

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073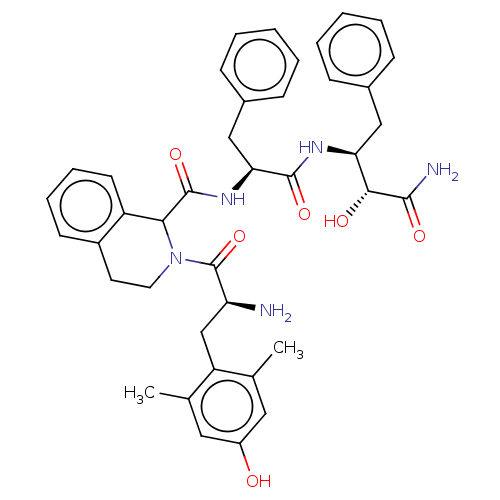 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072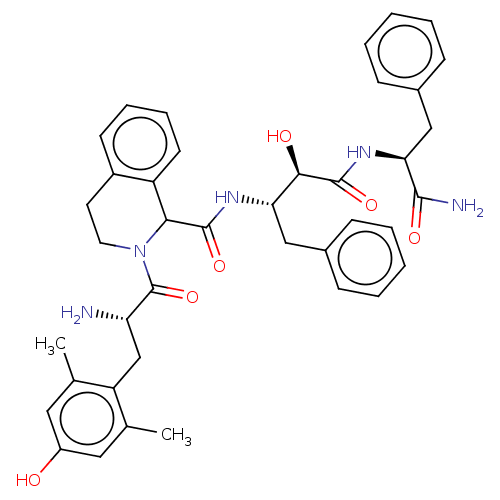 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50266734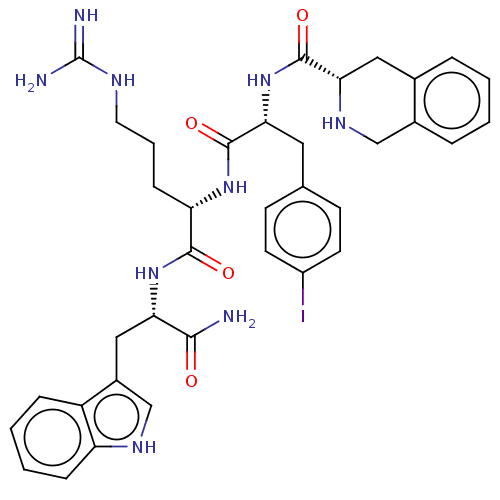 (CHEMBL4096081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at human MC4R expressed in CHO cells assessed as inhibition of aplha MSH-induced cAMP activation after 45 mins | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070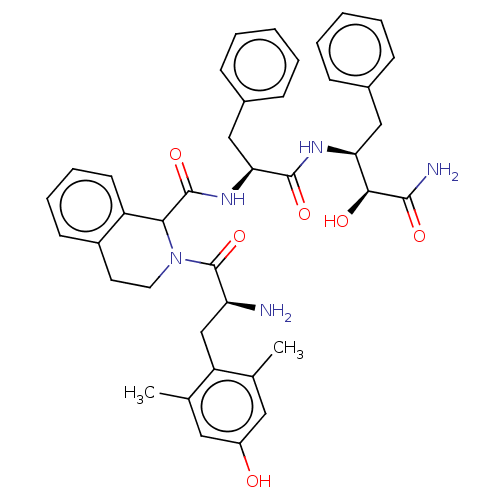 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094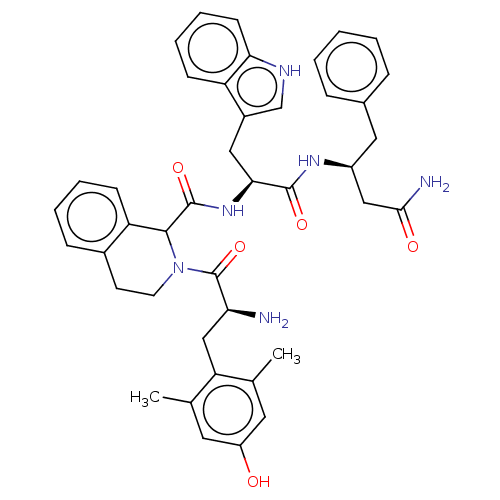 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069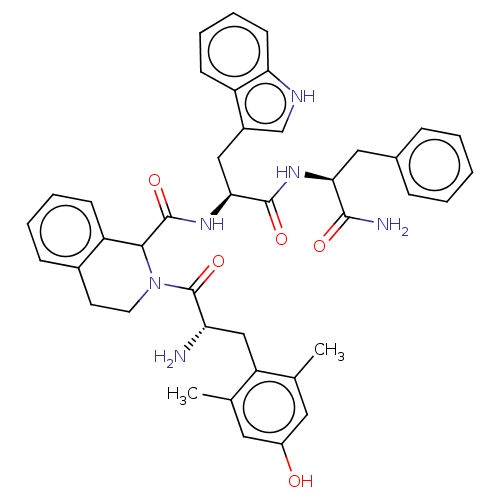 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068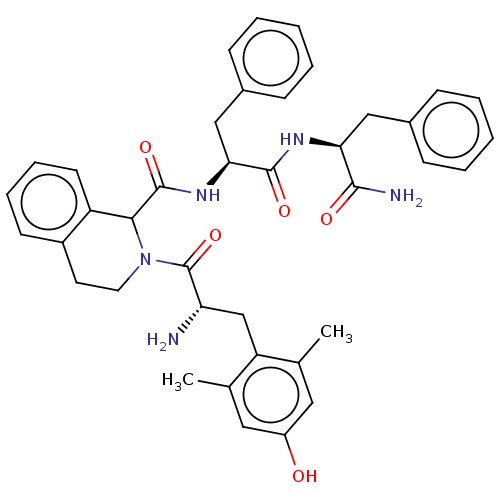 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071076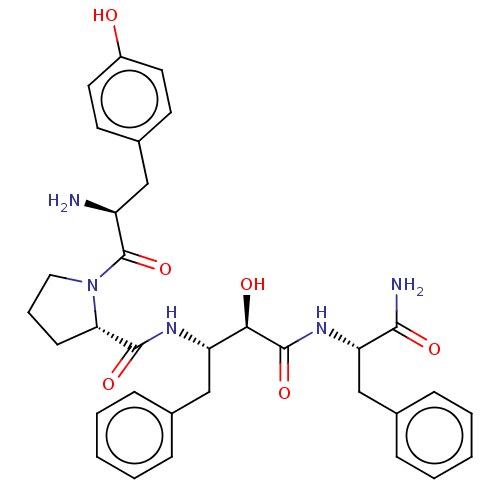 (CHEMBL3409749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071086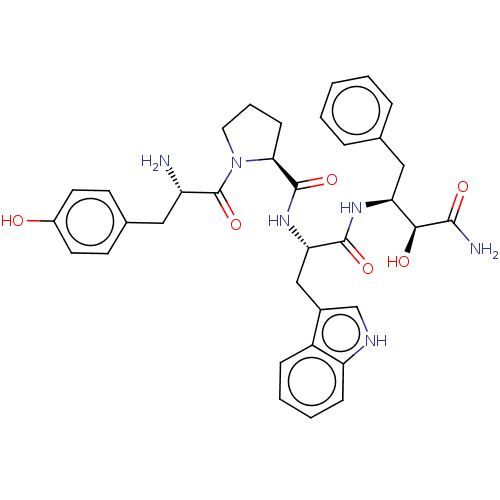 (CHEMBL3409741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50114010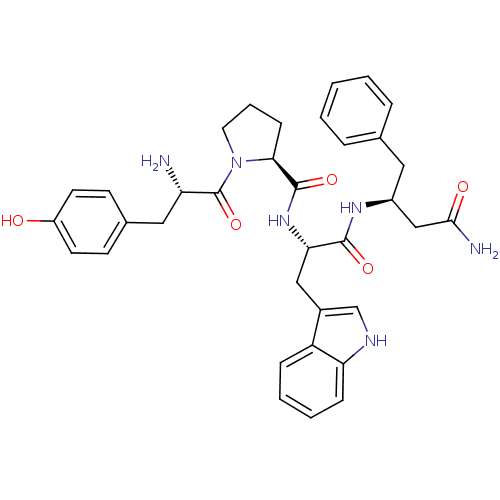 (1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246834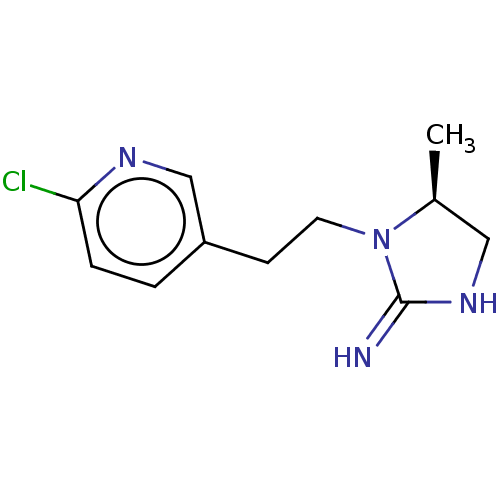 (CHEMBL4067640) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246823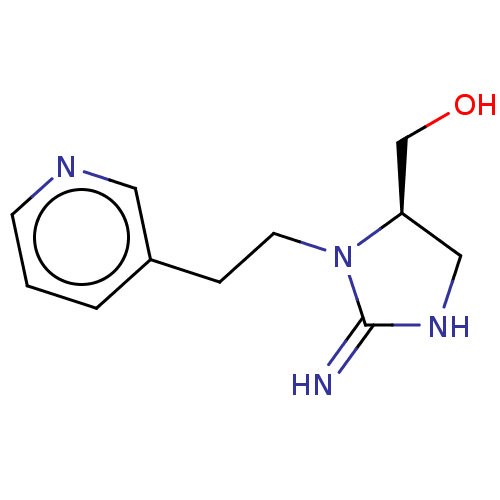 (CHEMBL4071277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071092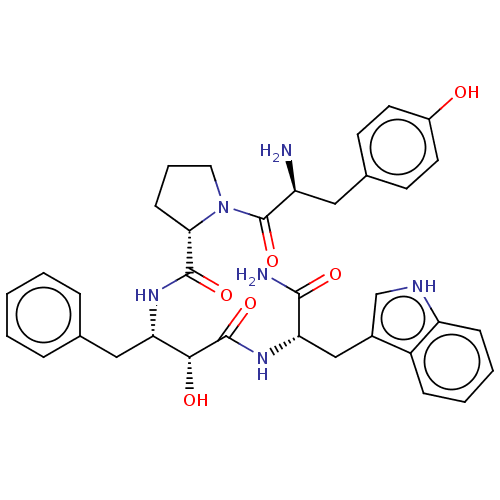 (CHEMBL3409755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266704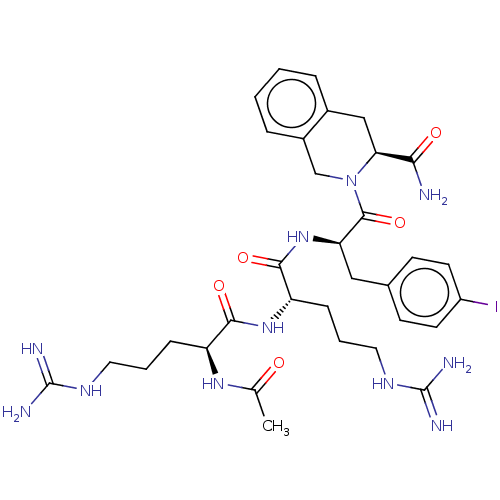 (CHEMBL4083717) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071090 (CHEMBL3409745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246826 (CHEMBL4084577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246835 (CHEMBL4077474) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071075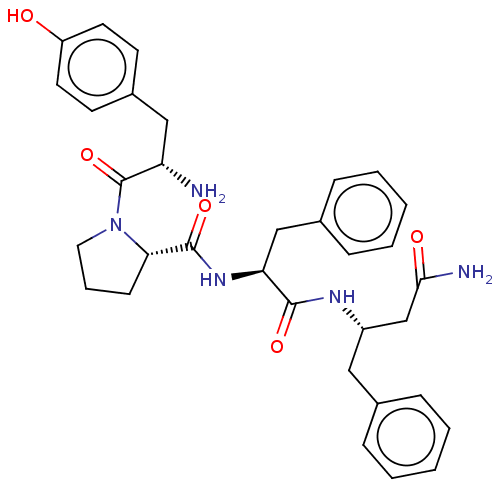 (CHEMBL3409748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071067 (CHEMBL3409757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071085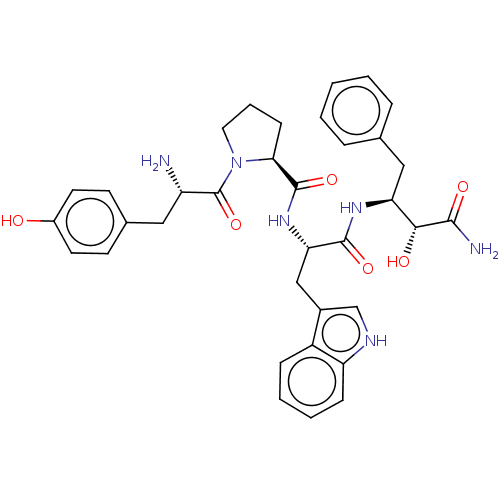 (CHEMBL3409740) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071093 (CHEMBL3409764) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071065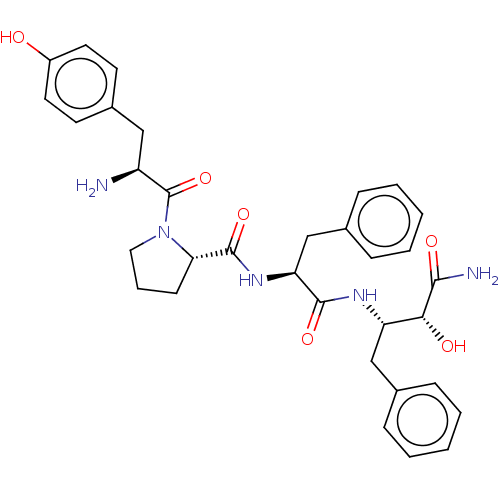 (CHEMBL3409744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071088 (CHEMBL3409753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071068 (CHEMBL3409758) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246819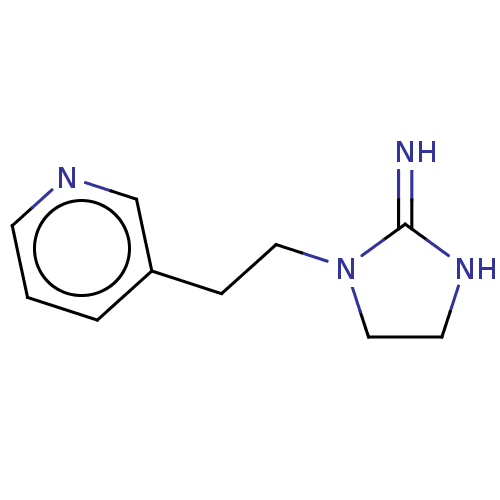 (CHEMBL4073220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071072 (CHEMBL3409762) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50246827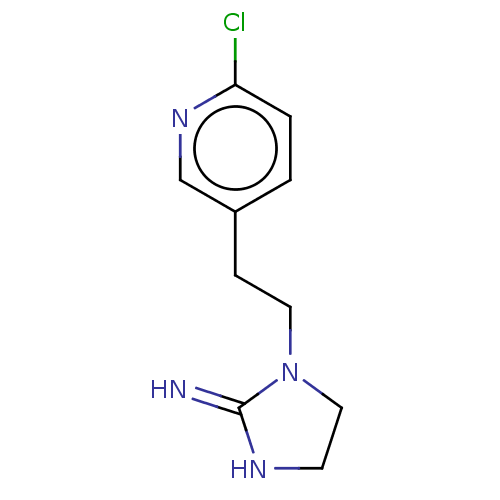 (CHEMBL4100236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha4beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071073 (CHEMBL3409763) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071070 (CHEMBL3409760) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50246823 (CHEMBL4071277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071066 (CHEMBL3409756) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071095 (CHEMBL3409766) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Rattus norvegicus (Rat)) | BDBM50246834 (CHEMBL4067640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta4 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Mus musculus) | BDBM50266713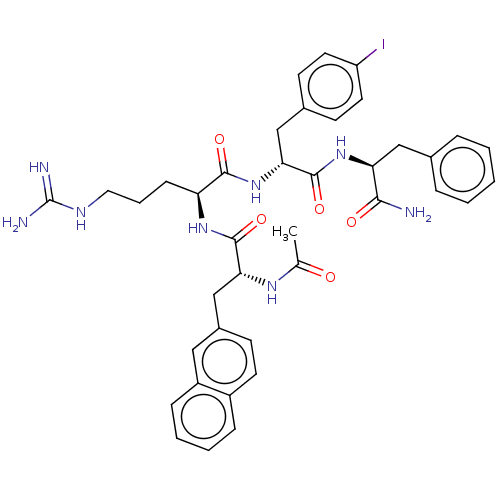 (CHEMBL4089759) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at mouse MC4R expressed in HEK293 cells assessed as inhibition of NDP-MSH induced-cAMP accumulation after 2 hrs by alpha screen a... | J Med Chem 60: 4342-4357 (2017) Article DOI: 10.1021/acs.jmedchem.7b00301 BindingDB Entry DOI: 10.7270/Q24170J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071071 (CHEMBL3409761) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-2 (Rattus norvegicus (Rat)) | BDBM50246826 (CHEMBL4084577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from rat alpha3beta2 nAChR expressed in HEK cell membranes after 2 hrs | J Med Chem 60: 10092-10104 (2017) Article DOI: 10.1021/acs.jmedchem.7b01250 BindingDB Entry DOI: 10.7270/Q2PG1V5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 687 total ) | Next | Last >> |