 Found 35 hits with Last Name = 'lippens' and Initial = 'g'
Found 35 hits with Last Name = 'lippens' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V2 receptor
(Sus scrofa) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition to V2 subtype receptor using [3H]- (VS2) as radioligand at 3 nM and arginine-vasopressin at 2 microM in LLCPKI cells |
Bioorg Med Chem Lett 7: 719-724 (1997)
Article DOI: 10.1016/S0960-894X(97)00050-4
BindingDB Entry DOI: 10.7270/Q2QC03HW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM50291329
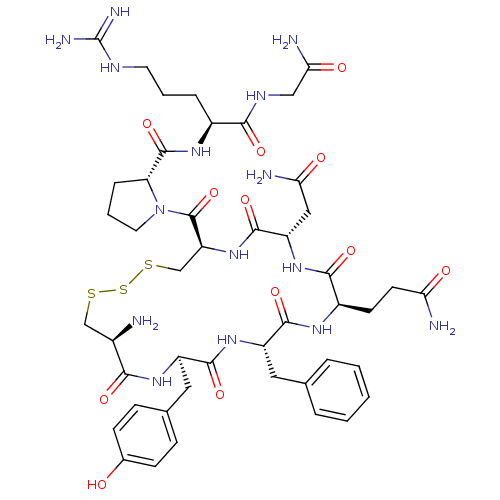
(8-arginine vasopressin trisulphide | CHEMBL267405)Show SMILES N[C@@H]1CSSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N15O12S3/c47-27-22-74-76-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28+,29-,30-,31+,32+,33+,34-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the TYR(Me)2 arginine-vasopressin as radioligand at 0.3 nM in A7r5 cells |
Bioorg Med Chem Lett 7: 719-724 (1997)
Article DOI: 10.1016/S0960-894X(97)00050-4
BindingDB Entry DOI: 10.7270/Q2QC03HW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Sus scrofa) | BDBM50291329

(8-arginine vasopressin trisulphide | CHEMBL267405)Show SMILES N[C@@H]1CSSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCC(N)=O)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccc(O)cc2)NC1=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O Show InChI InChI=1S/C46H65N15O12S3/c47-27-22-74-76-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28+,29-,30-,31+,32+,33+,34-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition to V2 subtype receptor using [3H]- (VS2) as radioligand at 3 nM and arginine-vasopressin at 2 microM in LLCPKI cells |
Bioorg Med Chem Lett 7: 719-724 (1997)
Article DOI: 10.1016/S0960-894X(97)00050-4
BindingDB Entry DOI: 10.7270/Q2QC03HW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a/V1b receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the TYR(Me)2 arginine-vasopressin as radioligand at 0.3 nM in A7r5 cells |
Bioorg Med Chem Lett 7: 719-724 (1997)
Article DOI: 10.1016/S0960-894X(97)00050-4
BindingDB Entry DOI: 10.7270/Q2QC03HW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170733
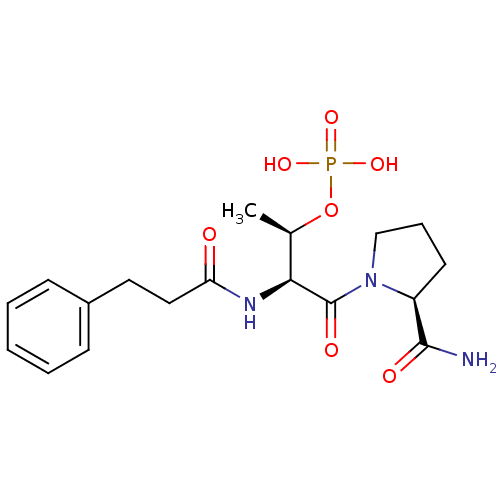
(CHEMBL365724 | Phosphoric acid mono-[(1S,3S)-3-(2-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C18H26N3O7P/c1-12(28-29(25,26)27)16(18(24)21-11-5-8-14(21)17(19)23)20-15(22)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14,16H,5,8-11H2,1H3,(H2,19,23)(H,20,22)(H2,25,26,27)/t12-,14+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170734
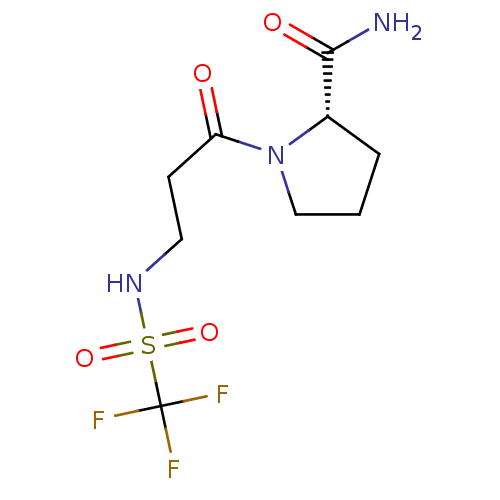
((S)-1-(3-Trifluoromethanesulfonylamino-propionyl)-...)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCNS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C9H14F3N3O4S/c10-9(11,12)20(18,19)14-4-3-7(16)15-5-1-2-6(15)8(13)17/h6,14H,1-5H2,(H2,13,17)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170735
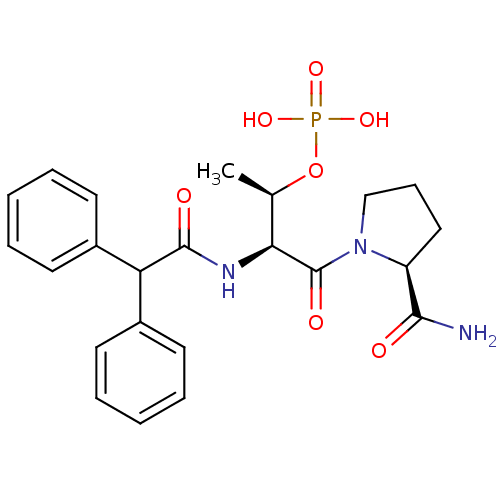
(CHEMBL364227 | Phosphoric acid mono-[(1S,3S)-3-(2-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C23H28N3O7P/c1-15(33-34(30,31)32)20(23(29)26-14-8-13-18(26)21(24)27)25-22(28)19(16-9-4-2-5-10-16)17-11-6-3-7-12-17/h2-7,9-12,15,18-20H,8,13-14H2,1H3,(H2,24,27)(H,25,28)(H2,30,31,32)/t15-,18+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170736

(CHEMBL188029 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1CSC[C@H]1C(N)=O Show InChI InChI=1S/C10H18N3O7PS/c1-5(20-21(17,18)19)8(12-6(2)14)10(16)13-4-22-3-7(13)9(11)15/h5,7-8H,3-4H2,1-2H3,(H2,11,15)(H,12,14)(H2,17,18,19)/t5-,7+,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170737
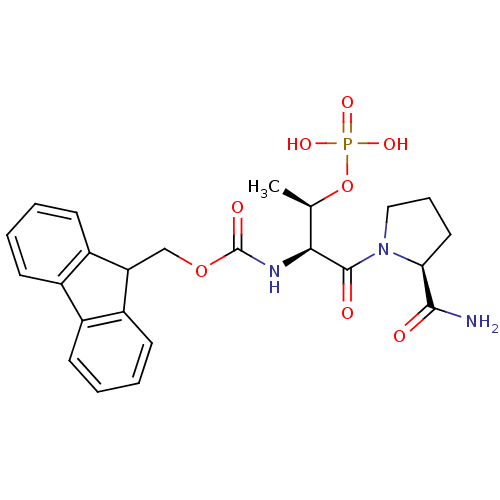
(CHEMBL360409 | [(S)-1-((S)-2-Carbamoyl-pyrrolidine...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C24H28N3O8P/c1-14(35-36(31,32)33)21(23(29)27-12-6-11-20(27)22(25)28)26-24(30)34-13-19-17-9-4-2-7-15(17)16-8-3-5-10-18(16)19/h2-5,7-10,14,19-21H,6,11-13H2,1H3,(H2,25,28)(H,26,30)(H2,31,32,33)/t14-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170742
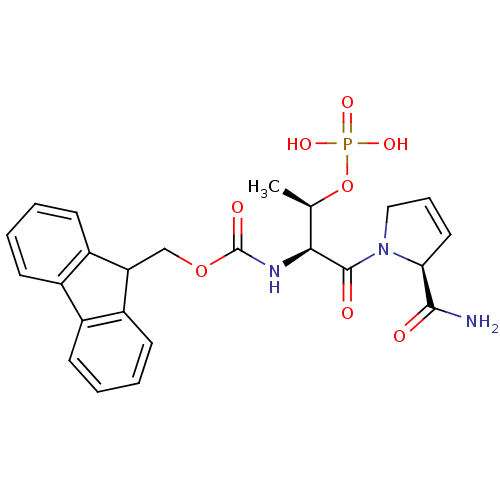
(CHEMBL187793 | [(S)-1-((S)-2-Carbamoyl-2,5-dihydro...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)N1CC=C[C@H]1C(N)=O |c:33| Show InChI InChI=1S/C24H26N3O8P/c1-14(35-36(31,32)33)21(23(29)27-12-6-11-20(27)22(25)28)26-24(30)34-13-19-17-9-4-2-7-15(17)16-8-3-5-10-18(16)19/h2-11,14,19-21H,12-13H2,1H3,(H2,25,28)(H,26,30)(H2,31,32,33)/t14-,20+,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170738
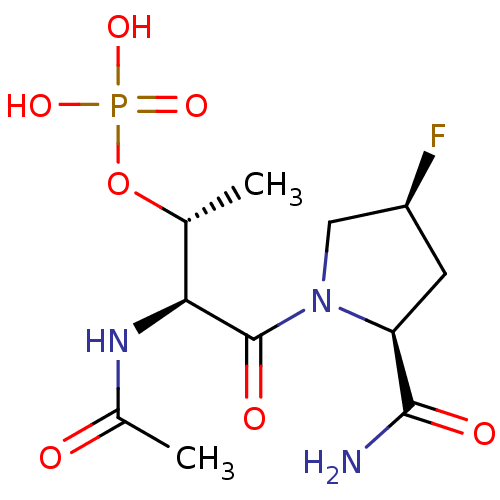
(CHEMBL365768 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1C[C@@H](F)C[C@H]1C(N)=O Show InChI InChI=1S/C11H19FN3O7P/c1-5(22-23(19,20)21)9(14-6(2)16)11(18)15-4-7(12)3-8(15)10(13)17/h5,7-9H,3-4H2,1-2H3,(H2,13,17)(H,14,16)(H2,19,20,21)/t5-,7+,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170739
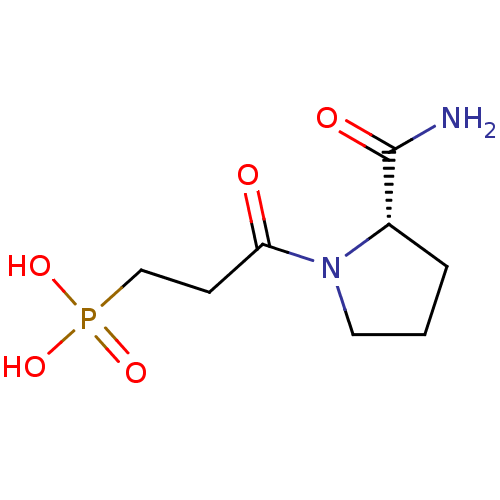
(CHEMBL187549 | [3-((S)-2-Carbamoyl-pyrrolidin-1-yl...)Show InChI InChI=1S/C8H15N2O5P/c9-8(12)6-2-1-4-10(6)7(11)3-5-16(13,14)15/h6H,1-5H2,(H2,9,12)(H2,13,14,15)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170741
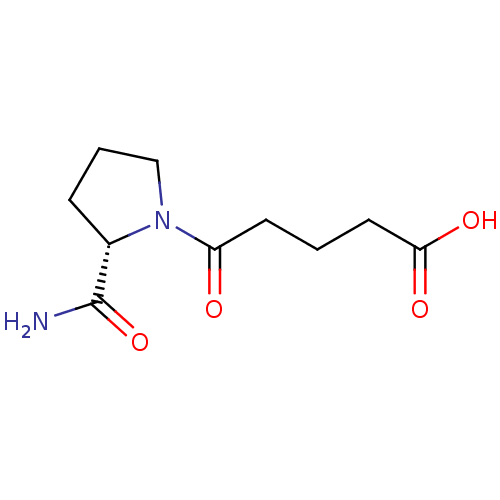
(5-((S)-2-Carbamoyl-pyrrolidin-1-yl)-5-oxo-pentanoi...)Show InChI InChI=1S/C10H16N2O4/c11-10(16)7-3-2-6-12(7)8(13)4-1-5-9(14)15/h7H,1-6H2,(H2,11,16)(H,14,15)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170740
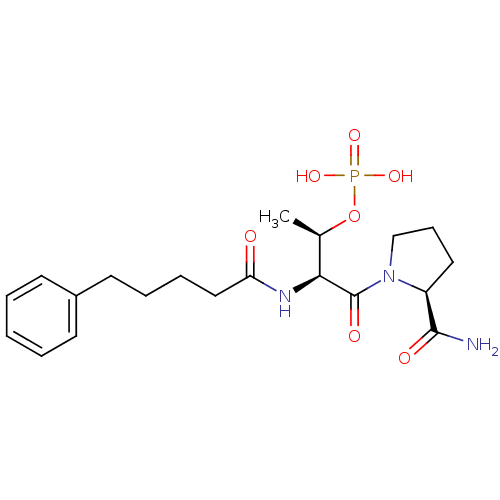
(CHEMBL188485 | Phosphoric acid mono-[(1S,3S)-3-(2-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)CCCCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C20H30N3O7P/c1-14(30-31(27,28)29)18(20(26)23-13-7-11-16(23)19(21)25)22-17(24)12-6-5-10-15-8-3-2-4-9-15/h2-4,8-9,14,16,18H,5-7,10-13H2,1H3,(H2,21,25)(H,22,24)(H2,27,28,29)/t14-,16+,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170743
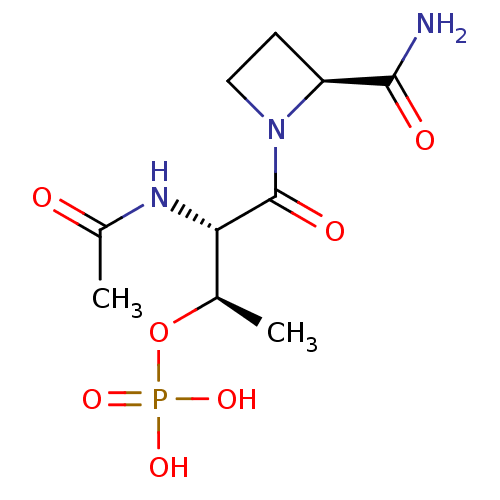
((R)-Phosphoric acid mono-[(1S,2S)-2-acetylamino-3-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1CC[C@H]1C(N)=O Show InChI InChI=1S/C10H18N3O7P/c1-5(20-21(17,18)19)8(12-6(2)14)10(16)13-4-3-7(13)9(11)15/h5,7-8H,3-4H2,1-2H3,(H2,11,15)(H,12,14)(H2,17,18,19)/t5-,7+,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170744

(CHEMBL190649 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1C[C@H](F)C[C@H]1C(N)=O Show InChI InChI=1S/C11H19FN3O7P/c1-5(22-23(19,20)21)9(14-6(2)16)11(18)15-4-7(12)3-8(15)10(13)17/h5,7-9H,3-4H2,1-2H3,(H2,13,17)(H,14,16)(H2,19,20,21)/t5-,7-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170746
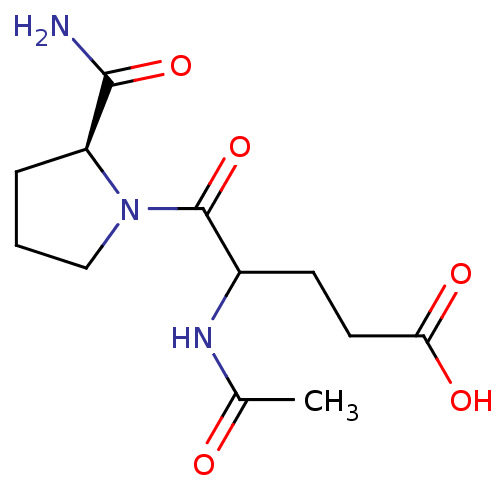
(4-Acetylamino-5-((S)-2-carbamoyl-pyrrolidin-1-yl)-...)Show InChI InChI=1S/C12H19N3O5/c1-7(16)14-8(4-5-10(17)18)12(20)15-6-2-3-9(15)11(13)19/h8-9H,2-6H2,1H3,(H2,13,19)(H,14,16)(H,17,18)/t8?,9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170747
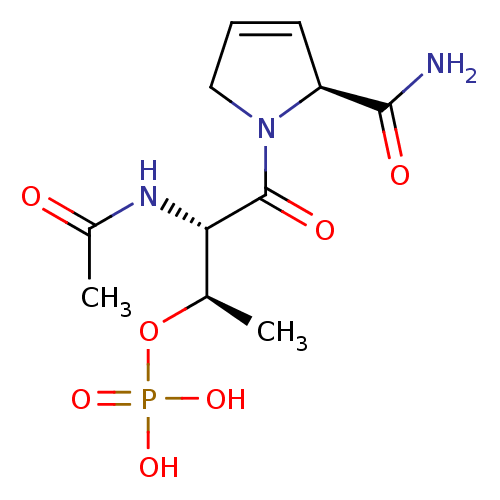
(CHEMBL189279 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1CC=C[C@H]1C(N)=O |c:16| Show InChI InChI=1S/C11H18N3O7P/c1-6(21-22(18,19)20)9(13-7(2)15)11(17)14-5-3-4-8(14)10(12)16/h3-4,6,8-9H,5H2,1-2H3,(H2,12,16)(H,13,15)(H2,18,19,20)/t6-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170745
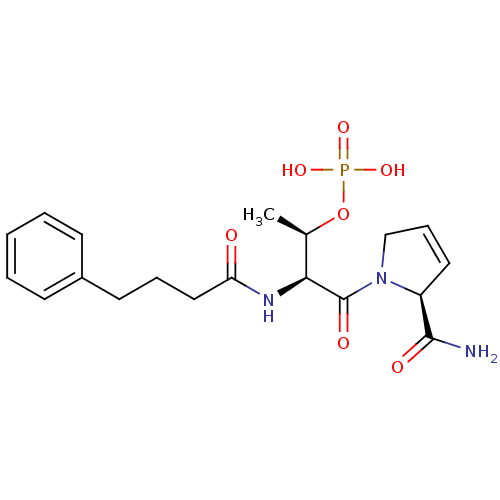
(CHEMBL187465 | Phosphoric acid mono-[(S)-3-(2-carb...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)CCCc1ccccc1)C(=O)N1CC=C[C@H]1C(N)=O |c:25| Show InChI InChI=1S/C19H26N3O7P/c1-13(29-30(26,27)28)17(19(25)22-12-6-10-15(22)18(20)24)21-16(23)11-5-9-14-7-3-2-4-8-14/h2-4,6-8,10,13,15,17H,5,9,11-12H2,1H3,(H2,20,24)(H,21,23)(H2,26,27,28)/t13-,15+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170749
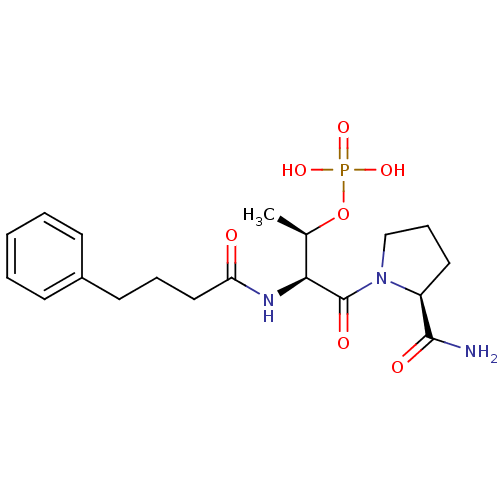
(CHEMBL189818 | Phosphoric acid mono-[(1S,3S)-3-(2-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)CCCc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C19H28N3O7P/c1-13(29-30(26,27)28)17(19(25)22-12-6-10-15(22)18(20)24)21-16(23)11-5-9-14-7-3-2-4-8-14/h2-4,7-8,13,15,17H,5-6,9-12H2,1H3,(H2,20,24)(H,21,23)(H2,26,27,28)/t13-,15+,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170750
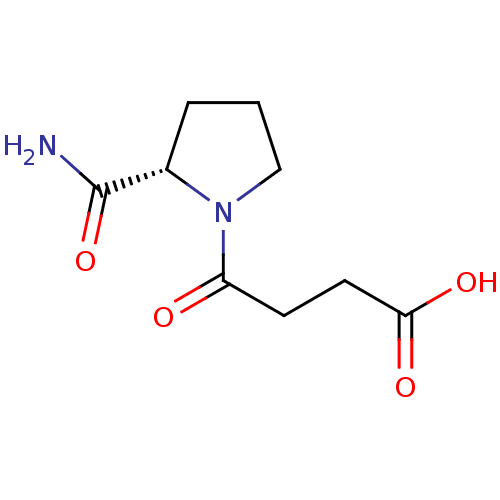
(4-((S)-2-Carbamoyl-pyrrolidin-1-yl)-4-oxo-butyric ...)Show InChI InChI=1S/C9H14N2O4/c10-9(15)6-2-1-5-11(6)7(12)3-4-8(13)14/h6H,1-5H2,(H2,10,15)(H,13,14)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170751
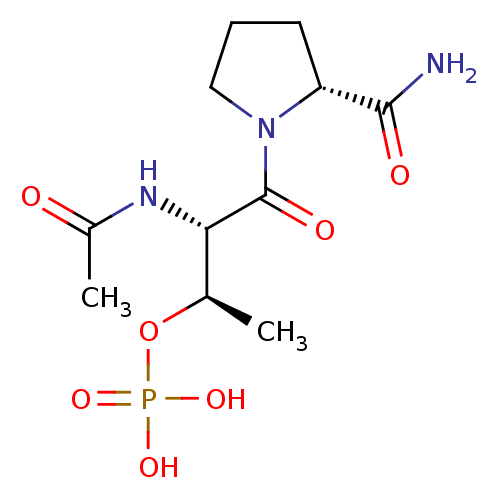
(CHEMBL190868 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1CCC[C@@H]1C(N)=O Show InChI InChI=1S/C11H20N3O7P/c1-6(21-22(18,19)20)9(13-7(2)15)11(17)14-5-3-4-8(14)10(12)16/h6,8-9H,3-5H2,1-2H3,(H2,12,16)(H,13,15)(H2,18,19,20)/t6-,8-,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170748
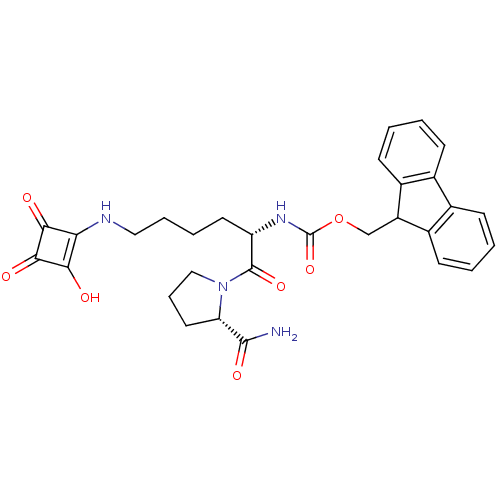
(CHEMBL366302 | [(S)-1-((S)-2-Carbamoyl-pyrrolidine...)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCNc1c(O)c(=O)c1=O)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C30H32N4O7/c31-28(38)23-13-7-15-34(23)29(39)22(12-5-6-14-32-24-25(35)27(37)26(24)36)33-30(40)41-16-21-19-10-3-1-8-17(19)18-9-2-4-11-20(18)21/h1-4,8-11,21-23,32,35H,5-7,12-16H2,(H2,31,38)(H,33,40)/t22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.40E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170752
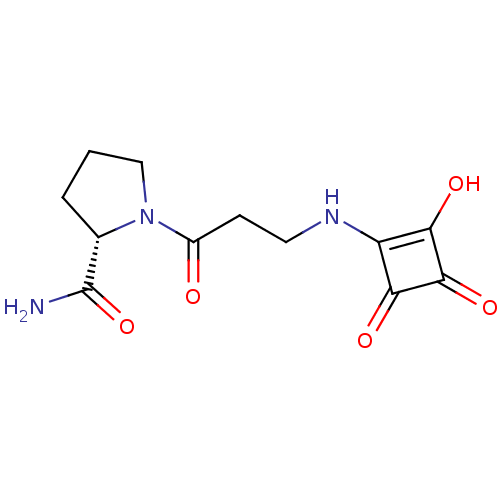
(1-[(S)-3-(2-Hydroxy-3,4-dioxo-cyclobut-1-enylamino...)Show InChI InChI=1S/C12H15N3O5/c13-12(20)6-2-1-5-15(6)7(16)3-4-14-8-9(17)11(19)10(8)18/h6,14,17H,1-5H2,(H2,13,20)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50403020
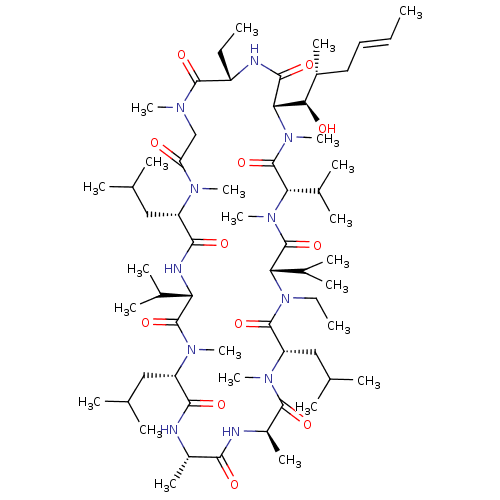
(CHEMBL2216758)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](C(C)C)N(CC)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-28-29-40(16)52(75)51-56(79)65-43(26-2)58(81)67(19)33-47(74)68(20)44(30-34(4)5)55(78)66-48(37(10)11)60(83)69(21)45(31-35(6)7)54(77)63-41(17)53(76)64-42(18)57(80)70(22)46(32-36(8)9)59(82)73(27-3)50(39(14)15)62(85)71(23)49(38(12)13)61(84)72(51)24/h25,28,34-46,48-52,75H,26-27,29-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b28-25+/t40-,41+,42-,43+,44+,45+,46+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by fluorescence polarization assay |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50403021
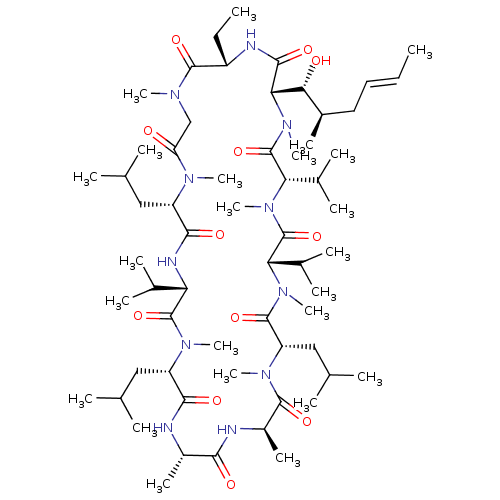
(CHEMBL2216757)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C61H109N11O12/c1-25-27-28-39(15)51(74)50-55(78)64-42(26-2)57(80)66(18)32-46(73)67(19)43(29-33(3)4)54(77)65-47(36(9)10)59(82)68(20)44(30-34(5)6)53(76)62-40(16)52(75)63-41(17)56(79)69(21)45(31-35(7)8)58(81)70(22)48(37(11)12)60(83)71(23)49(38(13)14)61(84)72(50)24/h25,27,33-45,47-51,74H,26,28-32H2,1-24H3,(H,62,76)(H,63,75)(H,64,78)(H,65,77)/b27-25+/t39-,40+,41-,42+,43+,44+,45+,47+,48+,49+,50+,51-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by fluorescence polarization assay |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50339127

((3S,6S,9S,12R,15S,18S,21S,24S,27R,30S,33S)-25,30-d...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(CC)C(=O)[C@@H](C)N(C)C1=O)C(C)C |r| Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+/t40-,41+,42-,43-,44+,45+,46+,47+,48+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by fluorescence polarization assay |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding affinity to biotinylated human recombinant T cell cyclophilin A expressed in Escherichia coli by spectrophotometric analysis |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to cyclophilin A by surface plasmon resonance analysis |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM50022815

((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...)Show SMILES CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C |r| Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human cyclophilin A by fluorescence polarization assay |
ACS Med Chem Lett 2: 485-487 (2011)
Article DOI: 10.1021/ml200039u
BindingDB Entry DOI: 10.7270/Q2K35VVC |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170732
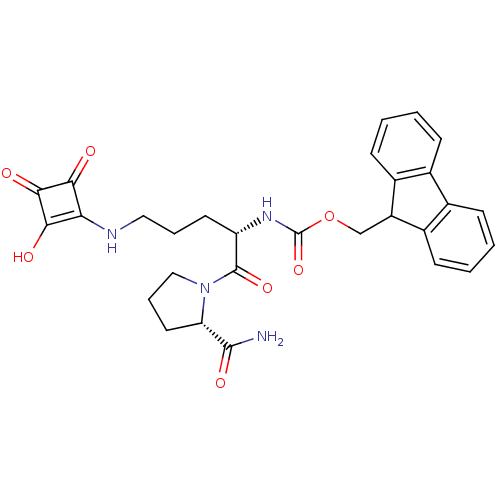
(CHEMBL191037 | [1-((S)-2-Carbamoyl-pyrrolidine-1-c...)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNc1c(O)c(=O)c1=O)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C29H30N4O7/c30-27(37)22-12-6-14-33(22)28(38)21(11-5-13-31-23-24(34)26(36)25(23)35)32-29(39)40-15-20-18-9-3-1-7-16(18)17-8-2-4-10-19(17)20/h1-4,7-10,20-22,31,34H,5-6,11-15H2,(H2,30,37)(H,32,39)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170730
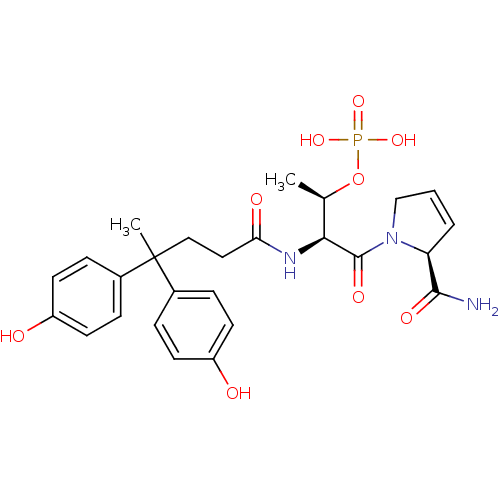
(CHEMBL371852 | Phosphoric acid mono-[(1R,2S)-2-[4,...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)CCC(C)(c1ccc(O)cc1)c1ccc(O)cc1)C(=O)N1CC=C[C@H]1C(N)=O |c:35| Show InChI InChI=1S/C26H32N3O9P/c1-16(38-39(35,36)37)23(25(34)29-15-3-4-21(29)24(27)33)28-22(32)13-14-26(2,17-5-9-19(30)10-6-17)18-7-11-20(31)12-8-18/h3-12,16,21,23,30-31H,13-15H2,1-2H3,(H2,27,33)(H,28,32)(H2,35,36,37)/t16-,21+,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170731
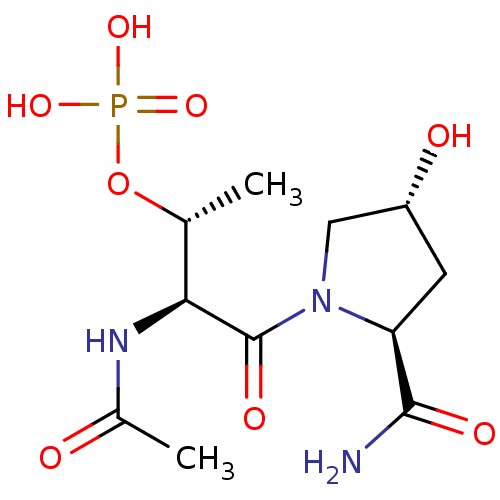
(CHEMBL191311 | Phosphoric acid mono-[(1R,2S)-2-ace...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1C[C@H](O)C[C@H]1C(N)=O Show InChI InChI=1S/C11H20N3O8P/c1-5(22-23(19,20)21)9(13-6(2)15)11(18)14-4-7(16)3-8(14)10(12)17/h5,7-9,16H,3-4H2,1-2H3,(H2,12,17)(H,13,15)(H2,19,20,21)/t5-,7-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170728
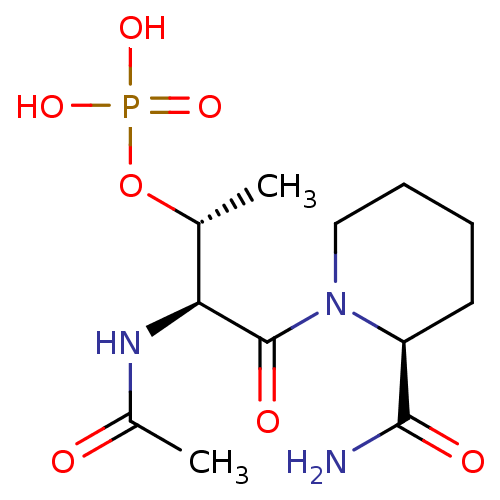
((R)-Phosphoric acid mono-[(1S,2S)-2-acetylamino-3-...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(C)=O)C(=O)N1CCCC[C@H]1C(N)=O Show InChI InChI=1S/C12H22N3O7P/c1-7(22-23(19,20)21)10(14-8(2)16)12(18)15-6-4-3-5-9(15)11(13)17/h7,9-10H,3-6H2,1-2H3,(H2,13,17)(H,14,16)(H2,19,20,21)/t7-,9+,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50170729
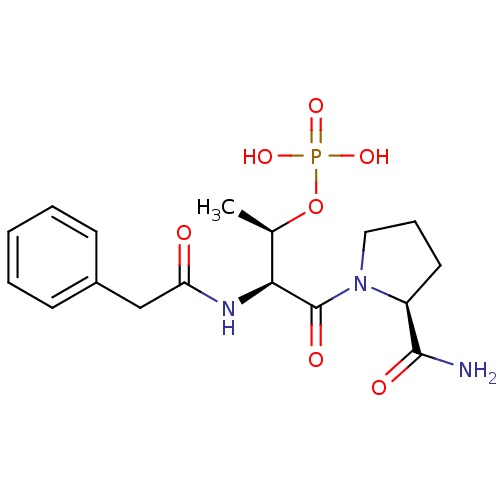
(CHEMBL191285 | Phosphoric acid mono-[(S)-3-(2-carb...)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C17H24N3O7P/c1-11(27-28(24,25)26)15(17(23)20-9-5-8-13(20)16(18)22)19-14(21)10-12-6-3-2-4-7-12/h2-4,6-7,11,13,15H,5,8-10H2,1H3,(H2,18,22)(H,19,21)(H2,24,25,26)/t11-,13+,15+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a |
CNRS-University of Lille 2 UMR 8525
Curated by ChEMBL
| Assay Description
Dissociation constant for human peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 |
J Med Chem 48: 4815-23 (2005)
Article DOI: 10.1021/jm0500119
BindingDB Entry DOI: 10.7270/Q2542N4Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data