

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139844 (CHEMBL3763503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004276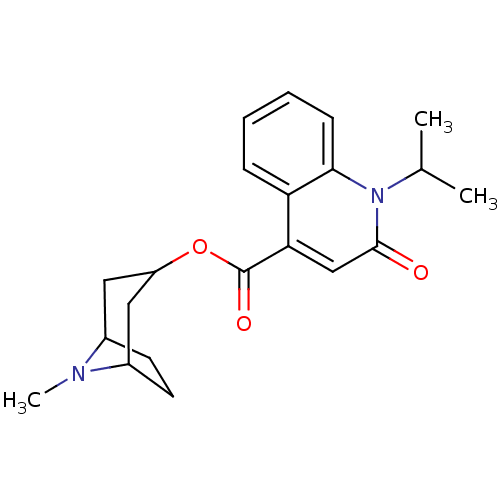 (1-Isopropyl-2-oxo-1,2-dihydro-quinoline-4-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139843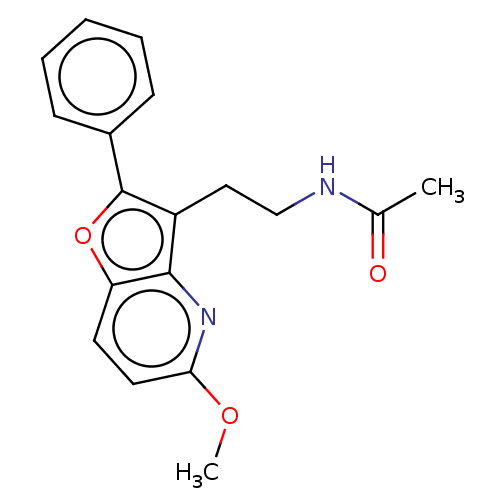 (CHEMBL3765540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139842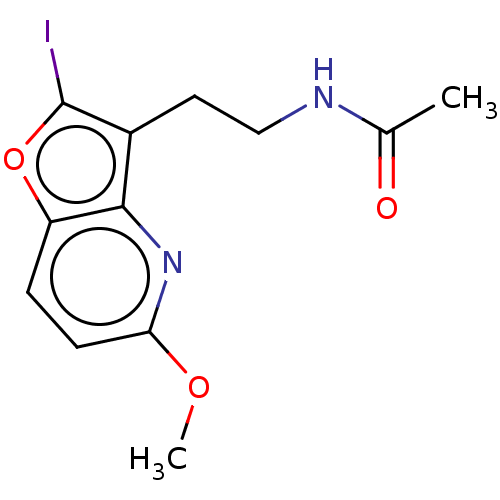 (CHEMBL3765401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139847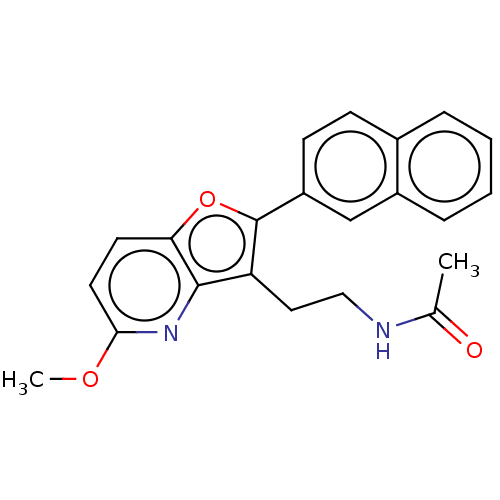 (CHEMBL3764765) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668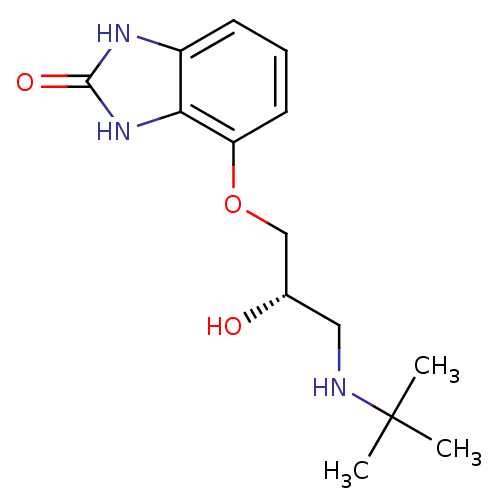 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518510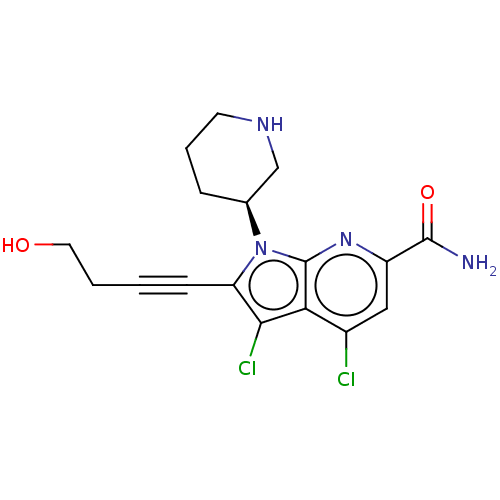 (CHEMBL4448325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50406750 (Firazyr | HOE-140 | Icatibant) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. | J Med Chem 36: 1450-60 (1993) BindingDB Entry DOI: 10.7270/Q2PG1QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50139843 (CHEMBL3765540) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50037242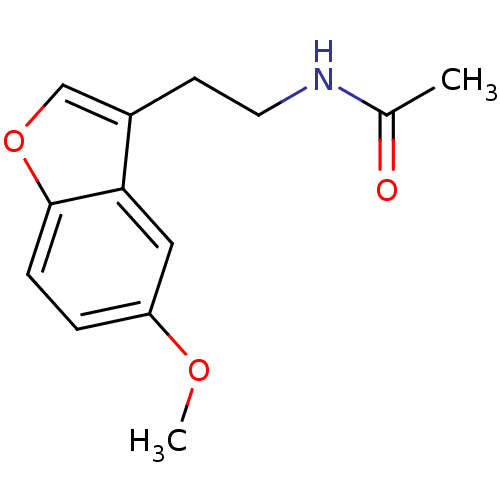 (CHEMBL323332 | N-(2-(5-methoxybenzofuran-3-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Cavia porcellus) | BDBM50406751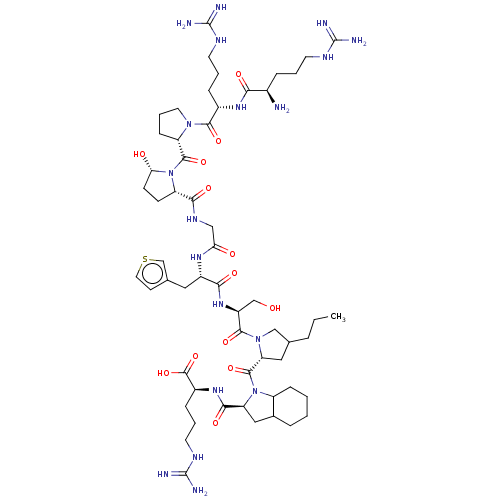 (CHEMBL2369941) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. | J Med Chem 36: 1450-60 (1993) BindingDB Entry DOI: 10.7270/Q2PG1QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin (GUINEA PIG) | BDBM85050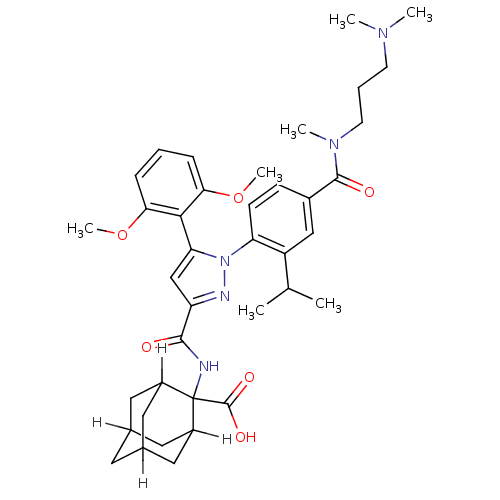 (CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 280: 802-12 (1997) BindingDB Entry DOI: 10.7270/Q2RR1WR1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50003019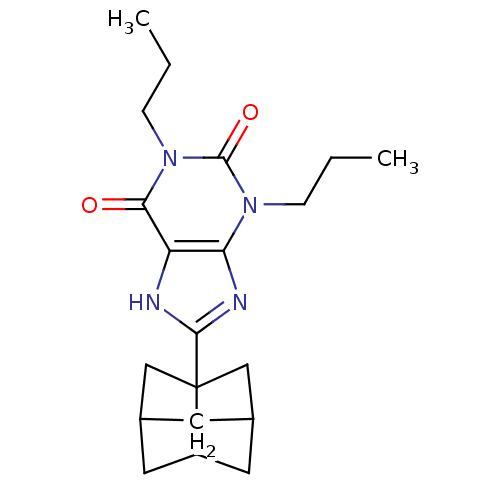 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor in rat forebrain membranes using N6-[3H]cyclohexyladenosine | J Med Chem 35: 3066-75 (1992) BindingDB Entry DOI: 10.7270/Q2DN45PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50044431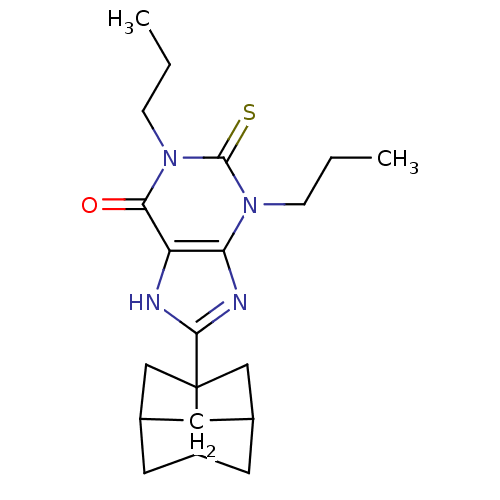 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Tested for binding affinity against Adenosine A1 receptor from rat forebrain membranes, using N6-[3H]- cyclohexyladenosine as radioligand | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139844 (CHEMBL3763503) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513620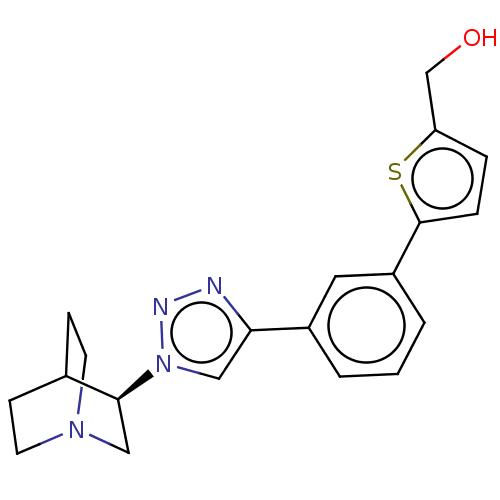 (CHEMBL4563574) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50139842 (CHEMBL3765401) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50044429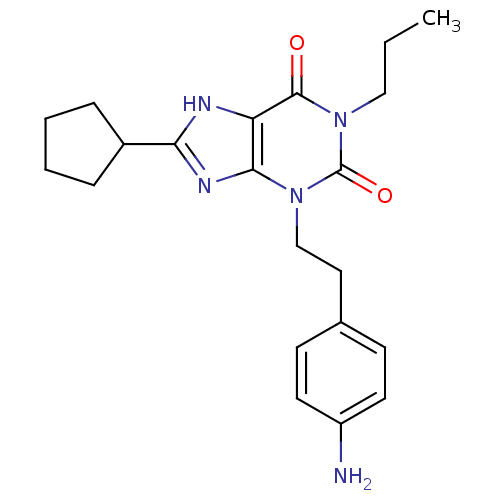 (3-[2-(4-Amino-phenyl)-ethyl]-8-cyclopentyl-1-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity against Adenosine A1 receptor from rat forebrain membranes with N6-[3H]- cyclohexyladenosine | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to adenosine A1 receptor from rat cortical membranes | J Med Chem 35: 2342-5 (1992) BindingDB Entry DOI: 10.7270/Q2T43S20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT1 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50340324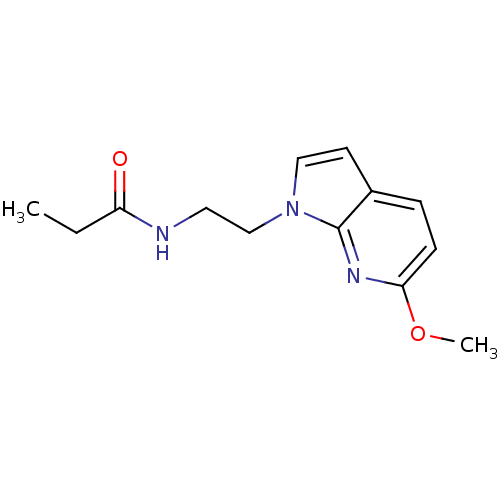 (CHEMBL1760946 | N-(2-(6-Methoxy-1H-pyrrolo[2,3-b]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for ability to compete with dansylamide for binding to human erythrocyte carbonic-anhydrase-II (HCA-II) | J Med Chem 37: 240-7 (1994) BindingDB Entry DOI: 10.7270/Q2N29XKD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neurotensin/neuromedin N (Homo sapiens (Human)) | BDBM85050 (CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 280: 802-12 (1997) BindingDB Entry DOI: 10.7270/Q2RR1WR1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513583 (CHEMBL4533685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513617 (CHEMBL4459802) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513605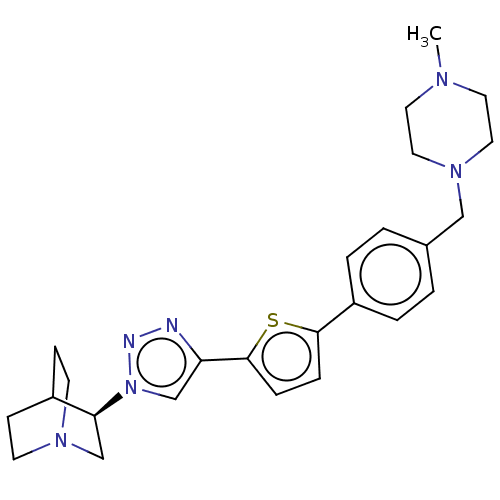 (CHEMBL4563839) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50513594 (CHEMBL4556722) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]-alpha-bungarotoxin from alpha7 nAChR in Wistar rat brain membrane incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 179: 449-469 (2019) Article DOI: 10.1016/j.ejmech.2019.06.049 BindingDB Entry DOI: 10.7270/Q2R49V3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210579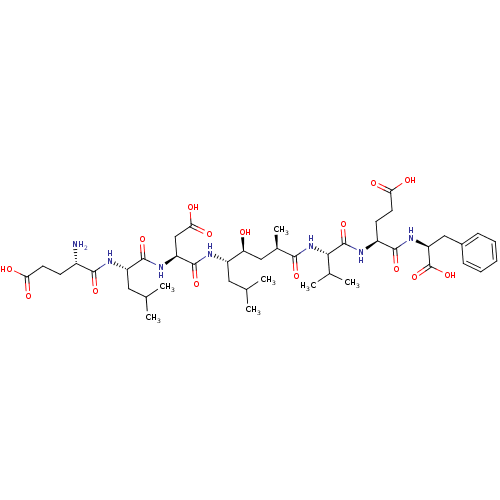 ((2S,5S,8S,11R,13S,14S,17S,20S,23S)-23-amino-2-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of human recombinant BACE1 ectodomain (1 to 460 amino acids) assessed as inhibition of proteolytic cleavage of Rhodamine-EVNLDAEFK-Quenche... | J Med Chem 54: 3081-5 (2011) Article DOI: 10.1021/jm101568y BindingDB Entry DOI: 10.7270/Q20C4W7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50307501 ((6S)-N,N-dipropyl-5,6,7,8-tetrahydro-1,1'-binaphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [3H]5CT from human cloned 5HT7B receptor expressed in HEK293 cells | Bioorg Med Chem 18: 1958-67 (2010) Article DOI: 10.1016/j.bmc.2010.01.035 BindingDB Entry DOI: 10.7270/Q2S182M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50340338 (CHEMBL1760947 | N-(2-(6-Methoxy-1H-pyrrolo[2,3-b]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004292 (2-Isopropoxy-quinoline-4-carboxylic acid 8-methyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004279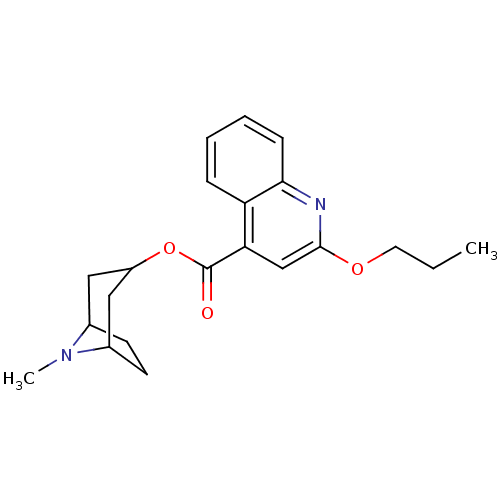 (2-Propoxy-quinoline-4-carboxylic acid 8-methyl-8-a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50267577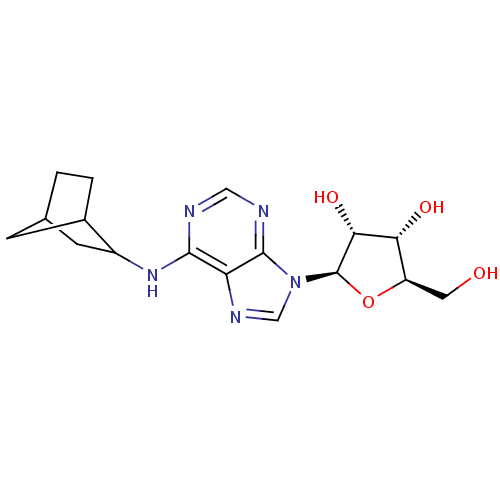 (CHEMBL489640 | N6-((+/-)-endo-norborn-2-yl)adenosi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using N6-[3H]cyclohexyladenosine as radioligand in guinea pig forebrain membranes | J Med Chem 35: 924-30 (1992) BindingDB Entry DOI: 10.7270/Q2NS0VJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to guinea pig forebrain membrane Adenosine A1 receptor | J Med Chem 35: 924-30 (1992) BindingDB Entry DOI: 10.7270/Q2NS0VJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of 2-[125I]-iodomelatonin from human MT2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 21: 2316-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.097 BindingDB Entry DOI: 10.7270/Q27H1JWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50037242 (CHEMBL323332 | N-(2-(5-methoxybenzofuran-3-yl)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50340329 (4-azamelatonin | CHEMBL1760944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Displacement of [125I]2-Iodomelatonin from human MT2 receptor expressed in HEK293 cells after 120 mins by radioligand competition assay | Eur J Med Chem 109: 268-75 (2016) Article DOI: 10.1016/j.ejmech.2016.01.008 BindingDB Entry DOI: 10.7270/Q2NC6318 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004288 (2-Butoxy-quinoline-4-carboxylic acid 8-methyl-8-az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50518517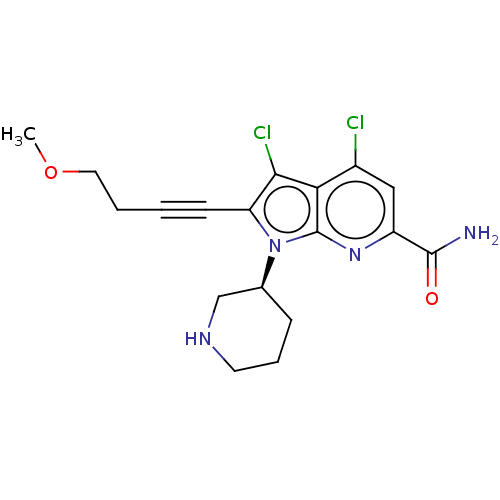 (CHEMBL4540910) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay | Bioorg Med Chem Lett 29: 491-495 (2019) Article DOI: 10.1016/j.bmcl.2018.12.015 BindingDB Entry DOI: 10.7270/Q21V5J93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004284 (2-Isobutoxy-quinoline-4-carboxylic acid 8-methyl-8...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004268 (2-Oxo-1-propyl-1,2-dihydro-quinoline-4-carboxylic ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Tested for binding affinity against Adenosine A1 receptor from rat forebrain membranes, using N6-[3H]- cyclohexyladenosine as radioligand | J Med Chem 36: 2508-18 (1993) BindingDB Entry DOI: 10.7270/Q2GX4C5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A1 receptor in rat forebrain membranes using N6-[3H]cyclohexyladenosine | J Med Chem 35: 3066-75 (1992) BindingDB Entry DOI: 10.7270/Q2DN45PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of N6-[3H]cyclohexyladenosine binding to adenosine A1 receptor from whole brain membranes | J Med Chem 35: 2342-5 (1992) BindingDB Entry DOI: 10.7270/Q2T43S20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5654 total ) | Next | Last >> |