

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pteridine reductase 1 (Leishmania major) | BDBM50551183 (CHEMBL4747846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551184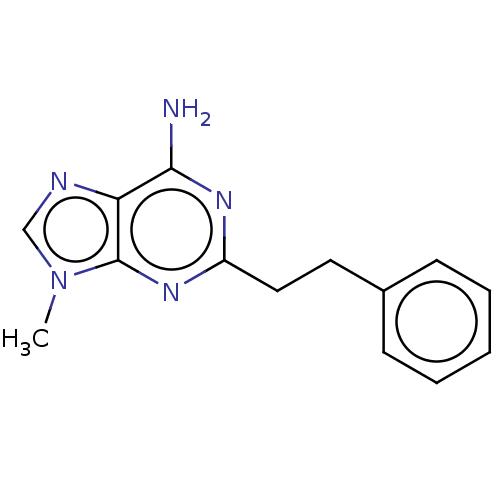 (CHEMBL4797185) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551180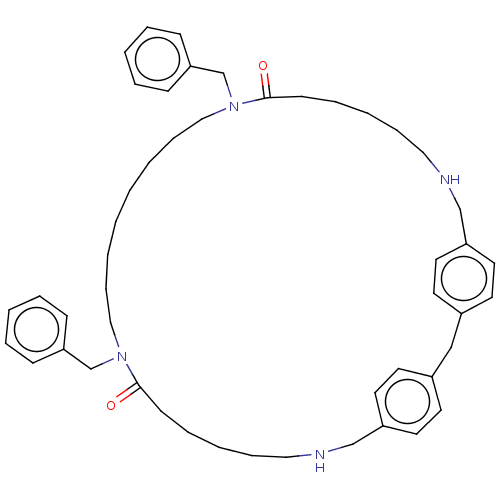 (CHEMBL4760251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551177 (CHEMBL4762279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551178 (CHEMBL4785591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551181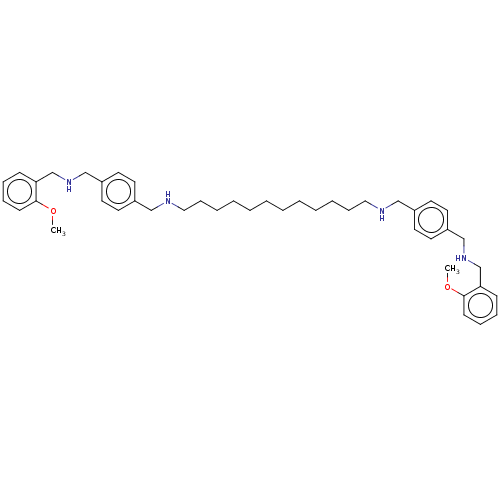 (CHEMBL54725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551182 (CHEMBL4754292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551179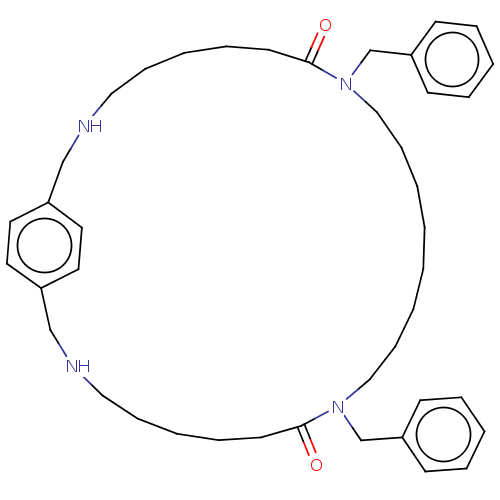 (CHEMBL4778279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551176 (CHEMBL4748094) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551175 (CHEMBL1993081) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551174 (CHEMBL158919) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551173 (CHEMBL4753331) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50062821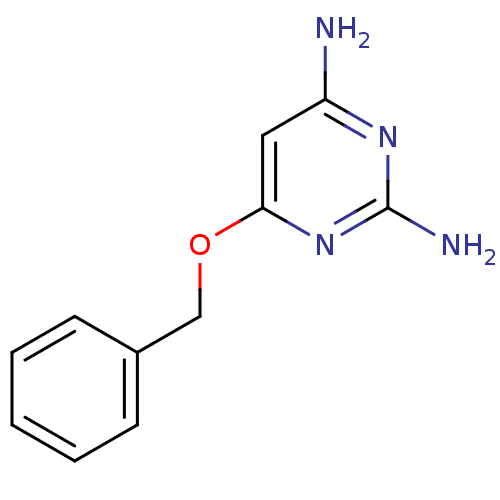 (6-Benzyloxy-pyrimidine-2,4-diamine | CHEMBL121445) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50386758 (CHEMBL2046893) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM29080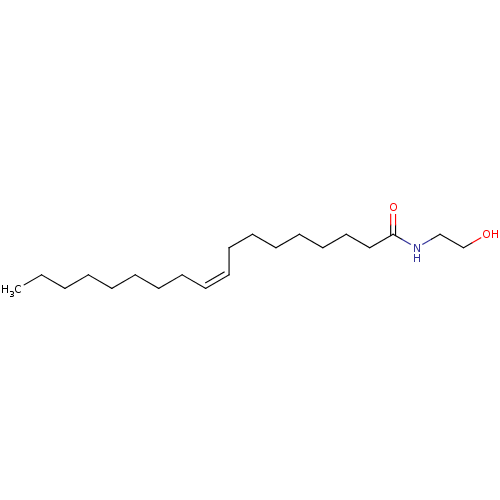 (CHEMBL280065 | N-oleoylethanolamine | Oleamide MEA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of acid ceramidase (unknown origin) | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233790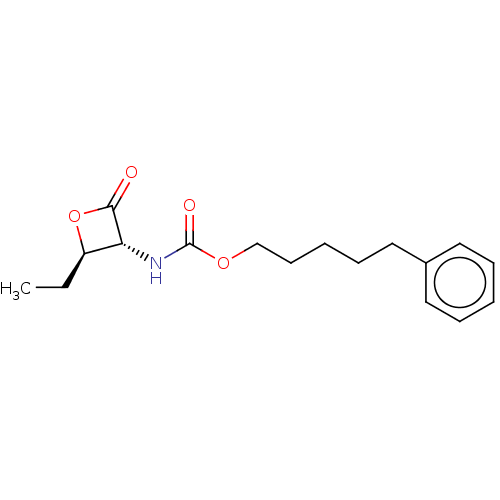 (US9353075, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM50431244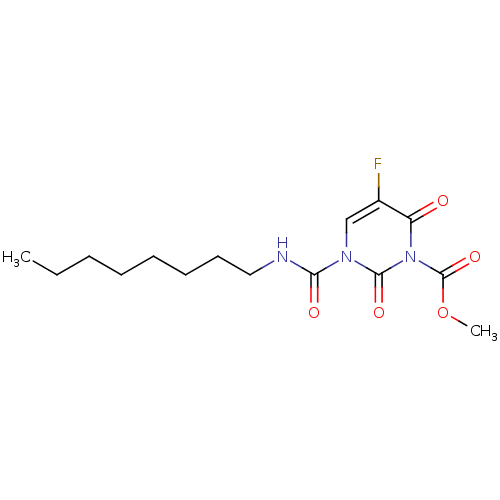 (CHEMBL2333064) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub... | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439653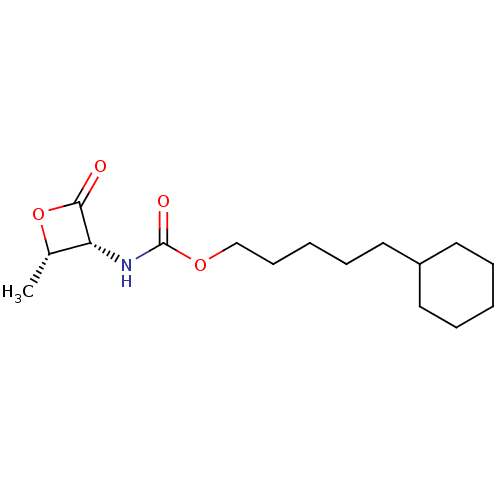 (CHEMBL2419830 | US9353075, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439649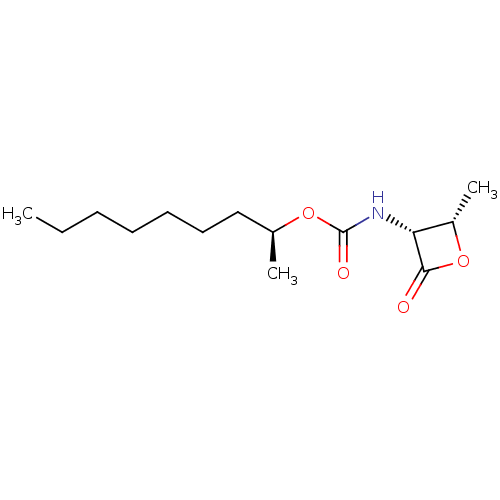 (CHEMBL2419811 | US9353075, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439653 (CHEMBL2419830 | US9353075, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439649 (CHEMBL2419811 | US9353075, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50234746 (CHEMBL4093333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio... | Eur J Med Chem 126: 561-575 (2017) Article DOI: 10.1016/j.ejmech.2016.11.039 BindingDB Entry DOI: 10.7270/Q25H7JHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50234744 (CHEMBL4101070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio... | Eur J Med Chem 126: 561-575 (2017) Article DOI: 10.1016/j.ejmech.2016.11.039 BindingDB Entry DOI: 10.7270/Q25H7JHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233791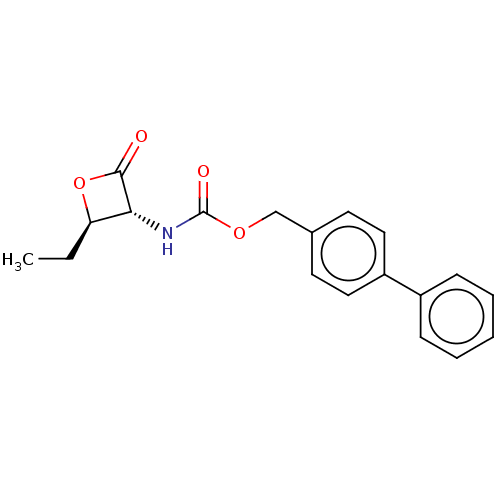 (US9353075, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032458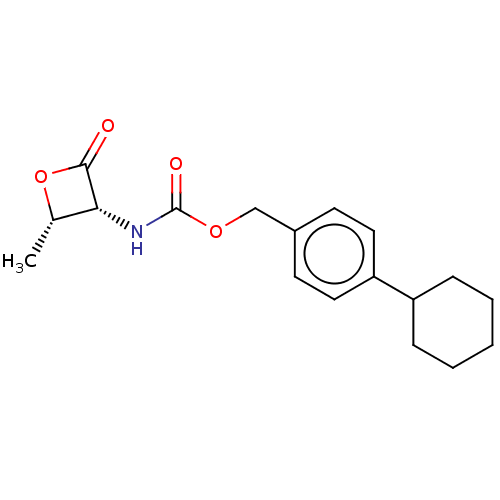 (CHEMBL3354144 | US9353075, 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151154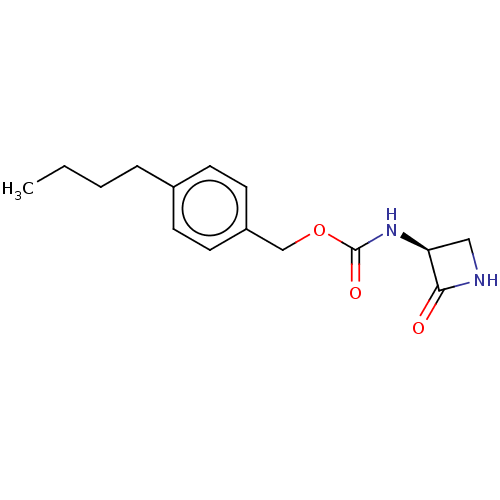 (CHEMBL3770896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032458 (CHEMBL3354144 | US9353075, 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description Lysosomal NAAA protein preparation were obtained by homogenizing male Sprague-Dawley rat lungs (Charles River) in 20 mM Tris-HCl buffer pH 7.4 contai... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM50431255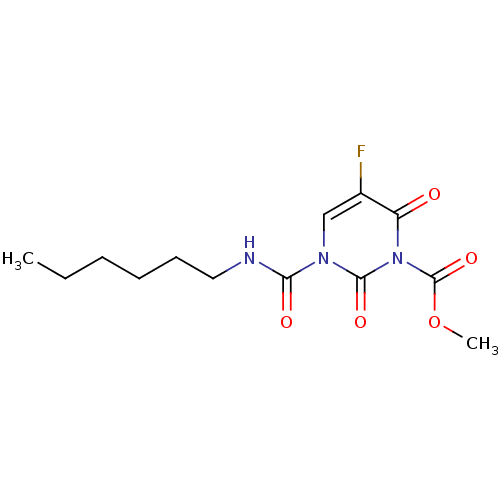 (CHEMBL2333053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub... | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of Sprague Dawley rat lung native NAAA enzyme using heptadecenoylethanolamide as substrate preincubated for 30 mins followed by substrate ... | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023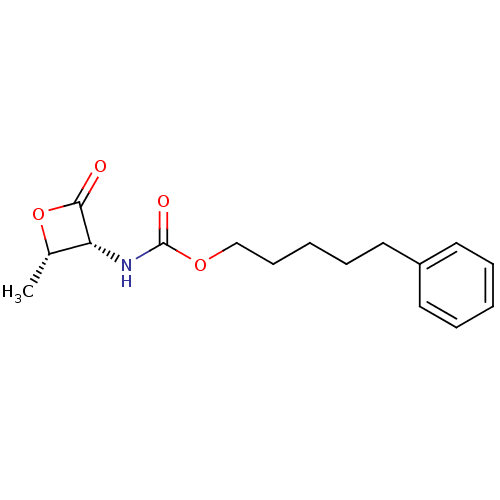 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description Lysosomal NAAA protein preparation were obtained by homogenizing male Sprague-Dawley rat lungs (Charles River) in 20 mM Tris-HCl buffer pH 7.4 contai... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032458 (CHEMBL3354144 | US9353075, 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM50431246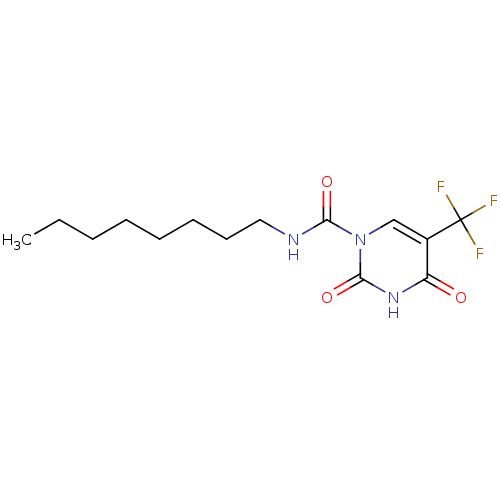 (CHEMBL2333062 | US9428465, 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub... | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant His6-tagged NAAA expressed in HEK293 cells using 10-cis-heptadecenoylethanolamide as substrate after 30 mins by UPLC/... | ACS Med Chem Lett 3: 422-426 (2012) Article DOI: 10.1021/ml300056y BindingDB Entry DOI: 10.7270/Q29W0GKS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50032463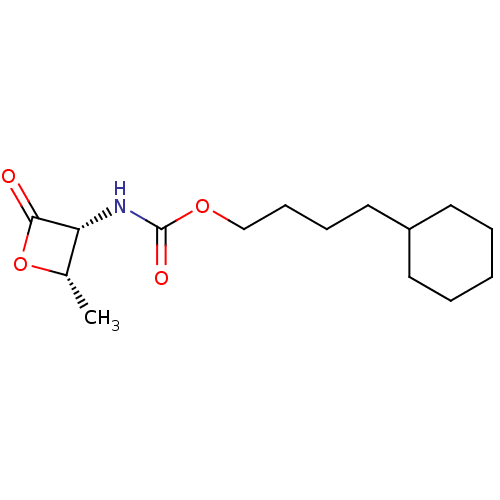 (CHEMBL3353547 | US9353075, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233792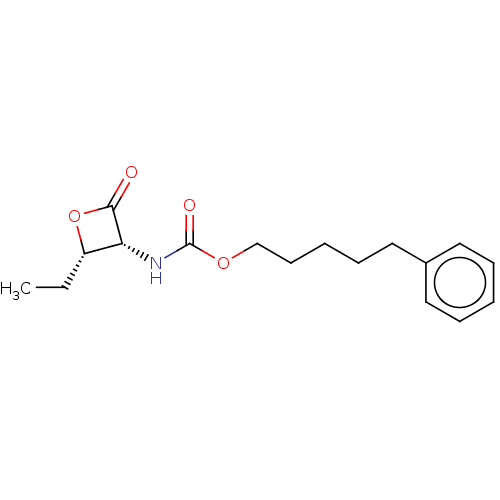 (US9353075, 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50032463 (CHEMBL3353547 | US9353075, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50234745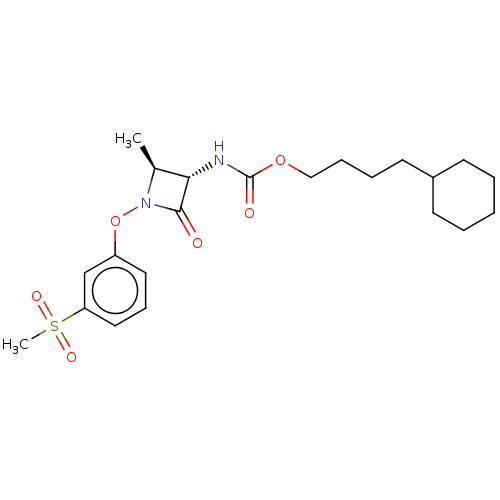 (CHEMBL4065712) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio... | Eur J Med Chem 126: 561-575 (2017) Article DOI: 10.1016/j.ejmech.2016.11.039 BindingDB Entry DOI: 10.7270/Q25H7JHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233776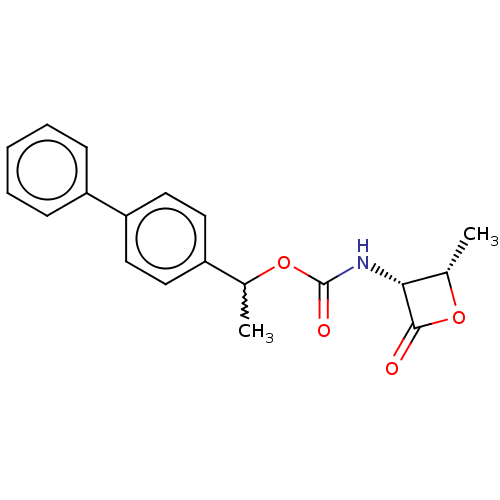 (US9353075, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032456 (CHEMBL3354145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032456 (CHEMBL3354145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM50431254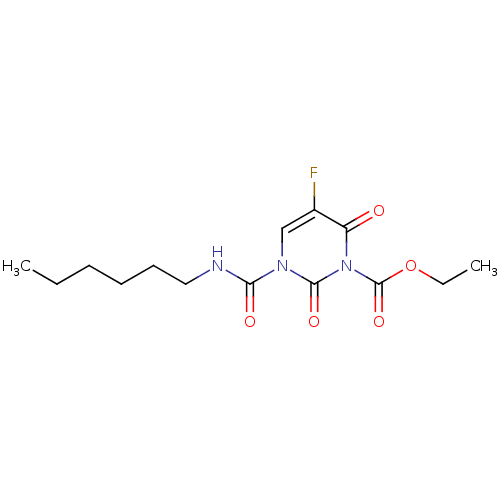 (CHEMBL2333054) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub... | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151242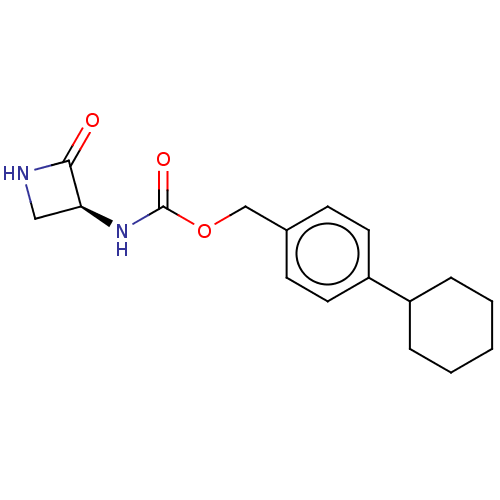 (CHEMBL3770525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Rattus norvegicus (Rat)) | BDBM50431271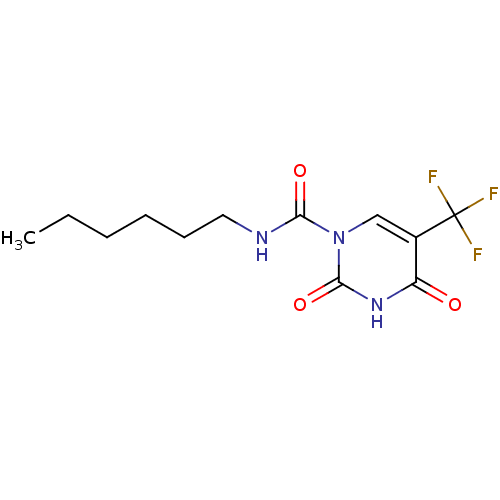 (CHEMBL2333033 | US9428465, 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of rat recombinant acid ceramidase expressed in human HEK293 cells using N-lauroylceramide as substrate incubated for 30 mins prior to sub... | J Med Chem 56: 3518-30 (2013) Article DOI: 10.1021/jm301879g BindingDB Entry DOI: 10.7270/Q27D2WH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233798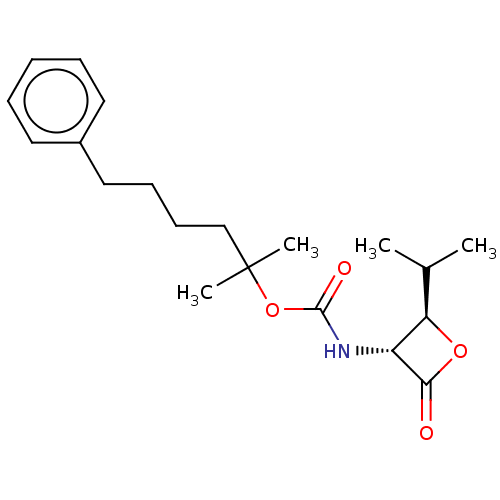 (US9353075, 55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 447 total ) | Next | Last >> |