 Found 1166 hits with Last Name = 'huang' and Initial = 'g'
Found 1166 hits with Last Name = 'huang' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 by radiometric assay |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129794

(CHEMBL328660 | [4-(6-Bromo-benzothiazol-2-yl)-phen...)Show InChI InChI=1S/C14H11BrN2S/c1-16-11-5-2-9(3-6-11)14-17-12-7-4-10(15)8-13(12)18-14/h2-8,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129784
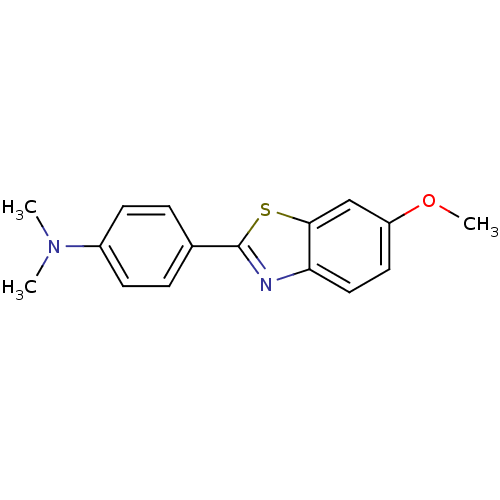
(4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylanil...)Show InChI InChI=1S/C16H16N2OS/c1-18(2)12-6-4-11(5-7-12)16-17-14-9-8-13(19-3)10-15(14)20-16/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100133
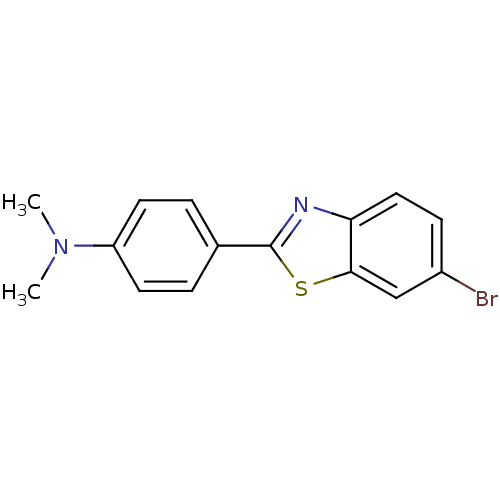
(CHEMBL55401 | [4-(6-Bromo-benzothiazol-2-yl)-pheny...)Show InChI InChI=1S/C15H13BrN2S/c1-18(2)12-6-3-10(4-7-12)15-17-13-8-5-11(16)9-14(13)19-15/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129791
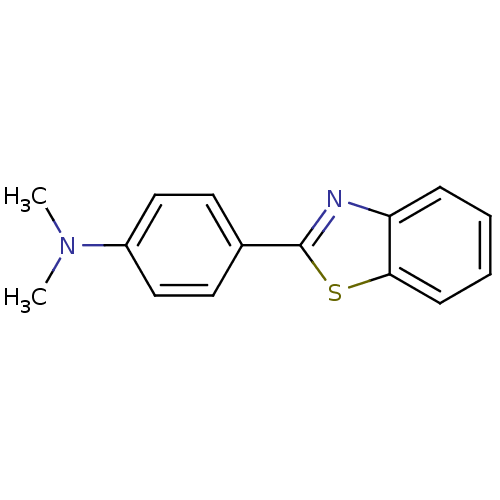
((4-Benzothiazol-2-yl-phenyl)-dimethyl-amine | 4-(B...)Show InChI InChI=1S/C15H14N2S/c1-17(2)12-9-7-11(8-10-12)15-16-13-5-3-4-6-14(13)18-15/h3-10H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129793
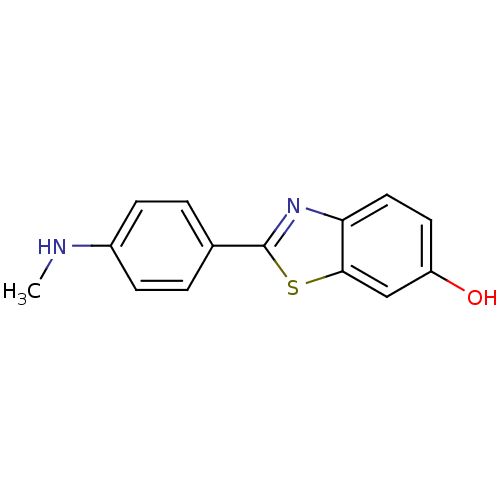
(2-(4''-methylaminophenyl)-6-hydroxybenzothiazole |...)Show InChI InChI=1S/C14H12N2OS/c1-15-10-4-2-9(3-5-10)14-16-12-7-6-11(17)8-13(12)18-14/h2-8,15,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129792
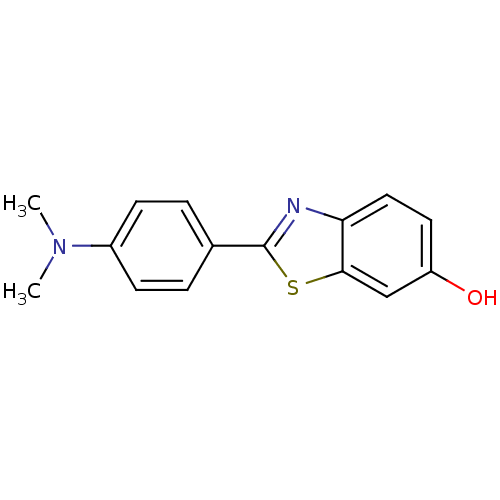
(2-(4-Dimethylamino-phenyl)-benzothiazol-6-ol | CHE...)Show InChI InChI=1S/C15H14N2OS/c1-17(2)11-5-3-10(4-6-11)15-16-13-8-7-12(18)9-14(13)19-15/h3-9,18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129786
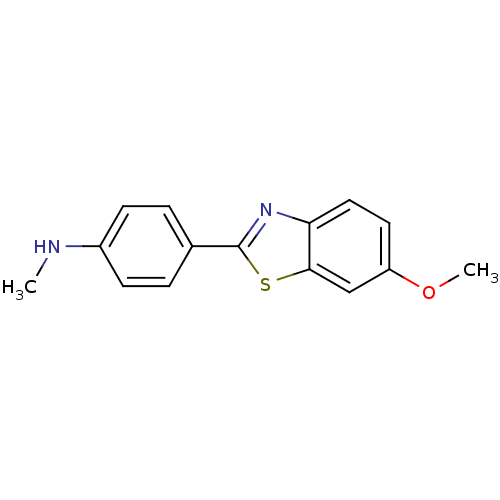
(CHEMBL94230 | [4-(6-Methoxy-benzothiazol-2-yl)-phe...)Show InChI InChI=1S/C15H14N2OS/c1-16-11-5-3-10(4-6-11)15-17-13-8-7-12(18-2)9-14(13)19-15/h3-9,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50052701
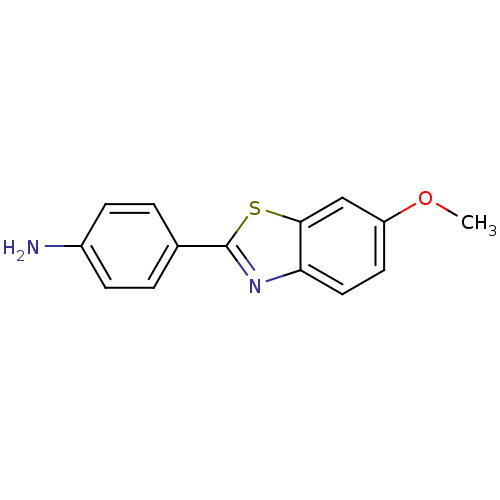
(2-(4-Aminophenyl)-6-methoxybenzothiazole | 4-(6-Me...)Show InChI InChI=1S/C14H12N2OS/c1-17-11-6-7-12-13(8-11)18-14(16-12)9-2-4-10(15)5-3-9/h2-8H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129783
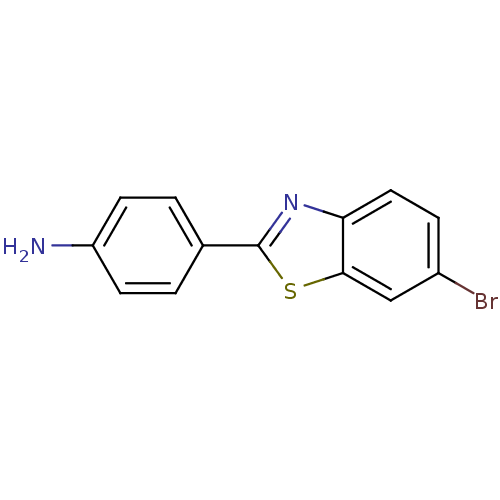
(4-(6-Bromo-benzothiazol-2-yl)-phenylamine | CHEMBL...)Show InChI InChI=1S/C13H9BrN2S/c14-9-3-6-11-12(7-9)17-13(16-11)8-1-4-10(15)5-2-8/h1-7H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129787
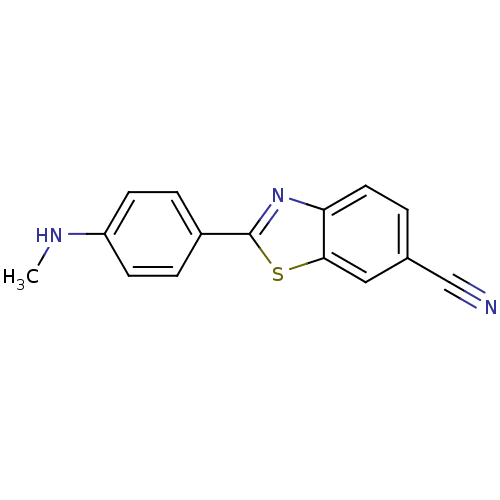
(2-(4-Methylamino-phenyl)-benzothiazole-6-carbonitr...)Show InChI InChI=1S/C15H11N3S/c1-17-12-5-3-11(4-6-12)15-18-13-7-2-10(9-16)8-14(13)19-15/h2-8,17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129788
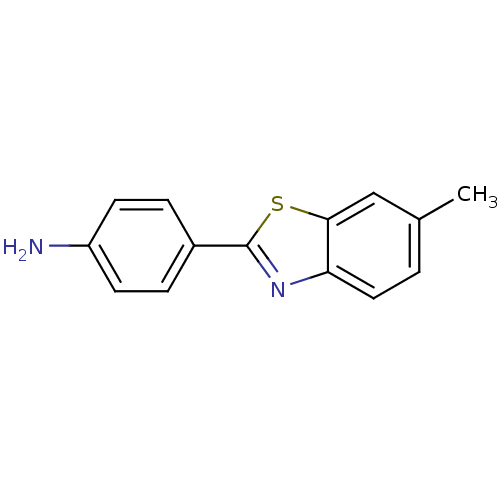
(2-(4-Aminophenyl)-6-methylbenzothiazole | 4-(6-Met...)Show InChI InChI=1S/C14H12N2S/c1-9-2-7-12-13(8-9)17-14(16-12)10-3-5-11(15)6-4-10/h2-8H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50109051
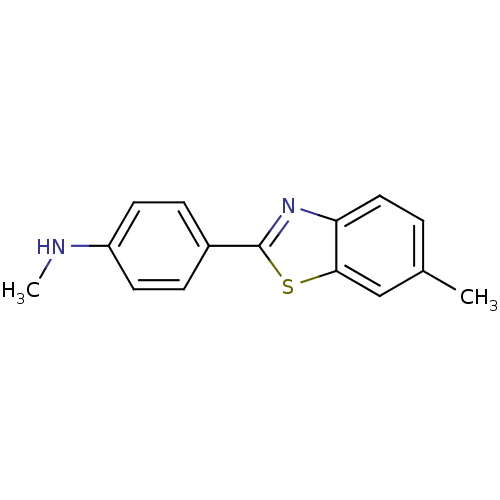
(CHEMBL330529 | Methyl-[4-(6-methyl-benzothiazol-2-...)Show InChI InChI=1S/C15H14N2S/c1-10-3-8-13-14(9-10)18-15(17-13)11-4-6-12(16-2)7-5-11/h3-9,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50109052
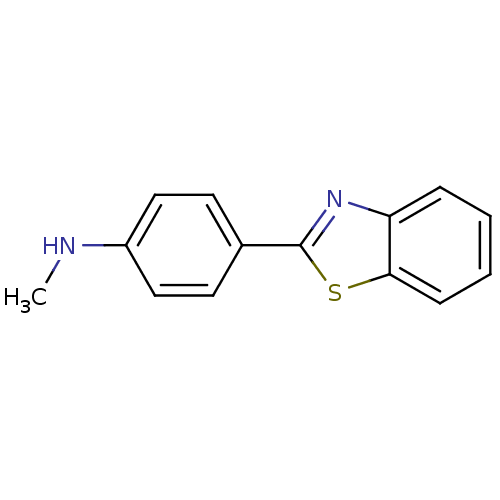
((4-Benzothiazol-2-yl-phenyl)-methyl-amine | 4-(ben...)Show InChI InChI=1S/C14H12N2S/c1-15-11-8-6-10(7-9-11)14-16-12-4-2-3-5-13(12)17-14/h2-9,15H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129785
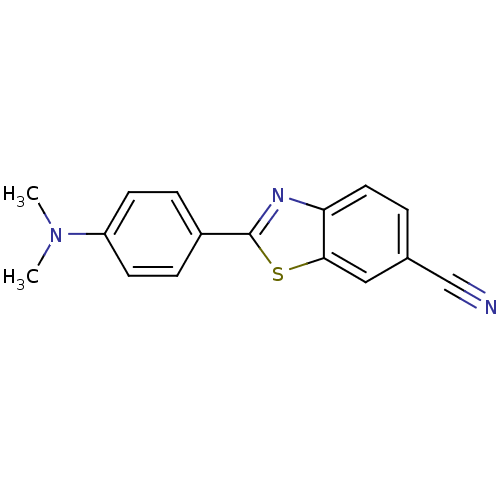
(2-(4-Dimethylamino-phenyl)-benzothiazole-6-carboni...)Show InChI InChI=1S/C16H13N3S/c1-19(2)13-6-4-12(5-7-13)16-18-14-8-3-11(10-17)9-15(14)20-16/h3-9H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50052702
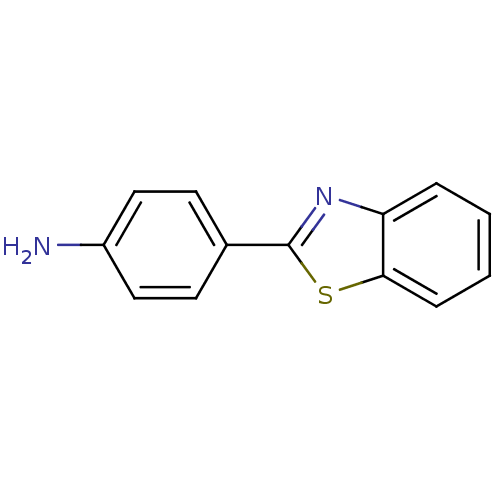
(2-(4-Aminophenyl)benzothiazole | 4-Benzothiazol-2-...)Show InChI InChI=1S/C13H10N2S/c14-10-7-5-9(6-8-10)13-15-11-3-1-2-4-12(11)16-13/h1-8H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of AXL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50052710
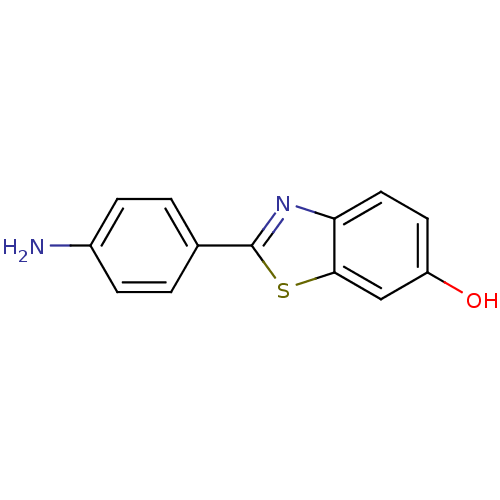
(2-(4-Amino-phenyl)-benzothiazol-6-ol | CHEMBL93884)Show InChI InChI=1S/C13H10N2OS/c14-9-3-1-8(2-4-9)13-15-11-6-5-10(16)7-12(11)17-13/h1-7,16H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129790

(CHEMBL329640 | Dimethyl-[4-(6-methyl-benzothiazol-...)Show InChI InChI=1S/C16H16N2S/c1-11-4-9-14-15(10-11)19-16(17-14)12-5-7-13(8-6-12)18(2)3/h4-10H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50129789
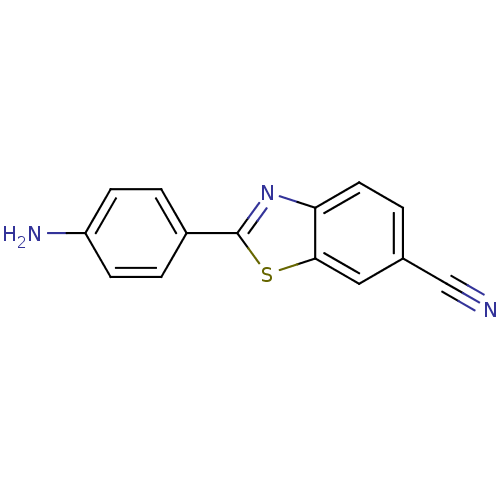
(2-(4-Amino-phenyl)-benzothiazole-6-carbonitrile | ...)Show InChI InChI=1S/C14H9N3S/c15-8-9-1-6-12-13(7-9)18-14(17-12)10-2-4-11(16)5-3-10/h1-7H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates in competition with [N-methyl-3H] BTA-1. |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053435
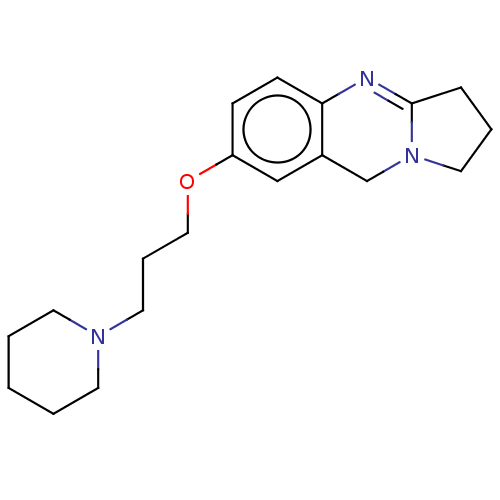
(CHEMBL3323035)Show InChI InChI=1S/C19H27N3O/c1-2-9-21(10-3-1)11-5-13-23-17-7-8-18-16(14-17)15-22-12-4-6-19(22)20-18/h7-8,14H,1-6,9-13,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
Bioorg Med Chem 22: 4867-81 (2014)
Article DOI: 10.1016/j.bmc.2014.06.045
BindingDB Entry DOI: 10.7270/Q25X2BK0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of cMET |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Lysine--tRNA ligase
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of SYK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Jak3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of ABL |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50100134

(2-(4-Dimethylamino-phenyl)-3,6-dimethyl-benzothiaz...)Show InChI InChI=1S/C17H19N2S/c1-12-5-10-15-16(11-12)20-17(19(15)4)13-6-8-14(9-7-13)18(2)3/h5-11H,1-4H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Binding affinity for Amyloid beta 1-40 aggregates fibrils in competition with BTA-1 |
J Med Chem 46: 2740-54 (2003)
Article DOI: 10.1021/jm030026b
BindingDB Entry DOI: 10.7270/Q23R0S87 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by ChEMBL
| Assay Description
Inhibition of Src |
J Med Chem 55: 725-34 (2012)
Article DOI: 10.1021/jm201198w
BindingDB Entry DOI: 10.7270/Q2GQ6Z6R |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50056333

(CHEMBL3322470)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCNCc1ccc(CNCCCCNC(=O)c2nn(c(c2C)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C50H48Cl6N8O2/c1-31-45(61-63(43-21-19-39(53)27-41(43)55)47(31)35-11-15-37(51)16-12-35)49(65)59-25-5-3-23-57-29-33-7-9-34(10-8-33)30-58-24-4-6-26-60-50(66)46-32(2)48(36-13-17-38(52)18-14-36)64(62-46)44-22-20-40(54)28-42(44)56/h7-22,27-28,57-58H,3-6,23-26,29-30H2,1-2H3,(H,59,65)(H,60,66) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 24: 4209-14 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.038
BindingDB Entry DOI: 10.7270/Q26M38HV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50056338
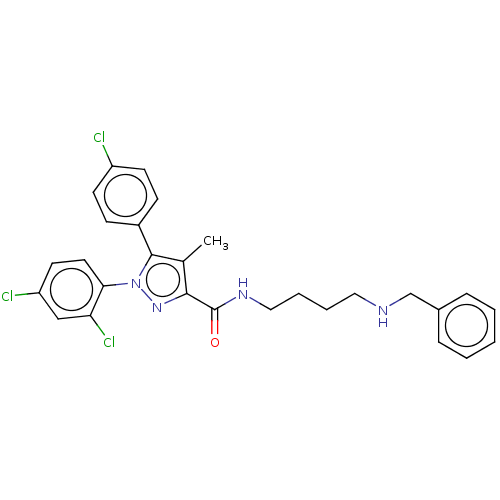
(CHEMBL3322475)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCNCc1ccccc1 Show InChI InChI=1S/C28H27Cl3N4O/c1-19-26(28(36)33-16-6-5-15-32-18-20-7-3-2-4-8-20)34-35(25-14-13-23(30)17-24(25)31)27(19)21-9-11-22(29)12-10-21/h2-4,7-14,17,32H,5-6,15-16,18H2,1H3,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 24: 4209-14 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.038
BindingDB Entry DOI: 10.7270/Q26M38HV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50056339
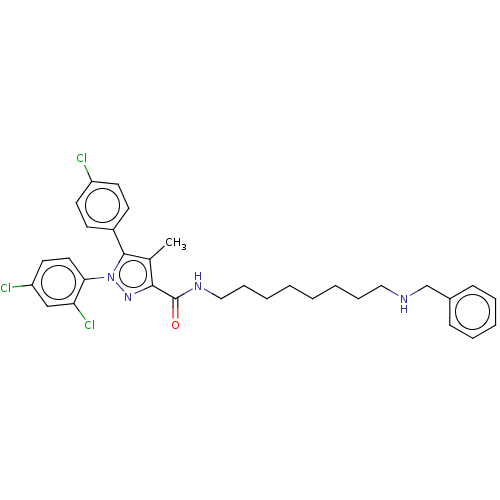
(CHEMBL3322476)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCCNCc1ccccc1 Show InChI InChI=1S/C32H35Cl3N4O/c1-23-30(32(40)37-20-10-5-3-2-4-9-19-36-22-24-11-7-6-8-12-24)38-39(29-18-17-27(34)21-28(29)35)31(23)25-13-15-26(33)16-14-25/h6-8,11-18,21,36H,2-5,9-10,19-20,22H2,1H3,(H,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius-Maximilians-Universit£t W£rzburg
Curated by ChEMBL
| Assay Description
Displacement of [3H] CP-55,940 from human CB2 receptor expressed in HEK cells at 10 uM after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 24: 4209-14 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.038
BindingDB Entry DOI: 10.7270/Q26M38HV |
More data for this
Ligand-Target Pair | |
mRNA cap guanine-N7 methyltransferase
(Homo sapiens) | BDBM50470562
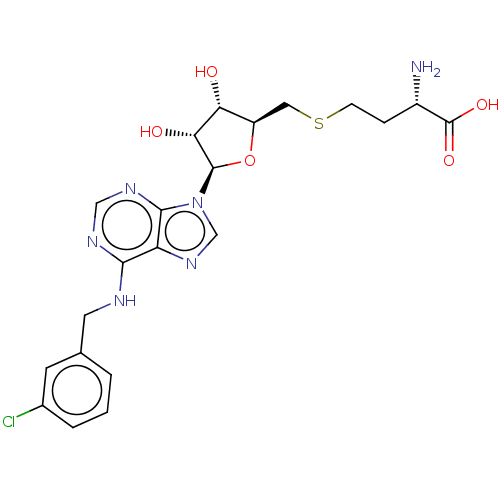
(CHEMBL1230055)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C21H25ClN6O5S/c22-12-3-1-2-11(6-12)7-24-18-15-19(26-9-25-18)28(10-27-15)20-17(30)16(29)14(33-20)8-34-5-4-13(23)21(31)32/h1-3,6,9-10,13-14,16-17,20,29-30H,4-5,7-8,23H2,(H,31,32)(H,24,25,26)/t13-,14+,16+,17+,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged RNMT (1 to 476 residues) expressed in Escherichia coli BL21 using 5'-GpppAGAACCUG-biotin-TEG-3 ... |
Eur J Med Chem 157: 994-1004 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.057
BindingDB Entry DOI: 10.7270/Q2V98BSZ |
More data for this
Ligand-Target Pair | |
DNA (cytosine-5)-methyltransferase 1
(Homo sapiens (Human)) | BDBM50470562

(CHEMBL1230055)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C21H25ClN6O5S/c22-12-3-1-2-11(6-12)7-24-18-15-19(26-9-25-18)28(10-27-15)20-17(30)16(29)14(33-20)8-34-5-4-13(23)21(31)32/h1-3,6,9-10,13-14,16-17,20,29-30H,4-5,7-8,23H2,(H,31,32)(H,24,25,26)/t13-,14+,16+,17+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human DNMT1A using double-stranded hemi-DNA oligonucleotide as substrate measured after 40 mins by [3H]methyl incorporation assay |
Eur J Med Chem 157: 994-1004 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.057
BindingDB Entry DOI: 10.7270/Q2V98BSZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562254
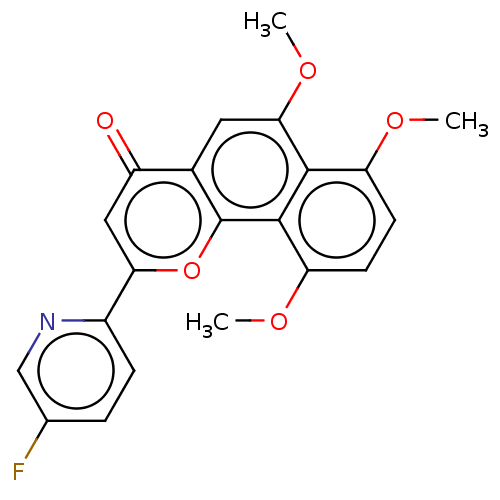
(CHEMBL4749123)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1ccc(F)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562252
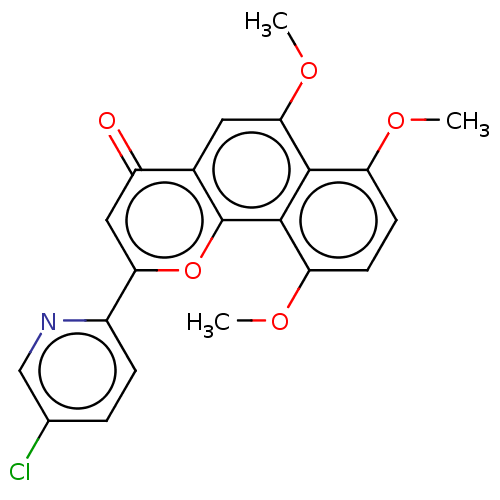
(CHEMBL4798651)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1ccc(Cl)cn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Ubiquitin thioesterase OTUB1
(Homo sapiens) | BDBM50591324
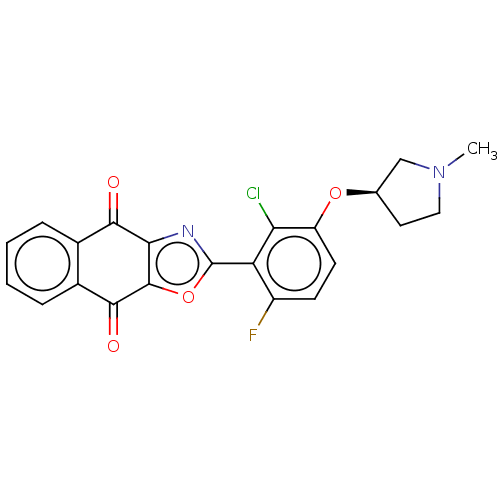
(CHEMBL5185273)Show SMILES CN1CC[C@H](C1)Oc1ccc(F)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1Cl |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 8
(Homo sapiens (Human)) | BDBM50591324

(CHEMBL5185273)Show SMILES CN1CC[C@H](C1)Oc1ccc(F)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562251
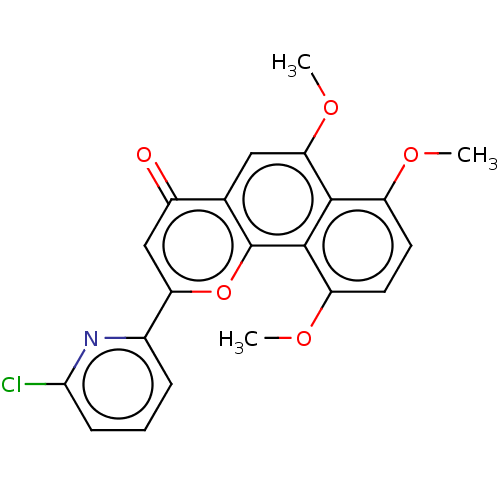
(CHEMBL4759410)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1cccc(Cl)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562250
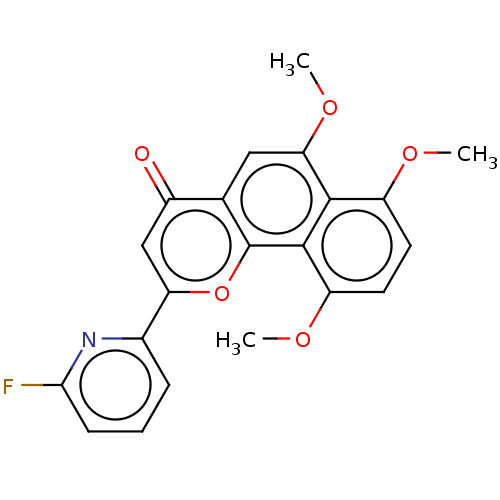
(CHEMBL4799247)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1cccc(F)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Ubiquitin thioesterase OTUB1
(Homo sapiens) | BDBM50591323
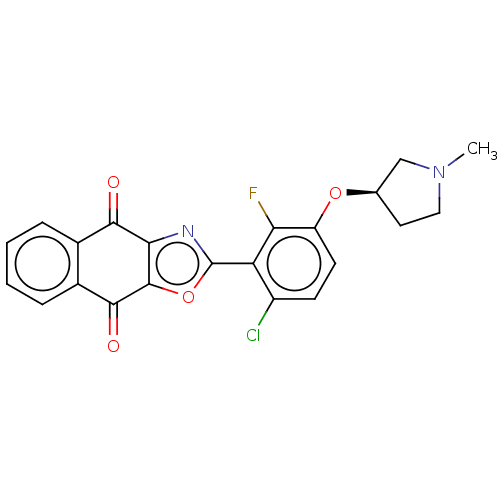
(CHEMBL5173239)Show SMILES CN1CC[C@H](C1)Oc1ccc(Cl)c(-c2nc3c(o2)C(=O)c2ccccc2C3=O)c1F |r| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00408
BindingDB Entry DOI: 10.7270/Q25H7M81 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562243
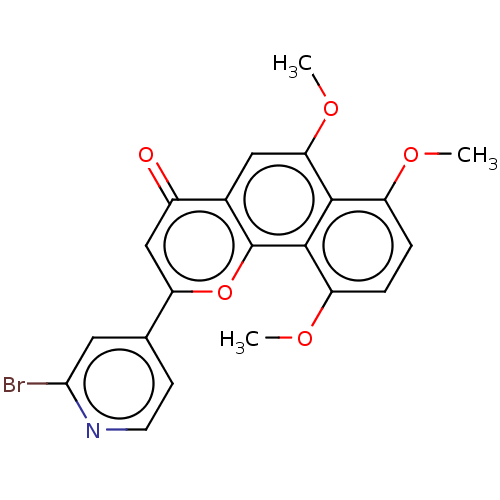
(CHEMBL4782753)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1ccnc(Br)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562229
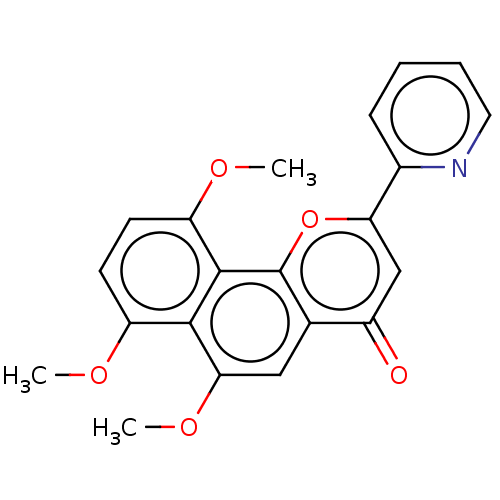
(CHEMBL4755282)Show SMILES COc1ccc(OC)c2c3oc(cc(=O)c3cc(OC)c12)-c1ccccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50593816
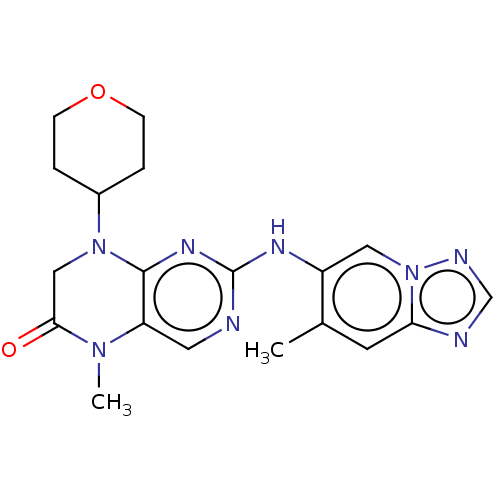
(CHEMBL5191459)Show SMILES CN1C(=O)CN(C2CCOCC2)c2nc(Nc3cn4ncnc4cc3C)ncc12 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114401
BindingDB Entry DOI: 10.7270/Q29C72D2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562253
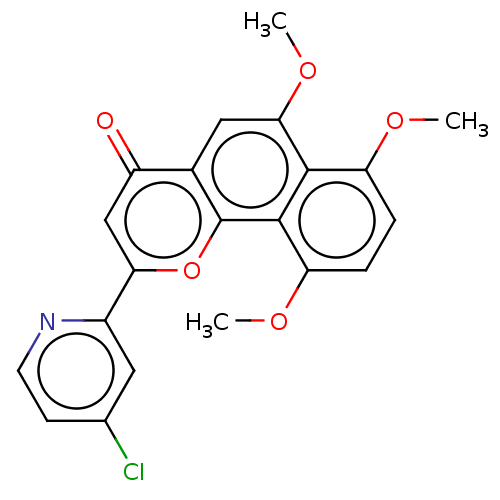
(CHEMBL4798694)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1cc(Cl)ccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50562222
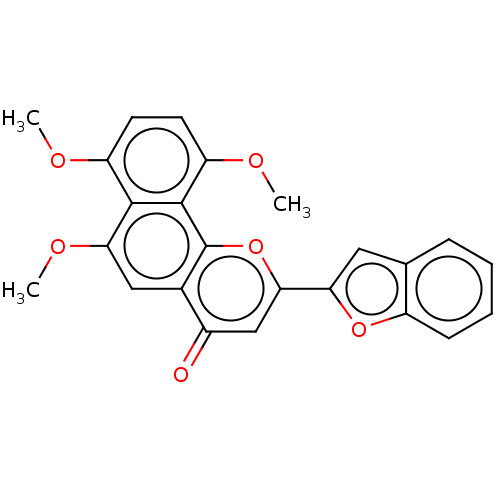
(CHEMBL4747913)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1cc2ccccc2o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1B1 using 7-ethoxyresorufin as substrate after 35 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data