

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475328 (CHEMBL414782) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475327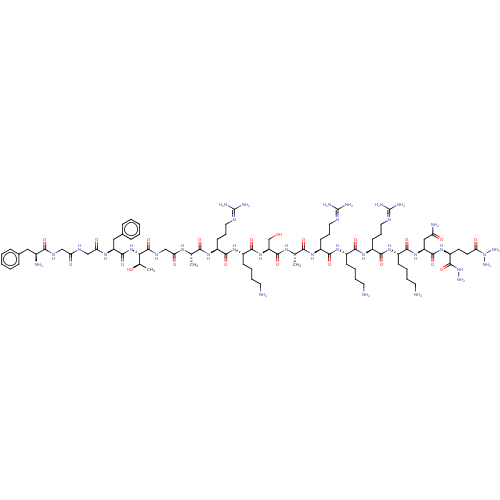 (CHEMBL410653) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50214385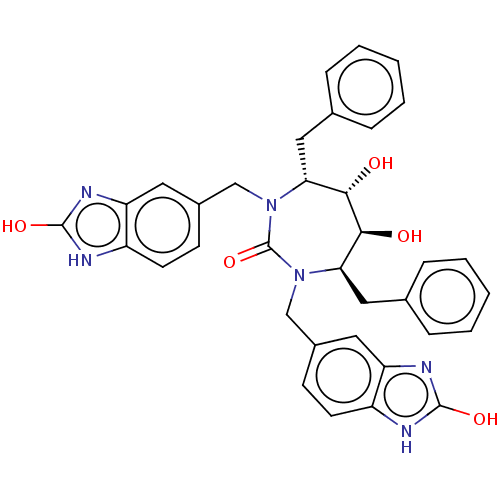 (CHEMBL316681) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160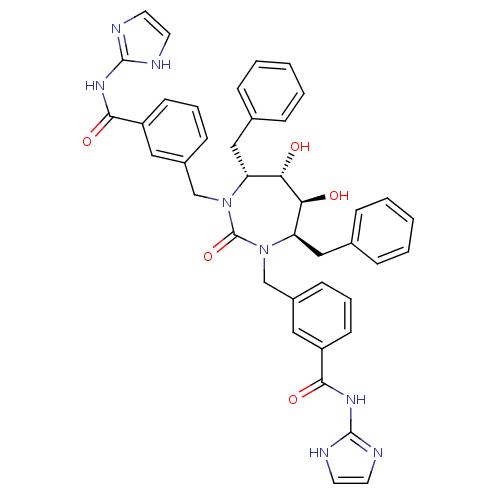 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065075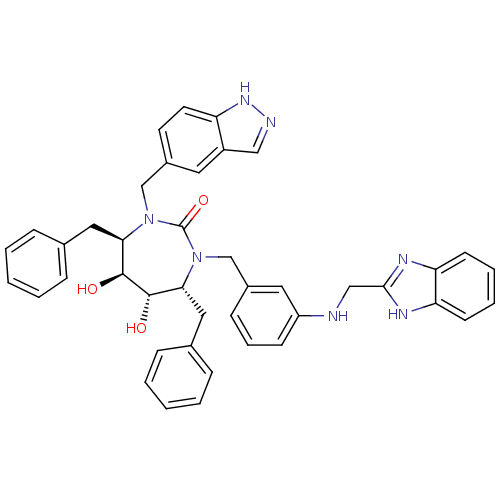 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065080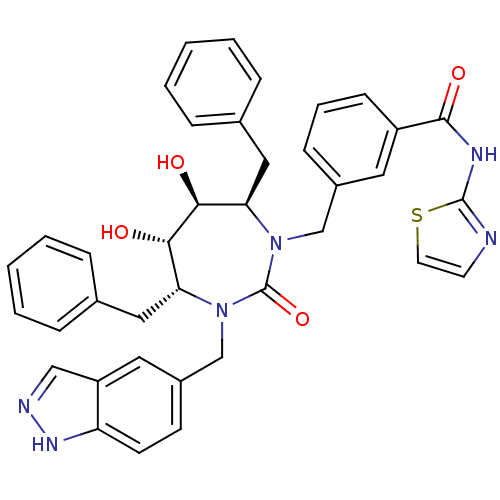 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065086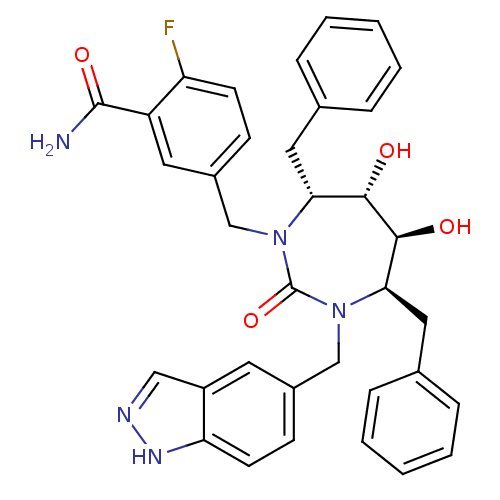 (5-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161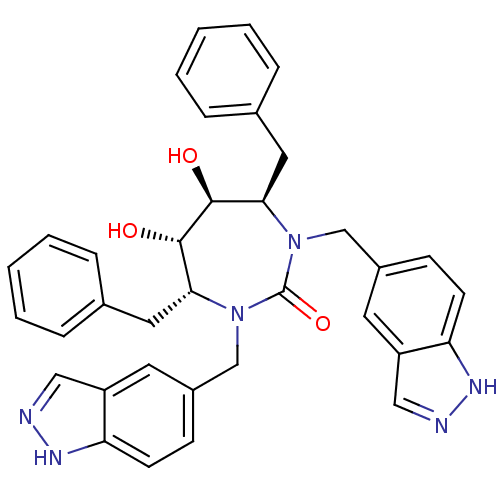 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288430 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089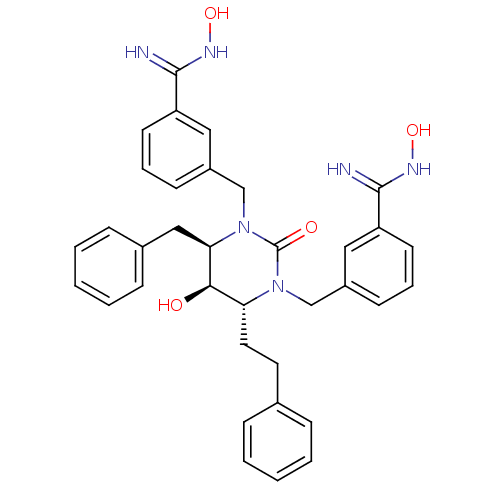 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065078 ((4R,5S,6S,7R)-1-(3-Amino-4-fluoro-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM161 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity was evaluated against HIV protease | Bioorg Med Chem Lett 6: 2919-2924 (1996) Article DOI: 10.1016/S0960-894X(96)00531-8 BindingDB Entry DOI: 10.7270/Q2QF8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475330 (CHEMBL442297) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM86660 (OFQ/N UFP-102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology Curated by PDSP Ki Database | J Pharmacol Exp Ther 312: 1114-23 (2005) Article DOI: 10.1124/jpet.104.077339 BindingDB Entry DOI: 10.7270/Q2222SC4 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065087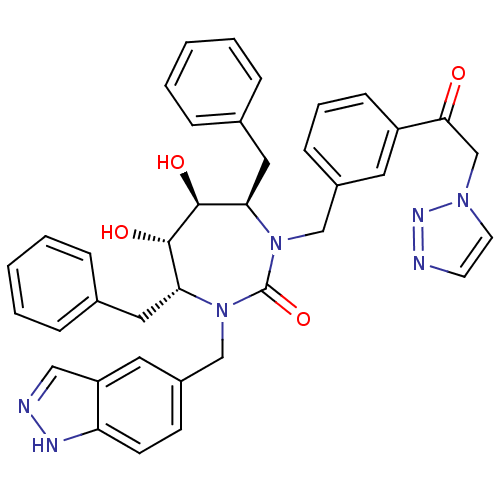 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065071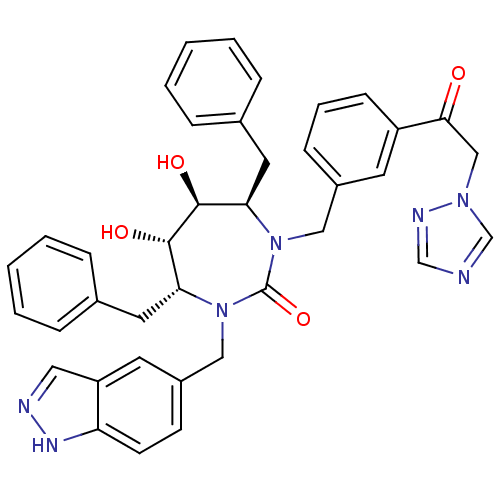 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475333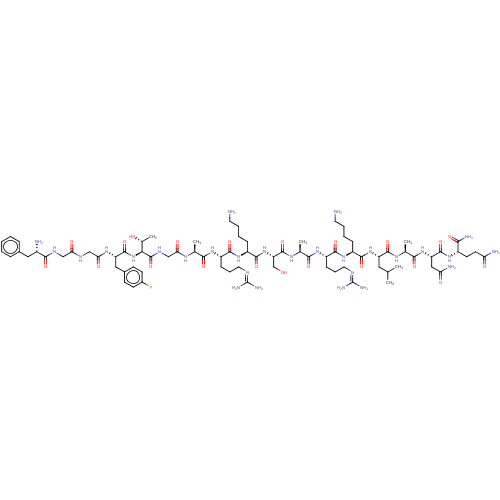 (CHEMBL264846) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM164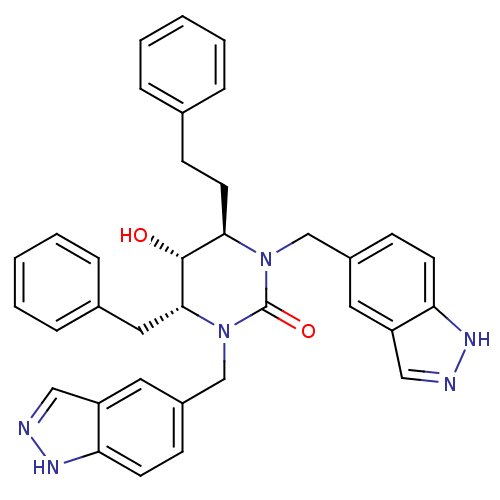 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155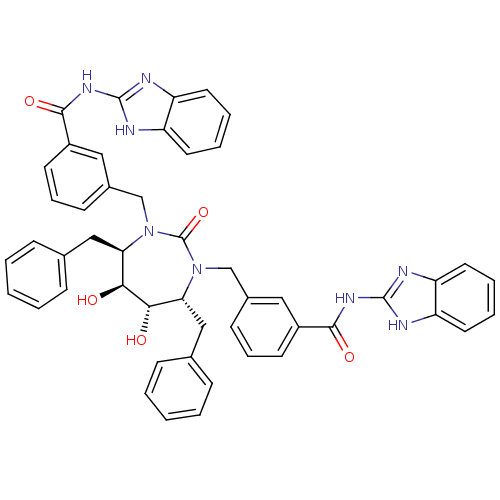 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065060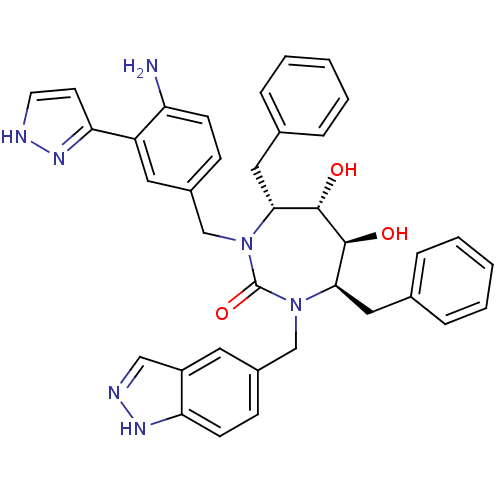 ((4R,5S,6S,7R)-1-[4-Amino-3-(2H-pyrazol-3-yl)-benzy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475331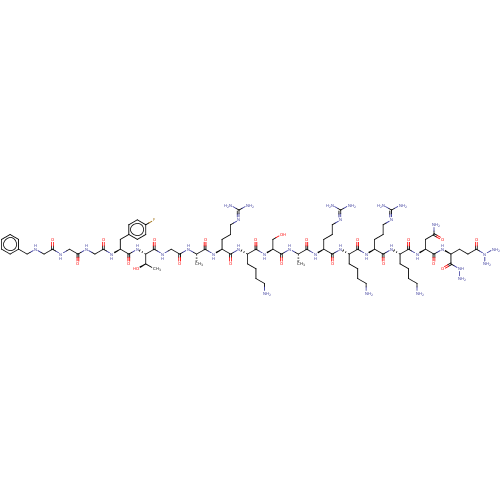 (CHEMBL411649) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065079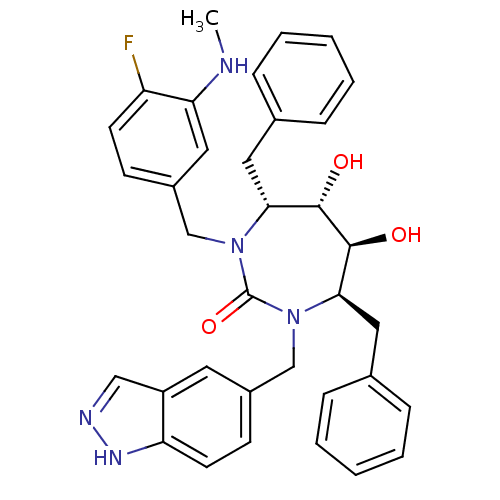 ((4R,5S,6S,7R)-4,7-Dibenzyl-1-(4-fluoro-3-methylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM154 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065069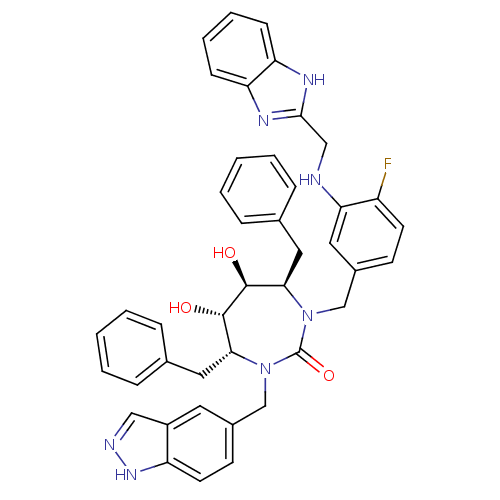 ((4R,5S,6S,7R)-1-{3-[(1H-Benzoimidazol-2-ylmethyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065067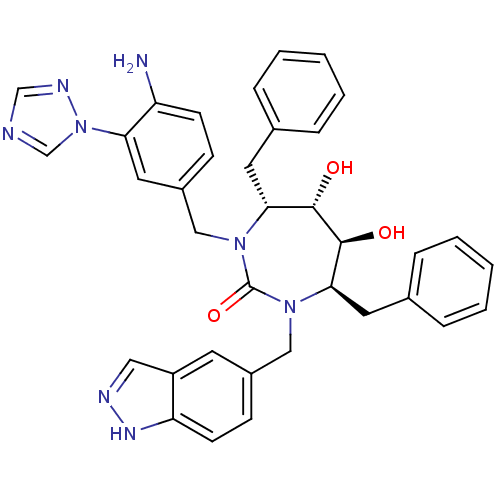 ((4R,5S,6S,7R)-1-(4-Amino-3-[1,2,4]triazol-1-yl-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7026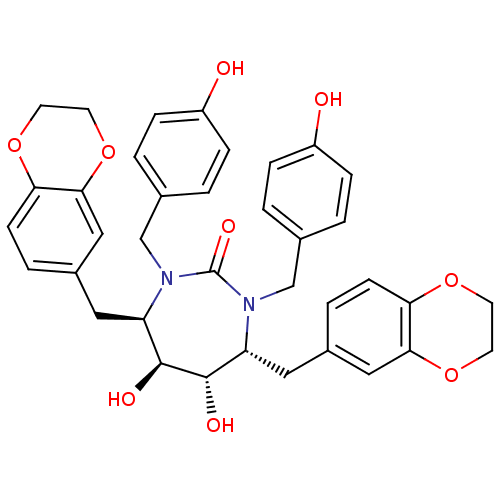 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065064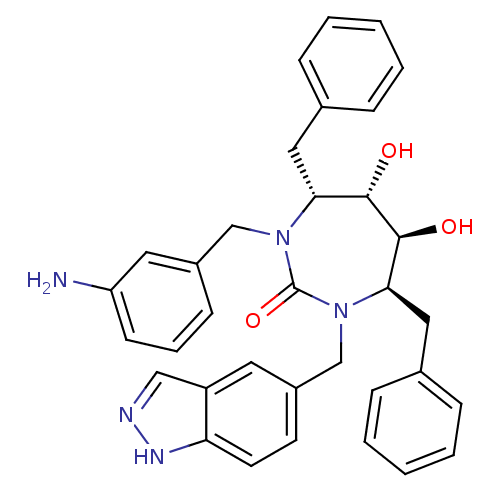 ((4R,5S,6S,7R)-1-(3-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065082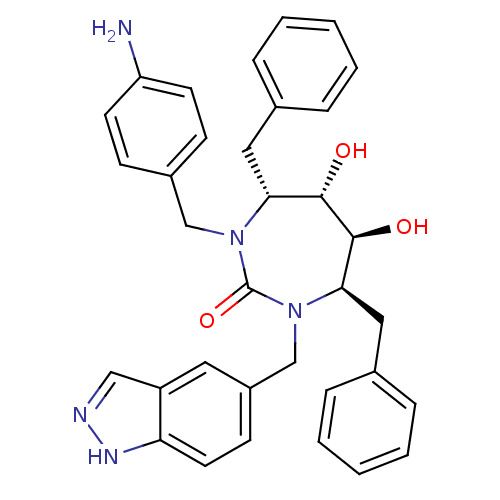 ((4R,5S,6S,7R)-1-(4-Amino-benzyl)-4,7-dibenzyl-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475325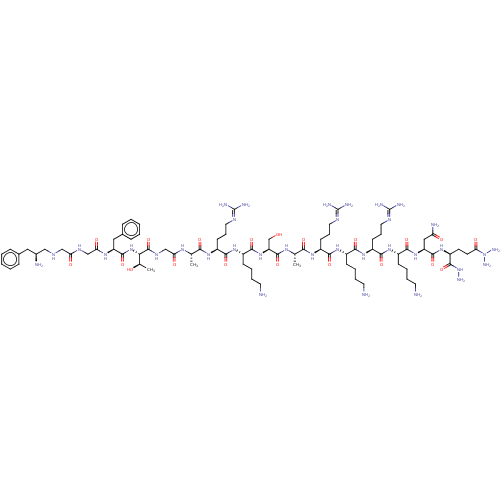 (CHEMBL262544) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065074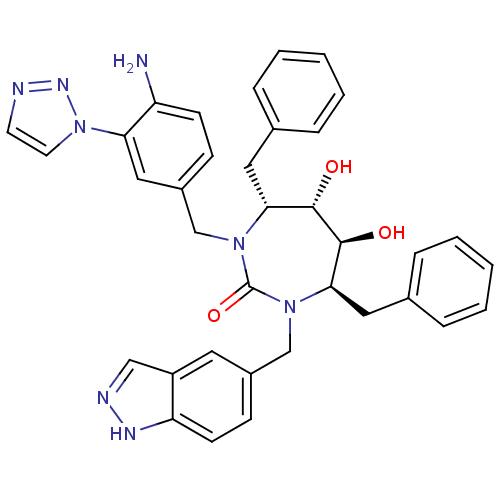 ((4R,5S,6S,7R)-1-(4-Amino-3-[1,2,3]triazol-1-yl-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065091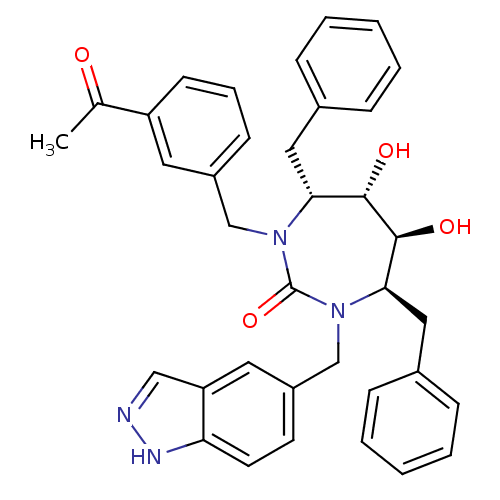 ((4R,5S,6S,7R)-1-(3-Acetyl-benzyl)-4,7-dibenzyl-5,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065068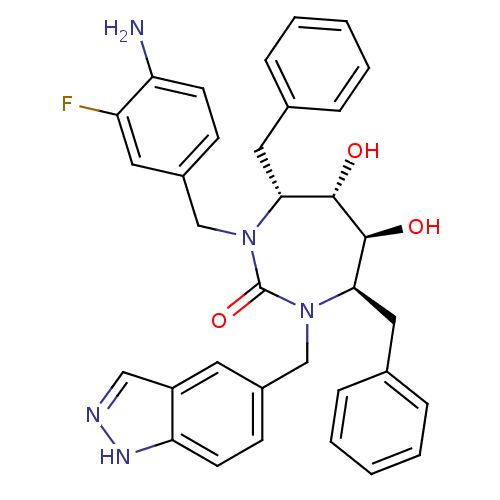 ((4R,5S,6S,7R)-1-(4-Amino-3-fluoro-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM156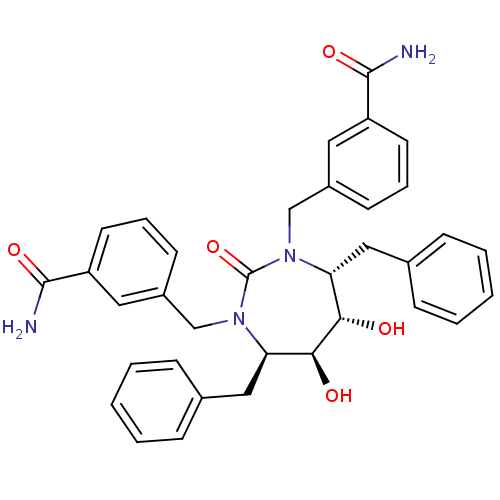 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(3-carbamoylphen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065065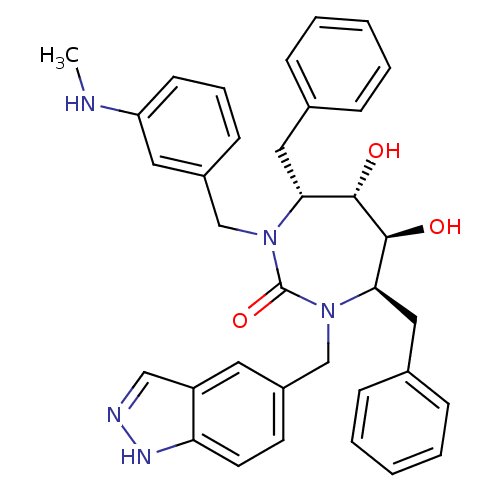 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7024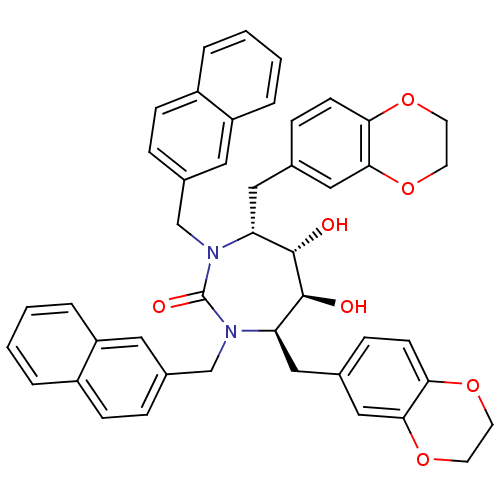 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7020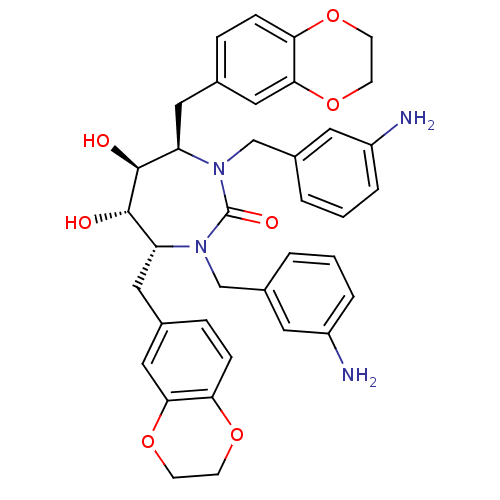 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7019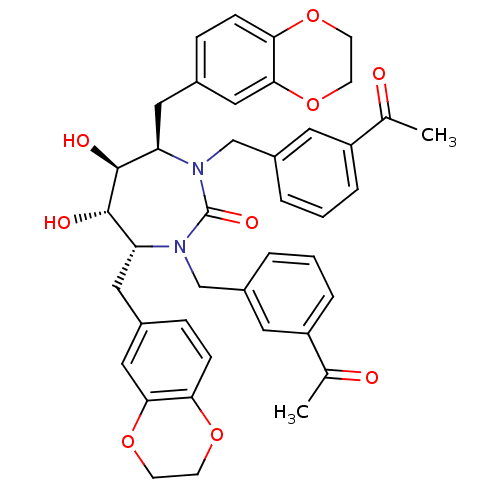 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1,4-benzodioxin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065073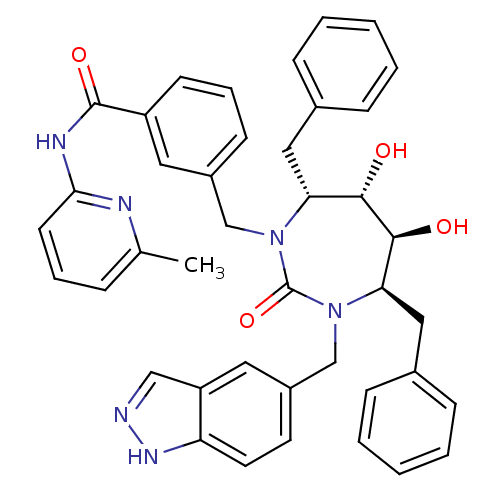 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065083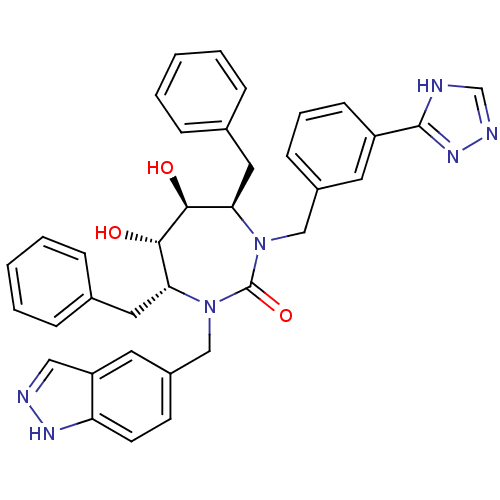 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM159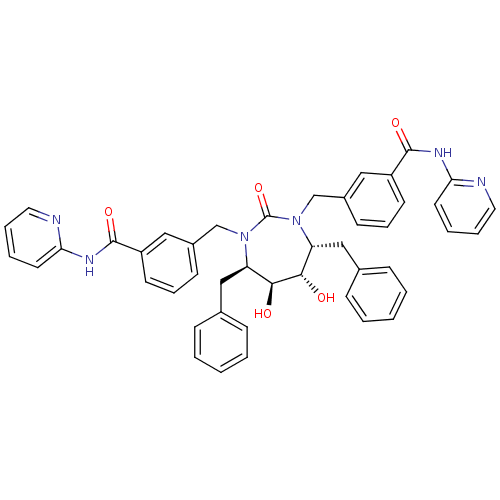 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065092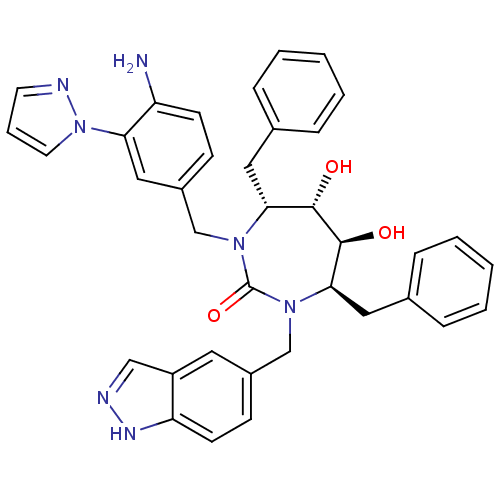 ((4R,5S,6S,7R)-1-(4-Amino-3-pyrazol-1-yl-benzyl)-4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM168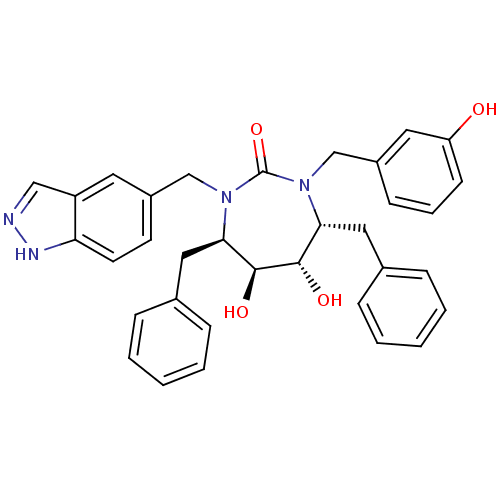 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-[(3-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065085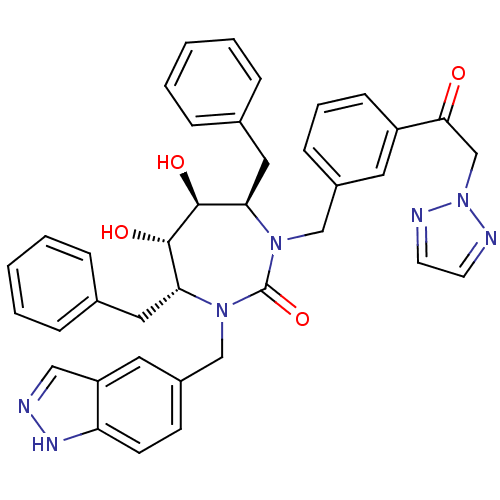 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM7028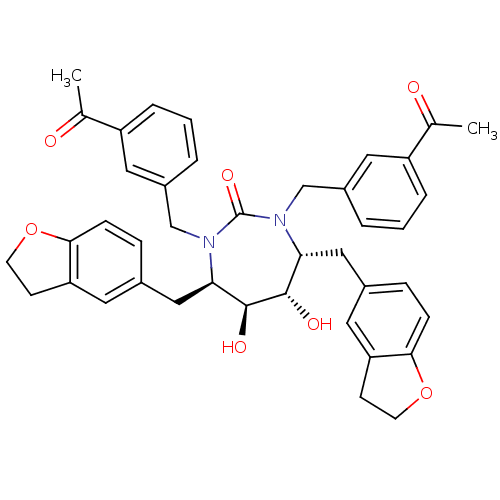 ((4R,5S,6S,7R)-4,7-bis(2,3-dihydro-1-benzofuran-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | -61.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 40: 1465-74 (1997) Article DOI: 10.1021/jm960839i BindingDB Entry DOI: 10.7270/Q29021Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065090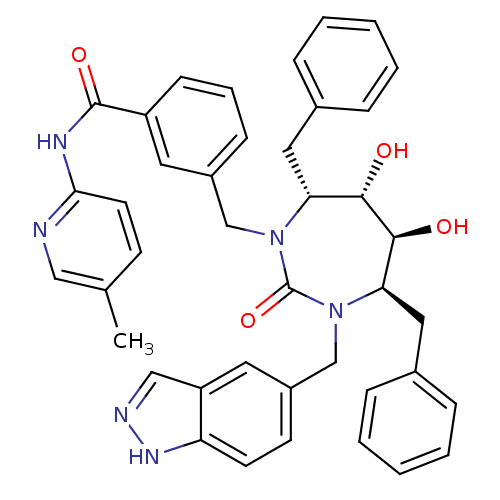 (3-[(4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50475329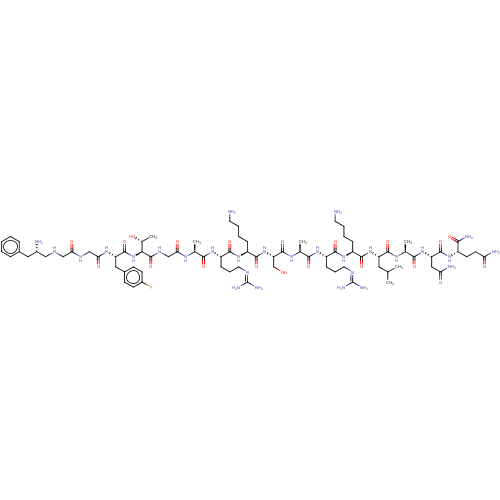 (CHEMBL263588) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Receptor binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178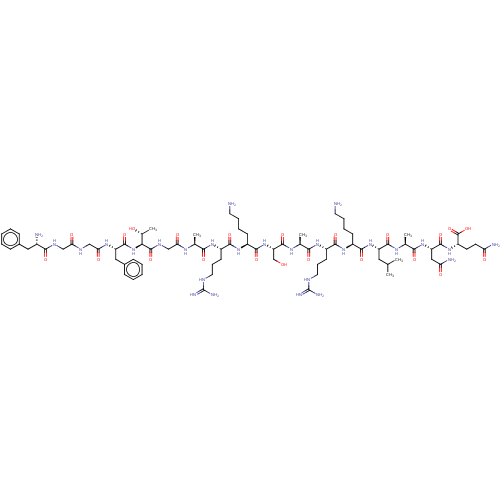 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Binding affinity for recombinant human N/OFQ peptide receptor (NOP) expressed in chinese hamster ovary cells | J Med Chem 48: 1421-7 (2005) Article DOI: 10.1021/jm040106v BindingDB Entry DOI: 10.7270/Q2J38WBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM167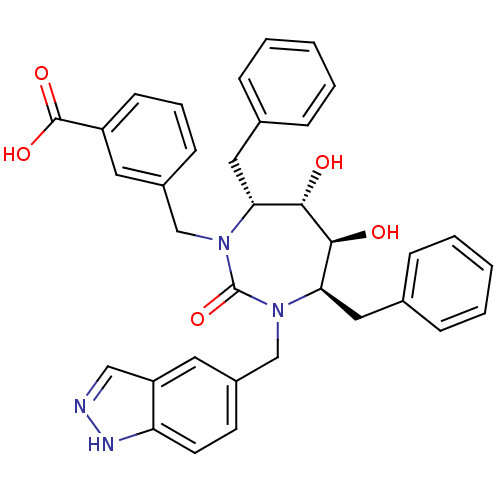 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-(1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065066 ((4R,5S,6S,7R)-1-(3-Amino-4-methyl-benzyl)-4,7-dibe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM169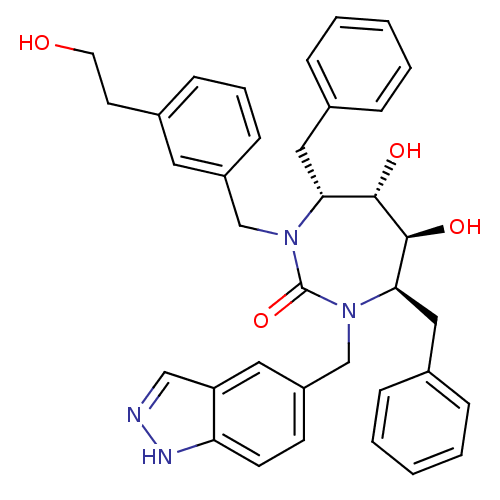 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3-(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dupont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | J Med Chem 41: 2411-23 (1998) Article DOI: 10.1021/jm980103g BindingDB Entry DOI: 10.7270/Q2H41QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2799 total ) | Next | Last >> |