-36.41,;3.08,-40.27,;1.76,-41.04,;1.75,-42.59,;3.09,-43.36,;3.09,-44.9,;4.42,-42.58,;5.75,-43.35,;7.09,-42.59,;8.42,-43.36,;7.09,-41.04,;5.75,-40.26,;4.42,-41.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595403

(CHEMBL5208201)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)C(NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.66,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-3.99,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;14.67,3.46,;14.67,5,;13.34,5.77,;14.05,4.54,;12.63,3.93,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.33,-6.54,;-13.33,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208047
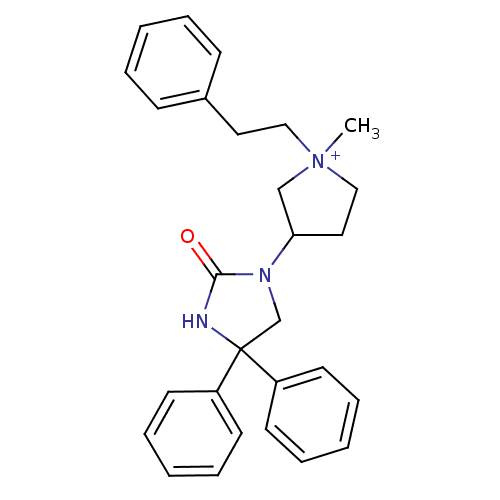
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O/c1-31(19-17-23-11-5-2-6-12-23)20-18-26(21-31)30-22-28(29-27(30)32,24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-16,26H,17-22H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208057
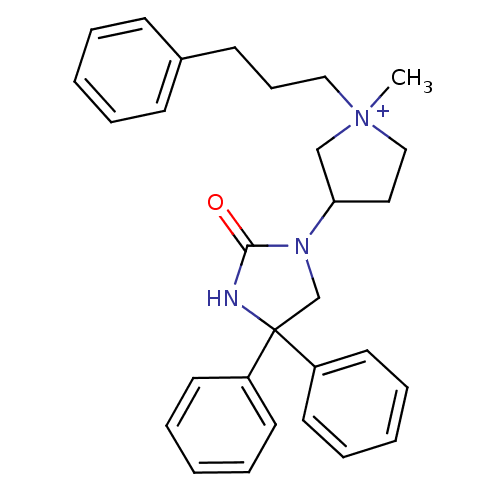
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O/c1-32(20-11-14-24-12-5-2-6-13-24)21-19-27(22-32)31-23-29(30-28(31)33,25-15-7-3-8-16-25)26-17-9-4-10-18-26/h2-10,12-13,15-18,27H,11,14,19-23H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208057

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O/c1-32(20-11-14-24-12-5-2-6-13-24)21-19-27(22-32)31-23-29(30-28(31)33,25-15-7-3-8-16-25)26-17-9-4-10-18-26/h2-10,12-13,15-18,27H,11,14,19-23H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208043
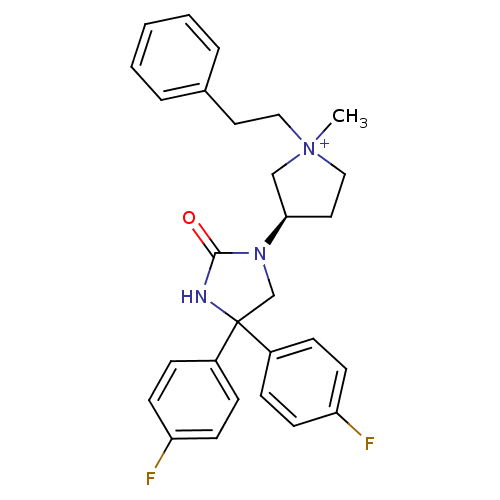
(1-methyl-3-(R)-[4,4-Bis-(4-fluoro-phenyl)-2-oxo-im...)Show SMILES C[N+]1(CCc2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c1-33(17-15-21-5-3-2-4-6-21)18-16-26(19-33)32-20-28(31-27(32)34,22-7-11-24(29)12-8-22)23-9-13-25(30)14-10-23/h2-14,26H,15-20H2,1H3/p+1/t26-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595405

(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595406
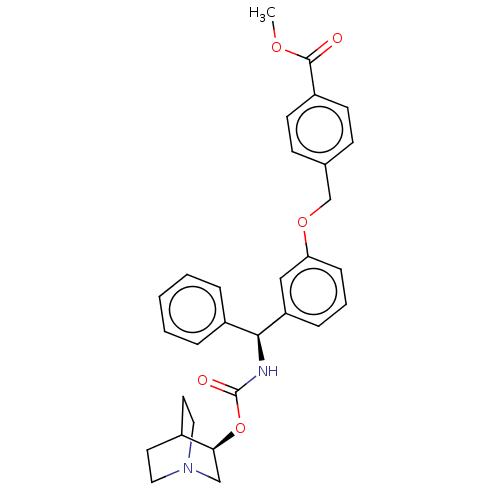
(CHEMBL5200448)Show SMILES COC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:16.17,wD:21.21,(-11.34,-1.54,;-10,-.77,;-8.67,-1.54,;-8.67,-3.08,;-7.34,-.77,;-6,-1.54,;-4.67,-.77,;-4.67,.77,;-3.33,1.54,;-2,.77,;-.67,1.54,;-.67,3.08,;.67,3.85,;2,3.08,;2,1.54,;.67,.77,;3.33,.77,;4.67,1.54,;6,.77,;6,-.77,;7.34,1.54,;8.67,.77,;8.67,-.77,;10,-1.54,;11.34,-.77,;11.34,.77,;10,1.54,;10.71,.31,;9.29,-.31,;3.33,-.77,;4.67,-1.54,;4.67,-3.08,;3.33,-3.85,;2,-3.08,;2,-1.54,;-6,1.54,;-7.34,.77,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595422
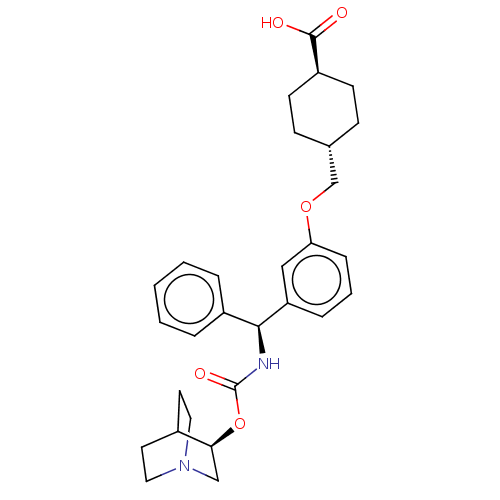
(CHEMBL5177499)Show SMILES OC(=O)[C@H]1CC[C@H](COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)CC1 |r,wU:6.6,15.16,wD:20.20,3.2,(-9.34,-3.08,;-9.34,-1.54,;-10.67,-.77,;-8,-.77,;-6.67,-1.54,;-5.33,-.77,;-5.33,.77,;-4,1.54,;-2.67,.77,;-1.33,1.54,;-1.33,3.08,;,3.85,;1.33,3.08,;1.33,1.54,;,.77,;2.67,.77,;4,1.54,;5.33,.77,;5.33,-.77,;6.67,1.54,;8,.77,;8,-.77,;9.34,-1.54,;10.67,-.77,;10.67,.77,;9.34,1.54,;10.05,.31,;8.62,-.31,;2.67,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;4,-3.08,;4,-1.54,;-6.67,1.54,;-8,.77,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208044
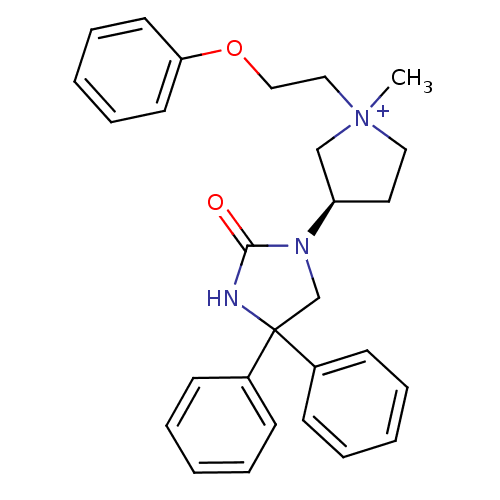
(1-methyl-3-(R)-(2-oxo-4,4-diphenyl-imidazolidin-1-...)Show SMILES C[N+]1(CCOc2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1/t25-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595408
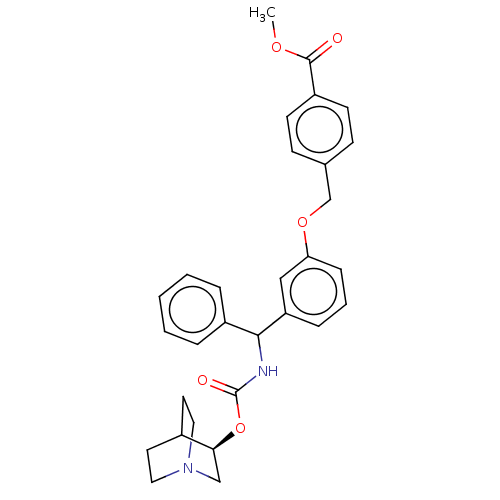
(CHEMBL5195203)Show SMILES COC(=O)c1ccc(COc2cccc(c2)C(NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wD:21.21,(-11.34,-1.54,;-10,-.77,;-8.67,-1.54,;-8.67,-3.08,;-7.34,-.77,;-6,-1.54,;-4.67,-.77,;-4.67,.77,;-3.33,1.54,;-2,.77,;-.67,1.54,;-.67,3.08,;.67,3.85,;2,3.08,;2,1.54,;.67,.77,;3.33,.77,;4.67,1.54,;6,.77,;6,-.77,;7.34,1.54,;8.67,.77,;8.67,-.77,;10,-1.54,;11.34,-.77,;11.34,.77,;10,1.54,;10.71,.31,;9.29,-.31,;3.33,-.77,;4.67,-1.54,;4.67,-3.08,;3.33,-3.85,;2,-3.08,;2,-1.54,;-6,1.54,;-7.34,.77,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208035
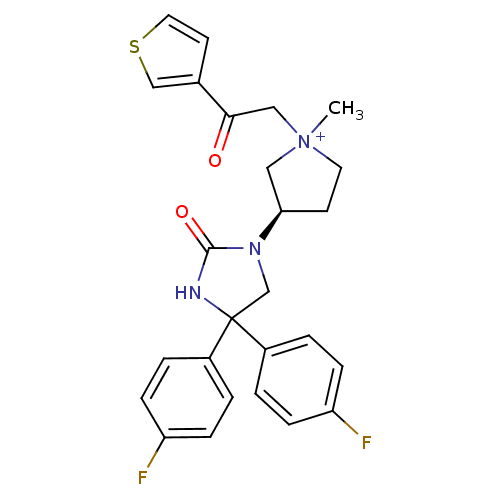
(1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...)Show SMILES C[N+]1(CC(=O)c2ccsc2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C26H25F2N3O2S/c1-31(15-24(32)18-11-13-34-16-18)12-10-23(14-31)30-17-26(29-25(30)33,19-2-6-21(27)7-3-19)20-4-8-22(28)9-5-20/h2-9,11,13,16,23H,10,12,14-15,17H2,1H3/p+1/t23-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208038
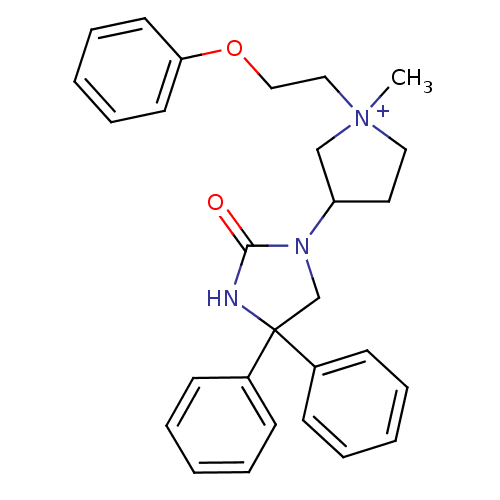
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208056

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O2/c1-32(19-11-21-34-27-16-9-4-10-17-27)20-18-26(22-32)31-23-29(30-28(31)33,24-12-5-2-6-13-24)25-14-7-3-8-15-25/h2-10,12-17,26H,11,18-23H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208051
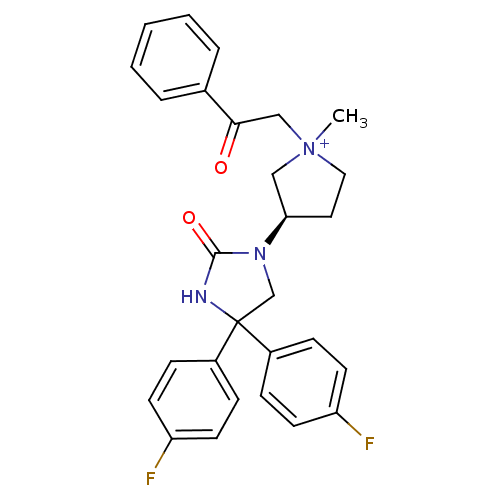
(1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...)Show SMILES C[N+]1(CC(=O)c2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H27F2N3O2/c1-33(18-26(34)20-5-3-2-4-6-20)16-15-25(17-33)32-19-28(31-27(32)35,21-7-11-23(29)12-8-21)22-9-13-24(30)14-10-22/h2-14,25H,15-19H2,1H3/p+1/t25-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208058
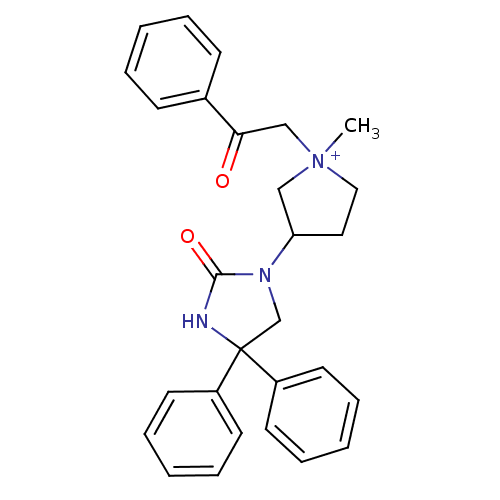
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CC(=O)c2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c1-31(20-26(32)22-11-5-2-6-12-22)18-17-25(19-31)30-21-28(29-27(30)33,23-13-7-3-8-14-23)24-15-9-4-10-16-24/h2-16,25H,17-21H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208038

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208044

(1-methyl-3-(R)-(2-oxo-4,4-diphenyl-imidazolidin-1-...)Show SMILES C[N+]1(CCOc2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1/t25-,31?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208041
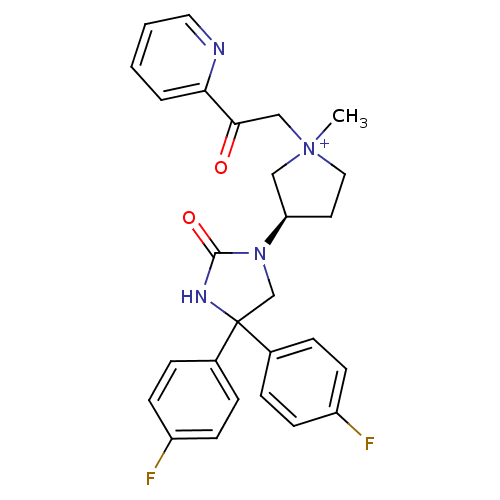
(1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...)Show SMILES C[N+]1(CC(=O)c2ccccn2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C27H26F2N4O2/c1-33(17-25(34)24-4-2-3-14-30-24)15-13-23(16-33)32-18-27(31-26(32)35,19-5-9-21(28)10-6-19)20-7-11-22(29)12-8-20/h2-12,14,23H,13,15-18H2,1H3/p+1/t23-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208029
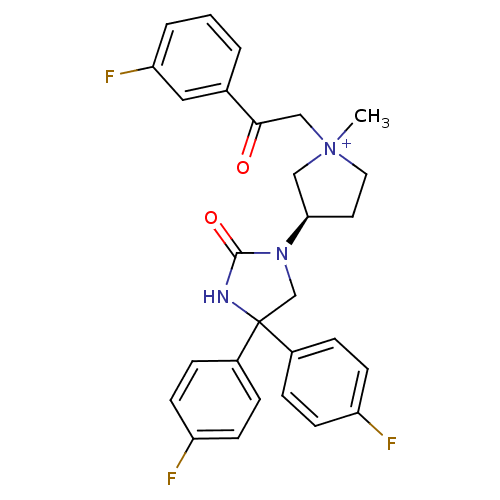
(1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...)Show SMILES C[N+]1(CC(=O)c2cccc(F)c2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H26F3N3O2/c1-34(17-26(35)19-3-2-4-24(31)15-19)14-13-25(16-34)33-18-28(32-27(33)36,20-5-9-22(29)10-6-20)21-7-11-23(30)12-8-21/h2-12,15,25H,13-14,16-18H2,1H3/p+1/t25-,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595418
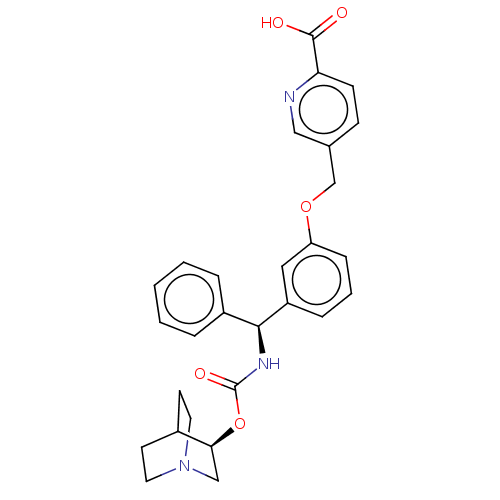
(CHEMBL5179380)Show SMILES OC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cn1 |r,wU:15.16,wD:20.20,THB:19:20:24.23:27.26,(-9.34,-3.08,;-9.34,-1.54,;-10.67,-.77,;-8,-.77,;-8,.77,;-6.67,1.54,;-5.33,.77,;-4,1.54,;-2.67,.77,;-1.33,1.54,;-1.33,3.08,;0,3.85,;1.33,3.08,;1.33,1.54,;0,.77,;2.67,.77,;4,1.54,;5.34,.77,;5.34,-.77,;6.67,1.54,;8,.77,;8,-.77,;9.34,-1.54,;8.62,-.31,;10.05,.31,;9.34,1.54,;10.67,.77,;10.67,-.77,;2.67,-.77,;4,-1.54,;4,-3.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;-5.33,-.77,;-6.67,-1.54,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595416
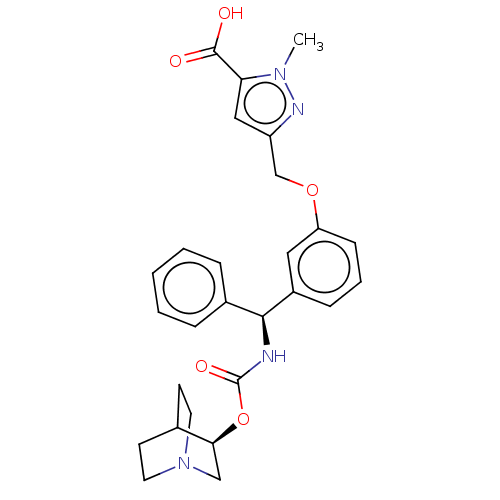
(CHEMBL5178217)Show SMILES Cn1nc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1C(O)=O |r,wU:12.13,wD:17.17,THB:16:17:20.21:23.24,(-9.99,.25,;-8.45,.25,;-7.42,1.4,;-6.01,.77,;-4.68,1.54,;-3.35,.77,;-2.01,1.54,;-2.01,3.08,;-.68,3.85,;.66,3.08,;.66,1.54,;-.68,.77,;1.99,.77,;3.32,1.54,;4.66,.77,;4.66,-.77,;5.99,1.54,;7.32,.77,;7.32,-.77,;8.66,-1.54,;7.95,-.31,;9.37,.31,;8.66,1.54,;9.99,.77,;9.99,-.77,;1.99,-.77,;3.32,-1.54,;3.32,-3.08,;1.99,-3.85,;.66,-3.08,;.66,-1.54,;-6.17,-.76,;-7.68,-1.08,;-8.31,-2.49,;-9.84,-2.65,;-7.4,-3.73,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595419
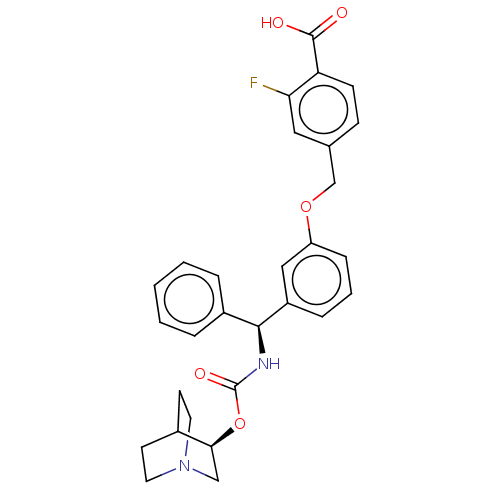
(CHEMBL5194569)Show SMILES OC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1F |r,wU:15.16,wD:20.20,(-9.34,-3.08,;-9.34,-1.54,;-10.67,-.77,;-8,-.77,;-6.67,-1.54,;-5.33,-.77,;-5.33,.77,;-4,1.54,;-2.67,.77,;-1.33,1.54,;-1.33,3.08,;0,3.85,;1.33,3.08,;1.33,1.54,;0,.77,;2.67,.77,;4,1.54,;5.34,.77,;5.34,-.77,;6.67,1.54,;8,.77,;8,-.77,;9.34,-1.54,;8.63,-.31,;10.05,.31,;9.34,1.54,;10.67,.77,;10.67,-.77,;2.67,-.77,;4,-1.54,;4,-3.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;-6.67,1.54,;-8,.77,;-9.34,1.54,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595414
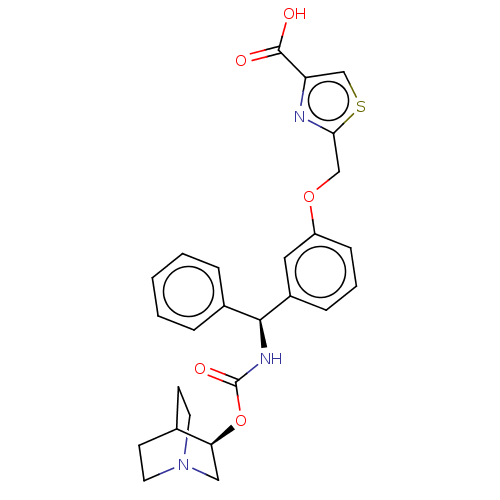
(CHEMBL5187593)Show SMILES OC(=O)c1csc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1 |r,wU:15.16,wD:20.20,THB:19:20:23.24:26.27,(-9.91,-2.65,;-8.38,-2.49,;-7.48,-3.73,;-7.76,-1.08,;-8.53,.25,;-7.5,1.4,;-6.09,.77,;-4.76,1.54,;-3.42,.77,;-2.09,1.54,;-2.09,3.08,;-.75,3.85,;.58,3.08,;.58,1.54,;-.75,.77,;1.91,.77,;3.25,1.54,;4.58,.77,;4.58,-.77,;5.91,1.54,;7.25,.77,;7.25,-.77,;8.58,-1.54,;7.87,-.31,;9.29,.31,;8.58,1.54,;9.91,.77,;9.91,-.77,;1.91,-.77,;3.25,-1.54,;3.25,-3.08,;1.91,-3.85,;.58,-3.08,;.58,-1.54,;-6.25,-.76,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595420
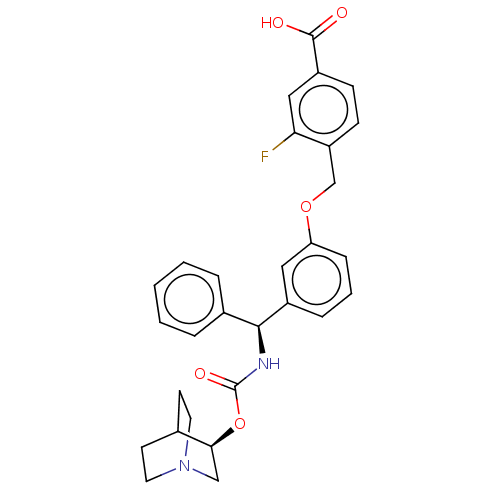
(CHEMBL5193776)Show SMILES OC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c(F)c1 |r,wU:15.16,wD:20.20,(-9.34,-3.08,;-9.34,-1.54,;-10.67,-.77,;-8,-.77,;-6.67,-1.54,;-5.33,-.77,;-5.33,.77,;-4,1.54,;-2.67,.77,;-1.33,1.54,;-1.33,3.08,;0,3.85,;1.33,3.08,;1.33,1.54,;0,.77,;2.67,.77,;4,1.54,;5.34,.77,;5.34,-.77,;6.67,1.54,;8,.77,;8,-.77,;9.34,-1.54,;8.63,-.31,;10.05,.31,;9.34,1.54,;10.67,.77,;10.67,-.77,;2.67,-.77,;4,-1.54,;4,-3.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;-6.67,1.54,;-6.67,3.08,;-8,.77,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208047

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O/c1-31(19-17-23-11-5-2-6-12-23)20-18-26(21-31)30-22-28(29-27(30)32,24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-16,26H,17-22H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595415
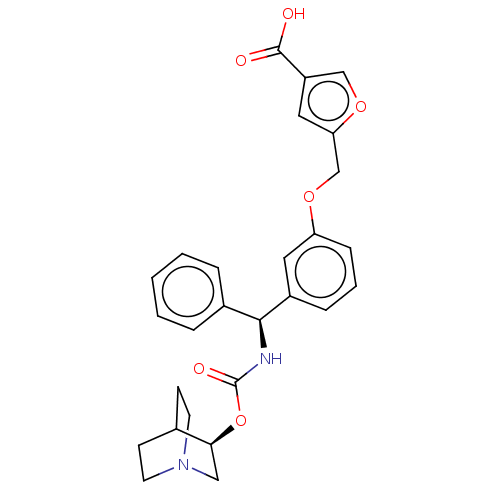
(CHEMBL5202045)Show SMILES OC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:15.16,wD:20.20,THB:19:20:23.24:26.27,(-9.91,-2.65,;-8.38,-2.49,;-7.48,-3.73,;-7.76,-1.08,;-8.53,.25,;-7.5,1.4,;-6.09,.77,;-4.76,1.54,;-3.42,.77,;-2.09,1.54,;-2.09,3.08,;-.75,3.85,;.58,3.08,;.58,1.54,;-.75,.77,;1.91,.77,;3.25,1.54,;4.58,.77,;4.58,-.77,;5.91,1.54,;7.25,.77,;7.25,-.77,;8.58,-1.54,;7.87,-.31,;9.29,.31,;8.58,1.54,;9.91,.77,;9.91,-.77,;1.91,-.77,;3.25,-1.54,;3.25,-3.08,;1.91,-3.85,;.58,-3.08,;.58,-1.54,;-6.25,-.76,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208036
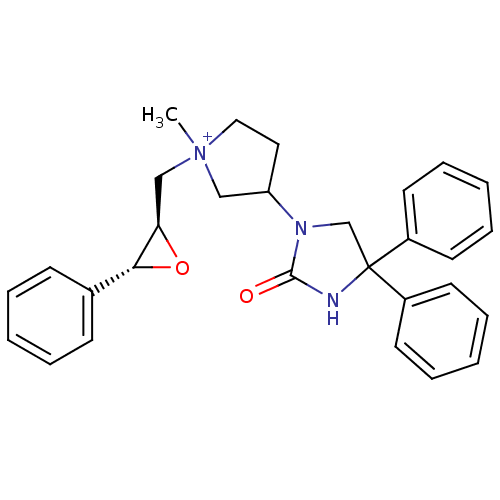
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(C[C@H]2O[C@@H]2c2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H31N3O2/c1-32(20-26-27(34-26)22-11-5-2-6-12-22)18-17-25(19-32)31-21-29(30-28(31)33,23-13-7-3-8-14-23)24-15-9-4-10-16-24/h2-16,25-27H,17-21H2,1H3/p+1/t25?,26-,27-,32?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208039
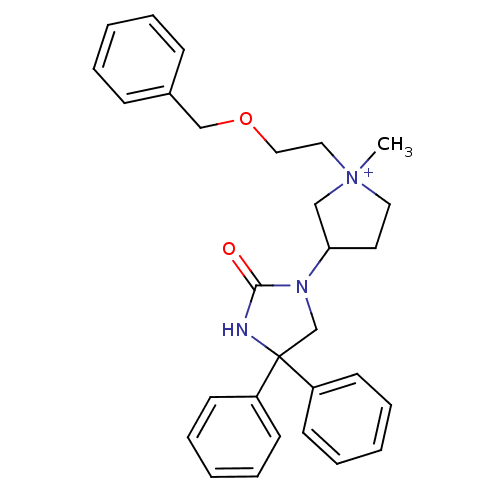
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCOCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O2/c1-32(19-20-34-22-24-11-5-2-6-12-24)18-17-27(21-32)31-23-29(30-28(31)33,25-13-7-3-8-14-25)26-15-9-4-10-16-26/h2-16,27H,17-23H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208054
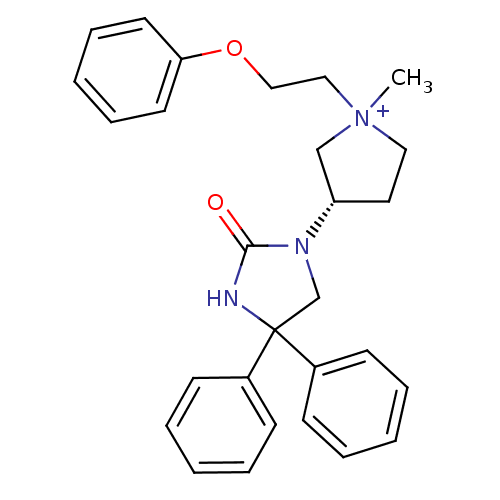
(1-methyl-3-(S)-(2-oxo-4,4-diphenyl-imidazolidin-1-...)Show SMILES C[N+]1(CCOc2ccccc2)CC[C@@H](C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1/t25-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
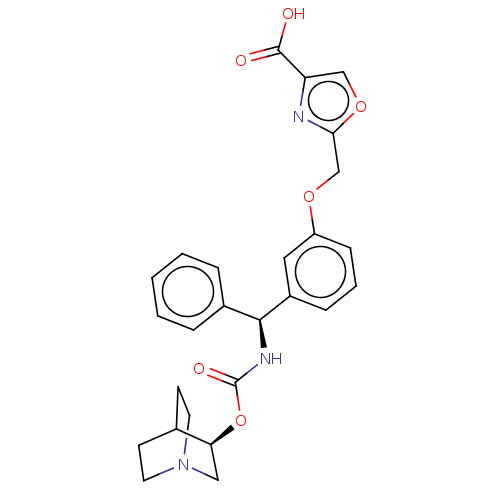
(Homo sapiens (Human)) | BDBM50595412
(CHEMBL5181855)Show SMILES OC(=O)c1coc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)n1 |r,wU:15.16,wD:20.20,THB:19:20:23.24:26.27,(-9.91,-2.65,;-8.38,-2.49,;-7.48,-3.73,;-7.76,-1.08,;-8.53,.25,;-7.5,1.4,;-6.09,.77,;-4.76,1.54,;-3.42,.77,;-2.09,1.54,;-2.09,3.08,;-.75,3.85,;.58,3.08,;.58,1.54,;-.75,.77,;1.91,.77,;3.25,1.54,;4.58,.77,;4.58,-.77,;5.91,1.54,;7.25,.77,;7.25,-.77,;8.58,-1.54,;7.87,-.31,;9.29,.31,;8.58,1.54,;9.91,.77,;9.91,-.77,;1.91,-.77,;3.25,-1.54,;3.25,-3.08,;1.91,-3.85,;.58,-3.08,;.58,-1.54,;-6.25,-.76,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
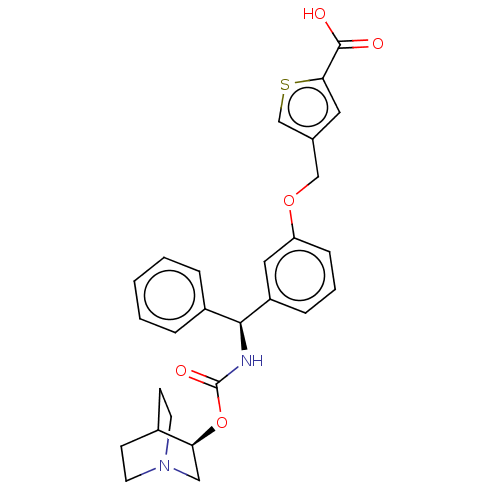
(Homo sapiens (Human)) | BDBM50595413
(CHEMBL5195224)Show SMILES OC(=O)c1cc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cs1 |r,wU:14.15,wD:19.19,THB:18:19:22.23:25.26,(-9.91,-2.65,;-8.38,-2.49,;-7.48,-3.73,;-7.76,-1.08,;-6.25,-.76,;-6.09,.77,;-4.76,1.54,;-3.42,.77,;-2.09,1.54,;-2.09,3.08,;-.75,3.85,;.58,3.08,;.58,1.54,;-.75,.77,;1.91,.77,;3.25,1.54,;4.58,.77,;4.58,-.77,;5.91,1.54,;7.25,.77,;7.25,-.77,;8.58,-1.54,;7.87,-.31,;9.29,.31,;8.58,1.54,;9.91,.77,;9.91,-.77,;1.91,-.77,;3.25,-1.54,;3.25,-3.08,;1.91,-3.85,;.58,-3.08,;.58,-1.54,;-7.5,1.4,;-8.53,.25,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595409
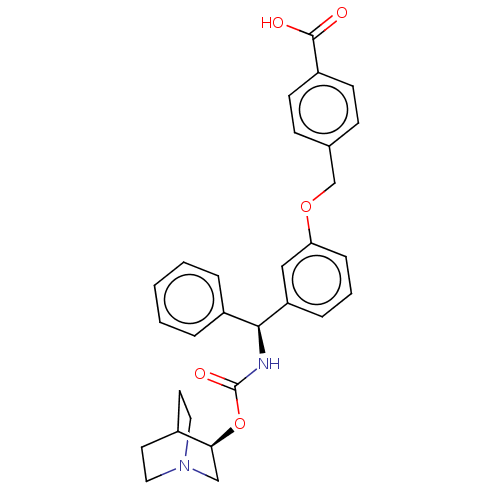
(CHEMBL5202567)Show SMILES OC(=O)c1ccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wD:15.16,20.20,(-10.67,.77,;-9.34,1.54,;-9.34,3.08,;-8,.77,;-8,-.77,;-6.67,-1.54,;-5.33,-.77,;-4,-1.54,;-2.67,-.77,;-1.33,-1.54,;-1.33,-3.08,;,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;2.67,-.77,;4,-1.54,;5.33,-.77,;5.33,.77,;6.67,-1.54,;8,-.77,;9.34,-1.54,;10.67,-.77,;10.11,.21,;9.13,.77,;8,.77,;9.34,1.54,;10.67,.77,;2.67,.77,;1.33,1.54,;1.33,3.08,;2.67,3.85,;4,3.08,;4,1.54,;-5.33,.77,;-6.67,1.54,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595410
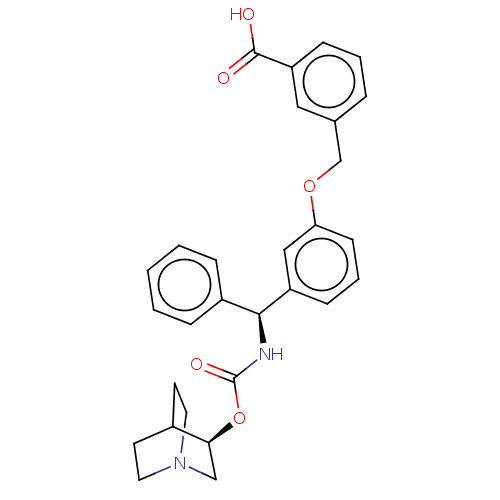
(CHEMBL5202787)Show SMILES OC(=O)c1cccc(COc2cccc(c2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:16.17,wD:21.21,(-10.67,.77,;-9.34,1.54,;-9.34,3.08,;-8,.77,;-8,-.77,;-6.67,-1.54,;-5.33,-.77,;-5.33,.77,;-4,1.54,;-2.67,.77,;-1.33,1.54,;-1.33,3.08,;0,3.85,;1.33,3.08,;1.33,1.54,;0,.77,;2.67,.77,;4,1.54,;5.33,.77,;5.33,-.77,;6.67,1.54,;8,.77,;8,-.77,;9.34,-1.54,;10.67,-.77,;10.67,.77,;9.34,1.54,;10.05,.31,;8.62,-.31,;2.67,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;4,-3.08,;4,-1.54,;-6.67,1.54,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208056

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O2/c1-32(19-11-21-34-27-16-9-4-10-17-27)20-18-26(22-32)31-23-29(30-28(31)33,24-12-5-2-6-13-24)25-14-7-3-8-15-25/h2-10,12-17,26H,11,18-23H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data