 Found 21356 hits with Last Name = 'si' and Initial = 'g'
Found 21356 hits with Last Name = 'si' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50346374

(3-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)benz...)Show SMILES O=c1n(CCN2CCN(CC2)c2cccc3ccccc23)nnc2ccccc12 Show InChI InChI=1S/C23H23N5O/c29-23-20-9-3-4-10-21(20)24-25-28(23)17-14-26-12-15-27(16-13-26)22-11-5-7-18-6-1-2-8-19(18)22/h1-11H,12-17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.000178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5HT1A receptor in Sprague-Dawley rat brain cortex homogenates after 30 mins by liquid scintillation counting |
Eur J Med Chem 46: 2206-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.001
BindingDB Entry DOI: 10.7270/Q2M32W4J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359960
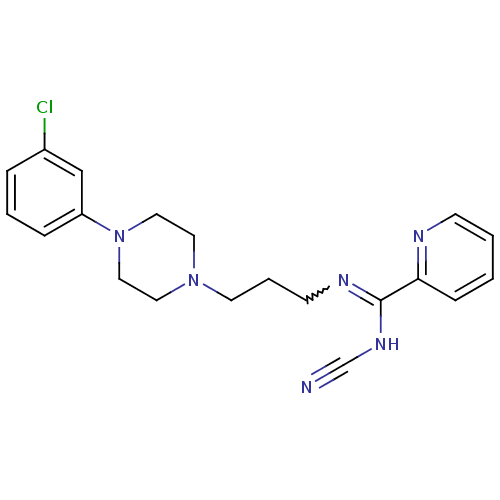
(CHEMBL1927094)Show SMILES Clc1cccc(c1)N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:14.14| Show InChI InChI=1S/C20H23ClN6/c21-17-5-3-6-18(15-17)27-13-11-26(12-14-27)10-4-9-24-20(25-16-22)19-7-1-2-8-23-19/h1-3,5-8,15H,4,9-14H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359961
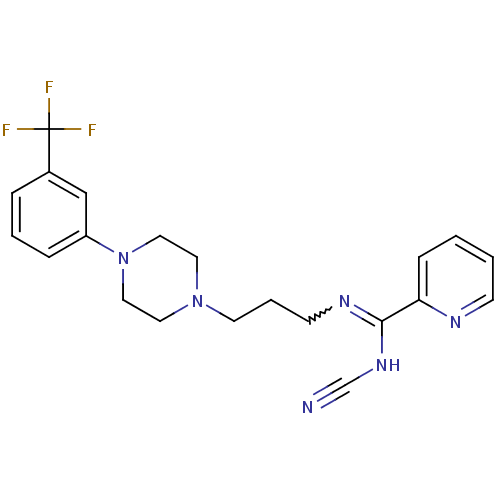
(CHEMBL1927095)Show SMILES FC(F)(F)c1cccc(c1)N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:17.17| Show InChI InChI=1S/C21H23F3N6/c22-21(23,24)17-5-3-6-18(15-17)30-13-11-29(12-14-30)10-4-9-27-20(28-16-25)19-7-1-2-8-26-19/h1-3,5-8,15H,4,9-14H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359954
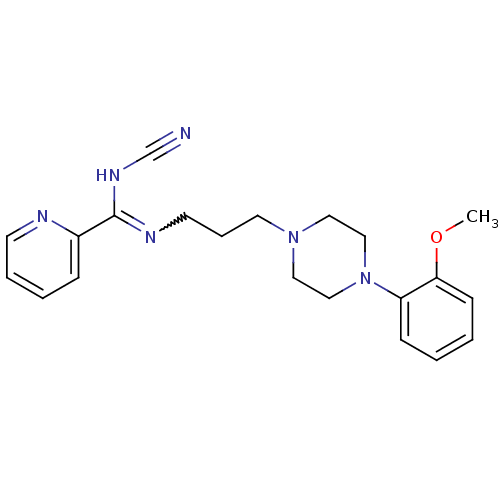
(CHEMBL1927088)Show SMILES COc1ccccc1N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:15.15| Show InChI InChI=1S/C21H26N6O/c1-28-20-9-3-2-8-19(20)27-15-13-26(14-16-27)12-6-11-24-21(25-17-22)18-7-4-5-10-23-18/h2-5,7-10H,6,11-16H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50180775
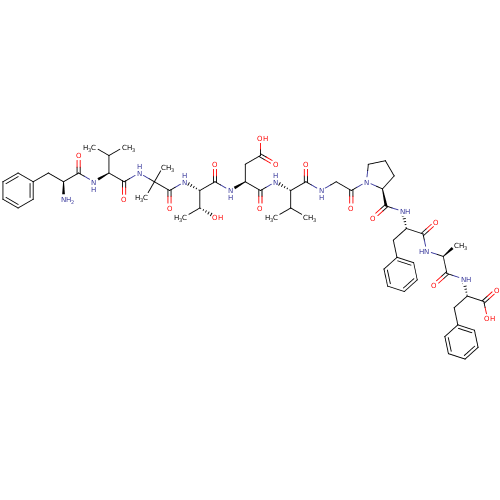
(CHEMBL386763 | FV-Aib-TDVGPFAF | [Aib29,Asp31,Pro3...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)C(C)(C)NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C59H81N11O15/c1-32(2)46(67-52(78)41(30-45(73)74)64-55(81)48(35(6)71)68-58(85)59(7,8)69-56(82)47(33(3)4)66-50(76)39(60)27-36-19-12-9-13-20-36)54(80)61-31-44(72)70-26-18-25-43(70)53(79)63-40(28-37-21-14-10-15-22-37)51(77)62-34(5)49(75)65-42(57(83)84)29-38-23-16-11-17-24-38/h9-17,19-24,32-35,39-43,46-48,71H,18,25-31,60H2,1-8H3,(H,61,80)(H,62,77)(H,63,79)(H,64,81)(H,65,75)(H,66,76)(H,67,78)(H,68,85)(H,69,82)(H,73,74)(H,83,84)/t34-,35+,39-,40-,41-,42-,43-,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig
Curated by ChEMBL
| Assay Description
Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells |
J Med Chem 49: 616-24 (2006)
Article DOI: 10.1021/jm050613s
BindingDB Entry DOI: 10.7270/Q280526H |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493145
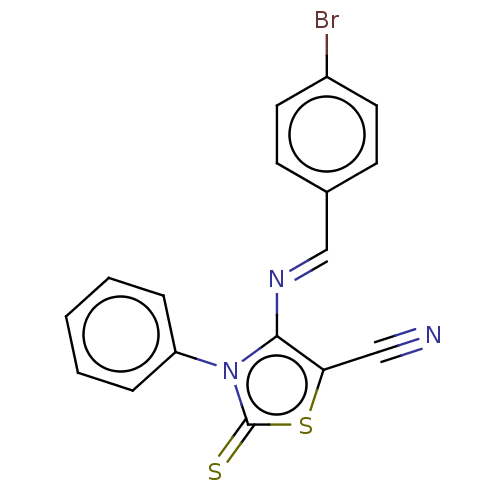
(CHEMBL2419149)Show InChI InChI=1S/C17H10BrN3S2/c18-13-8-6-12(7-9-13)11-20-16-15(10-19)23-17(22)21(16)14-4-2-1-3-5-14/h1-9,11H/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113907
BindingDB Entry DOI: 10.7270/Q20K2DJC |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50180778

(CHEMBL2371891 | FV-Hyp-TDVGPFAF)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H]1C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C60H81N11O16/c1-32(2)48(57(83)62-30-46(74)70-24-16-23-44(70)55(81)64-41(26-37-19-12-8-13-20-37)53(79)63-34(5)51(77)66-43(60(86)87)27-38-21-14-9-15-22-38)67-54(80)42(29-47(75)76)65-58(84)50(35(6)72)69-56(82)45-28-39(73)31-71(45)59(85)49(33(3)4)68-52(78)40(61)25-36-17-10-7-11-18-36/h7-15,17-22,32-35,39-45,48-50,72-73H,16,23-31,61H2,1-6H3,(H,62,83)(H,63,79)(H,64,81)(H,65,84)(H,66,77)(H,67,80)(H,68,78)(H,69,82)(H,75,76)(H,86,87)/t34-,35+,39+,40-,41-,42-,43-,44-,45-,48-,49-,50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig
Curated by ChEMBL
| Assay Description
Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells |
J Med Chem 49: 616-24 (2006)
Article DOI: 10.1021/jm050613s
BindingDB Entry DOI: 10.7270/Q280526H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551316
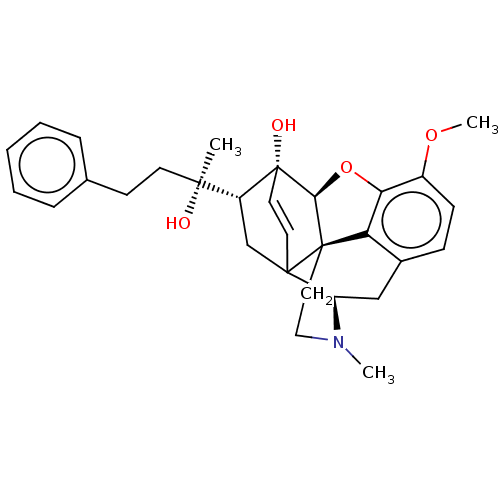
(CHEMBL4776706)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])([C@@](C)(O)CCc4ccccc4)[C@]2(O)C=C1)ccc3OC |r,c:36,TLB:3:4:12.11.9:14,33:5:12.11.9:14,4:13:16.15:31.32,12:13:16.15:31.32,THB:18:16:13.1:31.32| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142700
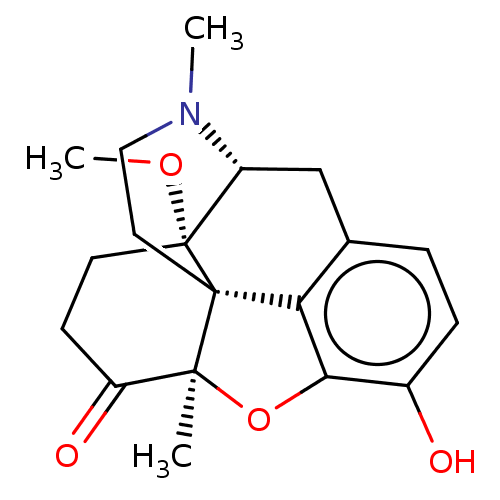
(CHEMBL326684)Show SMILES [H][C@@]12Cc3ccc(O)c4O[C@@]5(C)C(=O)CC[C@]1(OC)[C@@]5(CCN2C)c34 |r,TLB:23:22:16:2.3.24| Show InChI InChI=1S/C19H23NO4/c1-17-14(22)6-7-19(23-3)13-10-11-4-5-12(21)16(24-17)15(11)18(17,19)8-9-20(13)2/h4-5,13,21H,6-10H2,1-3H3/t13-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551315
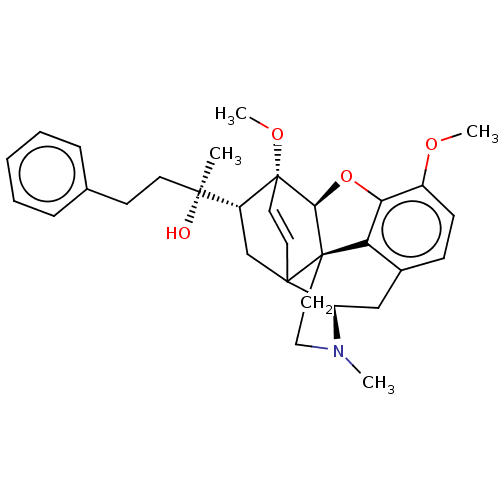
(CHEMBL4762529)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])([C@@](C)(O)CCc4ccccc4)[C@]2(OC)C=C1)ccc3OC |r,c:37,TLB:3:4:12.11.9:14,34:5:12.11.9:14,4:13:16.15:32.33,12:13:16.15:32.33,THB:18:16:13.1:32.33| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551313
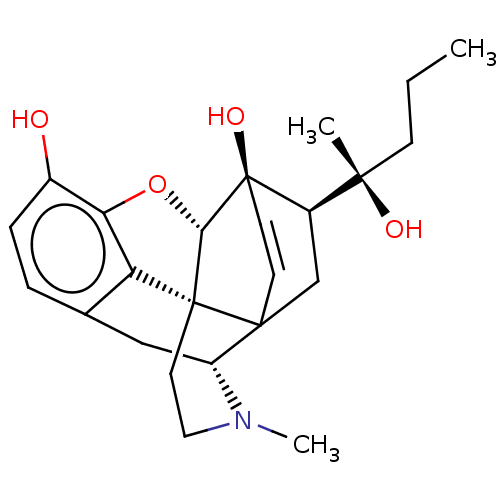
(CHEMBL4745764)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])([C@@](C)(O)CCC)[C@]2(O)C=C1)ccc3O |r,c:30,TLB:3:4:12.11.9:14,28:5:12.11.9:14,4:13:16.15:26.27,12:13:16.15:26.27,THB:18:16:13.1:26.27| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50299217
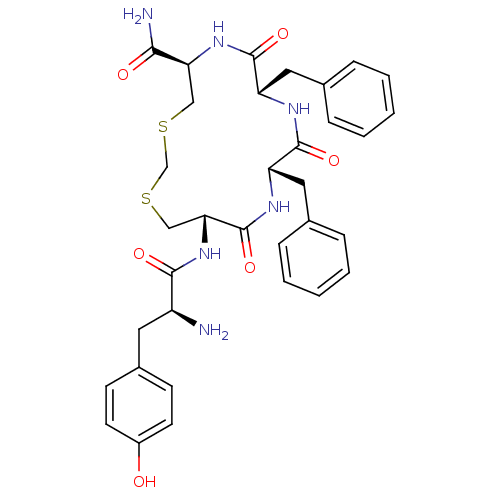
((5R,8S,11S,14S)-14-((S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCSC[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C34H40N6O6S2/c35-25(15-23-11-13-24(41)14-12-23)31(43)40-29-19-48-20-47-18-28(30(36)42)39-33(45)27(17-22-9-5-2-6-10-22)37-32(44)26(38-34(29)46)16-21-7-3-1-4-8-21/h1-14,25-29,41H,15-20,35H2,(H2,36,42)(H,37,44)(H,38,46)(H,39,45)(H,40,43)/t25-,26-,27-,28-,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50359962
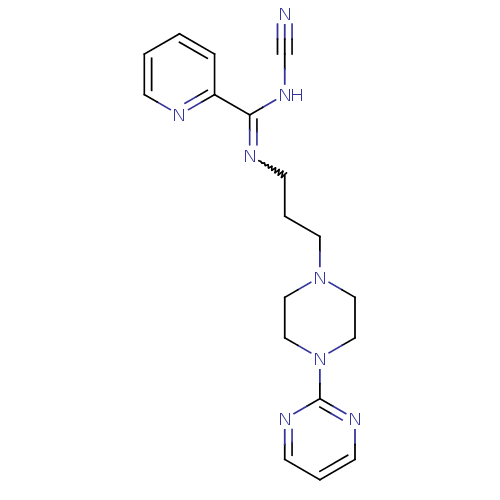
(CHEMBL1927096)Show SMILES N#CNC(=NCCCN1CCN(CC1)c1ncccn1)c1ccccn1 |w:4.4| Show InChI InChI=1S/C18H22N8/c19-15-24-17(16-5-1-2-6-20-16)21-9-4-10-25-11-13-26(14-12-25)18-22-7-3-8-23-18/h1-3,5-8H,4,9-14H2,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50180781

(CHEMBL2371890 | FV-Tic-TDVGPFAF)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C65H83N11O15/c1-36(2)53(62(87)67-34-51(78)75-28-18-27-49(75)60(85)69-46(30-41-21-12-8-13-22-41)58(83)68-38(5)56(81)71-48(65(90)91)31-42-23-14-9-15-24-42)72-59(84)47(33-52(79)80)70-63(88)55(39(6)77)74-61(86)50-32-43-25-16-17-26-44(43)35-76(50)64(89)54(37(3)4)73-57(82)45(66)29-40-19-10-7-11-20-40/h7-17,19-26,36-39,45-50,53-55,77H,18,27-35,66H2,1-6H3,(H,67,87)(H,68,83)(H,69,85)(H,70,88)(H,71,81)(H,72,84)(H,73,82)(H,74,86)(H,79,80)(H,90,91)/t38-,39+,45-,46-,47-,48-,49-,50-,53-,54-,55-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig
Curated by ChEMBL
| Assay Description
Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells |
J Med Chem 49: 616-24 (2006)
Article DOI: 10.1021/jm050613s
BindingDB Entry DOI: 10.7270/Q280526H |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50062162
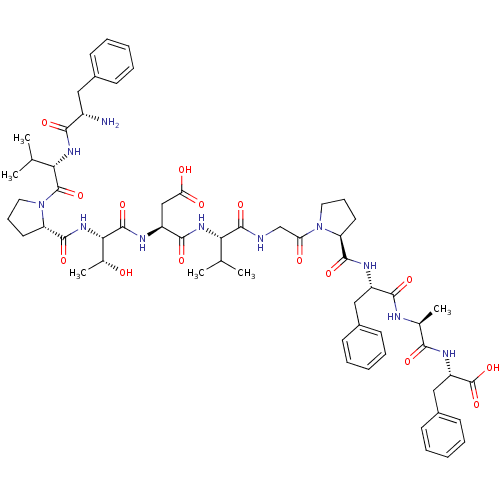
(CHEMBL264010 | FVPTDVGPFAF)Show SMILES CC(C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccccc1)C(C)C)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C60H81N11O15/c1-33(2)48(67-54(79)42(31-47(74)75)65-58(83)50(36(6)72)69-56(81)45-25-17-27-71(45)59(84)49(34(3)4)68-52(77)40(61)28-37-18-10-7-11-19-37)57(82)62-32-46(73)70-26-16-24-44(70)55(80)64-41(29-38-20-12-8-13-21-38)53(78)63-35(5)51(76)66-43(60(85)86)30-39-22-14-9-15-23-39/h7-15,18-23,33-36,40-45,48-50,72H,16-17,24-32,61H2,1-6H3,(H,62,82)(H,63,78)(H,64,80)(H,65,83)(H,66,76)(H,67,79)(H,68,77)(H,69,81)(H,74,75)(H,85,86)/t35-,36+,40-,41-,42-,43-,44-,45-,48-,49-,50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leipzig
Curated by ChEMBL
| Assay Description
Displacement of [3H-propionyl-K24] from halphaCGRP expressed in human neuroblastoma SK-N-MC cells |
J Med Chem 49: 616-24 (2006)
Article DOI: 10.1021/jm050613s
BindingDB Entry DOI: 10.7270/Q280526H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50142622
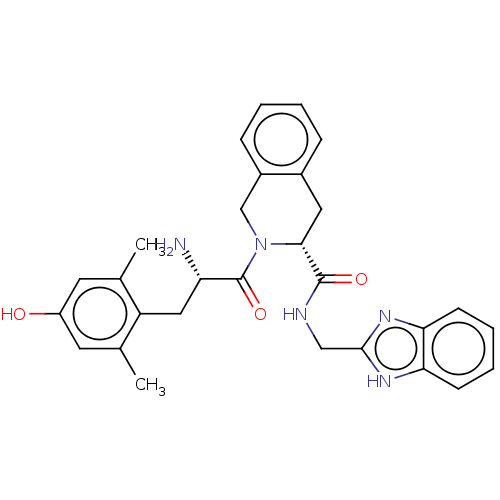
(CHEMBL3758292)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCc1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C29H31N5O3/c1-17-11-21(35)12-18(2)22(17)14-23(30)29(37)34-16-20-8-4-3-7-19(20)13-26(34)28(36)31-15-27-32-24-9-5-6-10-25(24)33-27/h3-12,23,26,35H,13-16,30H2,1-2H3,(H,31,36)(H,32,33)/t23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at delta opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50551314

(CHEMBL4751305)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])([C@@](C)(O)CCc4ccccc4)[C@]2(O)C=C1)ccc3O |r,c:36,TLB:3:4:12.11.9:14,33:5:12.11.9:14,4:13:16.15:31.32,12:13:16.15:31.32,THB:18:16:13.1:31.32| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50551311
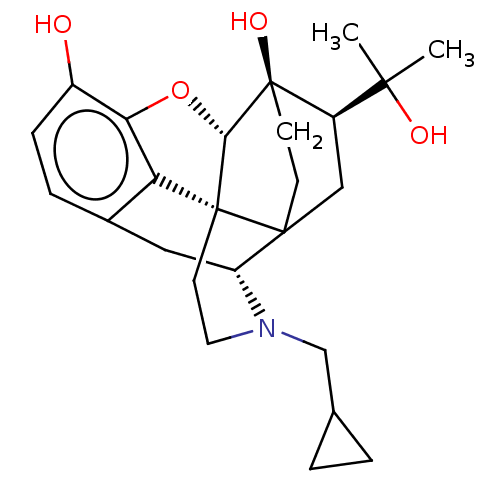
(CHEMBL4746512)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14C51CC[C@@]2(O)[C@H](C1)C(C)(C)O)ccc3O |r,TLB:3:4:15.14.9:17,28:5:15.14.9:17,THB:24:22:16.1:19.18,4:16:22.23:19.18,15:16:22.23:19.18| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]HS665 from KOR in guinea pig brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50303629
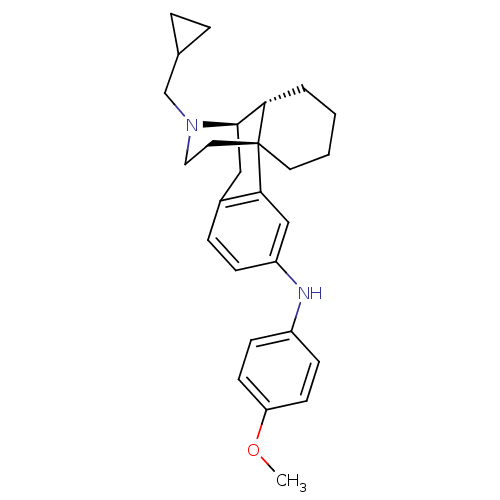
(17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...)Show SMILES COc1ccc(Nc2ccc3C[C@@H]4[C@@H]5CCCC[C@]5(CCN4CC4CC4)c3c2)cc1 |r| Show InChI InChI=1S/C27H34N2O/c1-30-23-11-9-21(10-12-23)28-22-8-7-20-16-26-24-4-2-3-13-27(24,25(20)17-22)14-15-29(26)18-19-5-6-19/h7-12,17,19,24,26,28H,2-6,13-16,18H2,1H3/t24-,26+,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM85681
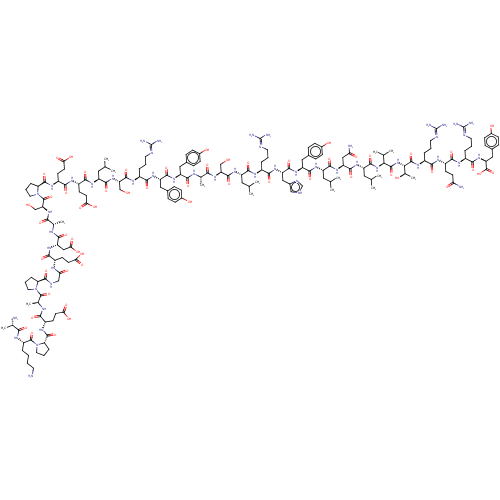
(PYY 3-36)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C175H271N51O56/c1-84(2)67-113(154(262)200-103(26-18-60-189-172(180)181)146(254)215-120(75-98-78-188-83-194-98)159(267)214-119(73-96-37-45-101(233)46-38-96)157(265)209-114(68-85(3)4)155(263)216-121(76-130(179)236)160(268)210-116(70-87(7)8)161(269)222-138(88(9)10)167(275)223-139(93(15)230)168(276)206-106(29-21-63-192-175(186)187)144(252)202-108(49-54-129(178)235)149(257)199-105(28-20-62-191-174(184)185)147(255)218-123(171(279)280)74-97-39-47-102(234)48-40-97)211-163(271)124(80-227)219-141(249)90(12)195-152(260)117(71-94-33-41-99(231)42-34-94)213-158(266)118(72-95-35-43-100(232)44-36-95)212-145(253)104(27-19-61-190-173(182)183)201-162(270)125(81-228)220-156(264)115(69-86(5)6)208-151(259)110(52-57-135(242)243)203-150(258)111(53-58-136(244)245)205-166(274)128-32-24-66-226(128)282(281)132(82-229)221-142(250)91(13)196-153(261)122(77-137(246)247)217-148(256)107(50-55-133(238)239)198-131(237)79-193-164(272)126-30-22-64-224(126)169(277)92(14)197-143(251)109(51-56-134(240)241)204-165(273)127-31-23-65-225(127)170(278)112(25-16-17-59-176)207-140(248)89(11)177/h33-48,78,83-93,103-128,132,138-139,227-234H,16-32,49-77,79-82,176-177H2,1-15H3,(H2,178,235)(H2,179,236)(H,188,194)(H,193,272)(H,195,260)(H,196,261)(H,197,251)(H,198,237)(H,199,257)(H,200,262)(H,201,270)(H,202,252)(H,203,258)(H,204,273)(H,205,274)(H,206,276)(H,207,248)(H,208,259)(H,209,265)(H,210,268)(H,211,271)(H,212,253)(H,213,266)(H,214,267)(H,215,254)(H,216,263)(H,217,256)(H,218,255)(H,219,249)(H,220,264)(H,221,250)(H,222,269)(H,223,275)(H,238,239)(H,240,241)(H,242,243)(H,244,245)(H,246,247)(H,279,280)(H4,180,181,189)(H4,182,183,190)(H4,184,185,191)(H4,186,187,192)/t89-,90-,91-,92-,93+,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,132+,138-,139-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal Institute of Technology of Zurich
Curated by PDSP Ki Database
| |
J Biol Chem 275: 36043-8 (2000)
Article DOI: 10.1074/jbc.M000626200
BindingDB Entry DOI: 10.7270/Q2PV6HX1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21865
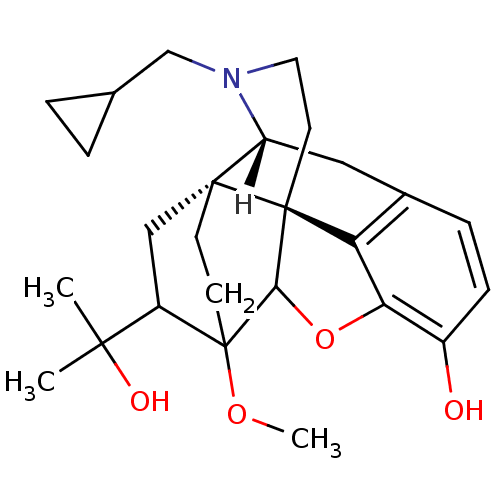
((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...)Show SMILES [H][C@]12Cc3ccc(O)c4OC5[C@](CCN1CC1CC1)(c34)[C@@]21CCC5(OC)C(C1)C(C)(C)O |TLB:28:26:11.10:21.22,8:19:20:14.12.13,4:3:20:14.12.13| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18?,19-,22?,24-,25+,26?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 286: 1007-13 (1998)
BindingDB Entry DOI: 10.7270/Q2QC022N |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50303629

(17-(Cyclopropylmethyl)-N-(4-methoxyphenyl)morphina...)Show SMILES COc1ccc(Nc2ccc3C[C@@H]4[C@@H]5CCCC[C@]5(CCN4CC4CC4)c3c2)cc1 |r| Show InChI InChI=1S/C27H34N2O/c1-30-23-11-9-21(10-12-23)28-22-8-7-20-16-26-24-4-2-3-13-27(24,25(20)17-22)14-15-29(26)18-19-5-6-19/h7-12,17,19,24,26,28H,2-6,13-16,18H2,1H3/t24-,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142621
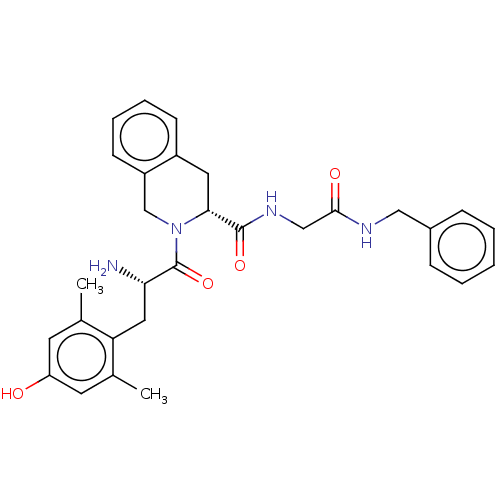
(CHEMBL3759292)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C30H34N4O4/c1-19-12-24(35)13-20(2)25(19)15-26(31)30(38)34-18-23-11-7-6-10-22(23)14-27(34)29(37)33-17-28(36)32-16-21-8-4-3-5-9-21/h3-13,26-27,35H,14-18,31H2,1-2H3,(H,32,36)(H,33,37)/t26-,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(GUINEA PIG) | BDBM82421
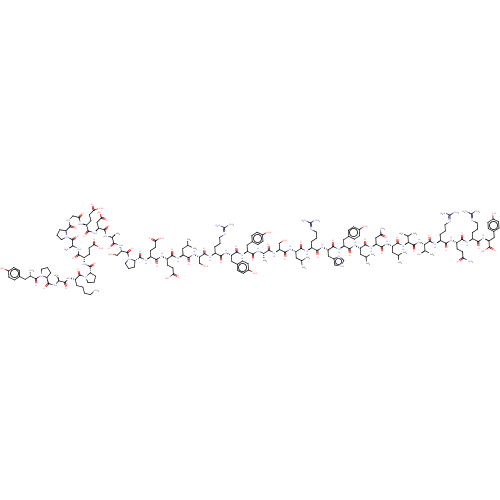
(CAS_81858-94-8 | PYY, porcine)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C189H287N53O59/c1-92(2)74-124(166(279)215-114(27-18-66-203-186(194)195)158(271)230-131(83-107-86-202-91-208-107)171(284)229-130(81-105-41-51-111(250)52-42-105)169(282)224-125(75-93(3)4)167(280)231-132(84-142(193)253)172(285)225-127(77-95(7)8)173(286)237-150(96(9)10)180(293)238-151(101(15)246)181(294)221-117(30-21-69-206-189(200)201)156(269)217-119(55-60-141(192)252)161(274)214-116(29-20-68-205-188(198)199)159(272)233-134(185(298)299)82-106-43-53-112(251)54-44-106)226-175(288)135(88-243)234-153(266)97(11)209-164(277)128(79-103-37-47-109(248)48-38-103)228-170(283)129(80-104-39-49-110(249)50-40-104)227-157(270)115(28-19-67-204-187(196)197)216-174(287)136(89-244)235-168(281)126(76-94(5)6)223-163(276)121(58-63-147(259)260)218-162(275)122(59-64-148(261)262)220-179(292)140-34-25-73-242(140)301(300)144(90-245)236-154(267)99(13)210-165(278)133(85-149(263)264)232-160(273)118(56-61-145(255)256)213-143(254)87-207-176(289)137-31-22-70-239(137)182(295)100(14)212-155(268)120(57-62-146(257)258)219-178(291)139-33-24-72-241(139)184(297)123(26-16-17-65-190)222-152(265)98(12)211-177(290)138-32-23-71-240(138)183(296)113(191)78-102-35-45-108(247)46-36-102/h35-54,86,91-101,113-140,144,150-151,243-251H,16-34,55-85,87-90,190-191H2,1-15H3,(H2,192,252)(H2,193,253)(H,202,208)(H,207,289)(H,209,277)(H,210,278)(H,211,290)(H,212,268)(H,213,254)(H,214,274)(H,215,279)(H,216,287)(H,217,269)(H,218,275)(H,219,291)(H,220,292)(H,221,294)(H,222,265)(H,223,276)(H,224,282)(H,225,285)(H,226,288)(H,227,270)(H,228,283)(H,229,284)(H,230,271)(H,231,280)(H,232,273)(H,233,272)(H,234,266)(H,235,281)(H,236,267)(H,237,286)(H,238,293)(H,255,256)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,298,299)(H4,194,195,203)(H4,196,197,204)(H4,198,199,205)(H4,200,201,206)/t97-,98-,99-,100-,101+,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,139-,140-,144+,150-,151-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by PDSP Ki Database
| |
Regul Pept 23-8 (1998)
Article DOI: 10.1016/s0167-0115(98)00049-4
BindingDB Entry DOI: 10.7270/Q2JM2853 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551309

(CHEMBL4758369)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])(C(C)(C)O)[C@]2(OC)C=C1)ccc3O |r,c:29,TLB:3:4:12.11.9:14,27:5:12.11.9:14,4:13:16.15:25.26,12:13:16.15:25.26,THB:18:16:13.1:25.26| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551311

(CHEMBL4746512)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@]14C51CC[C@@]2(O)[C@H](C1)C(C)(C)O)ccc3O |r,TLB:3:4:15.14.9:17,28:5:15.14.9:17,THB:24:22:16.1:19.18,4:16:22.23:19.18,15:16:22.23:19.18| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50215552
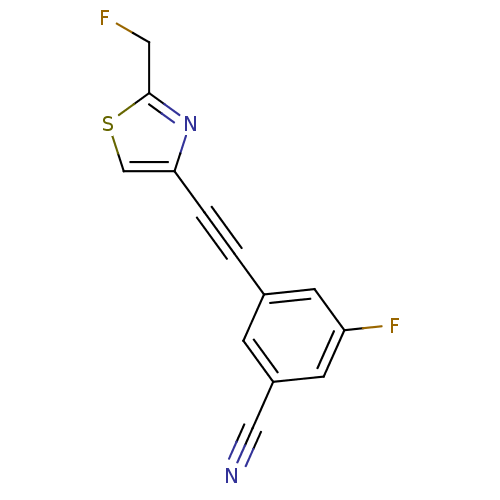
(3-fluoro-5-(2-(2-(fluoromethyl)thiazol-4-yl)ethyny...)Show InChI InChI=1S/C13H6F2N2S/c14-6-13-17-12(8-18-13)2-1-9-3-10(7-16)5-11(15)4-9/h3-5,8H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGluR5 in rat brain |
J Med Chem 54: 901-8 (2012)
Article DOI: 10.1021/jm101430m
BindingDB Entry DOI: 10.7270/Q26Q1Z7T |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50015490
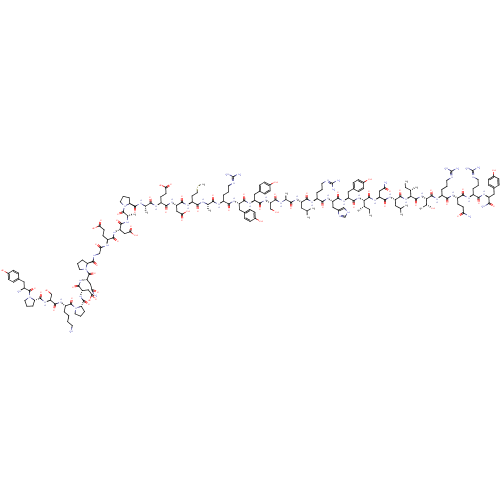
(CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(N)=O Show InChI InChI=1S/C189H285N55O57S/c1-15-93(7)148(179(295)234-128(81-140(193)254)168(284)226-123(74-92(5)6)171(287)239-149(94(8)16-2)180(296)240-150(99(13)247)181(297)222-115(31-22-67-208-189(202)203)156(272)220-117(56-59-139(192)253)161(277)218-113(29-20-65-206-187(198)199)157(273)224-121(151(195)267)76-101-38-48-107(249)49-39-101)238-172(288)126(79-104-44-54-110(252)55-45-104)229-167(283)127(80-105-86-204-90-210-105)230-159(275)114(30-21-66-207-188(200)201)219-164(280)122(73-91(3)4)225-154(270)96(10)212-173(289)133(88-245)236-166(282)125(78-103-42-52-109(251)53-43-103)228-165(281)124(77-102-40-50-108(250)51-41-102)227-158(274)112(28-19-64-205-186(196)197)216-152(268)95(9)211-155(271)119(62-72-302-14)221-169(285)130(84-146(263)264)232-162(278)118(58-61-144(259)260)217-153(269)97(11)213-176(292)136-33-24-68-241(136)182(298)98(12)214-163(279)129(83-145(261)262)231-160(276)116(57-60-143(257)258)215-142(256)87-209-175(291)135-32-23-70-243(135)185(301)132(82-141(194)255)235-170(286)131(85-147(265)266)233-177(293)138-35-26-71-244(138)184(300)120(27-17-18-63-190)223-174(290)134(89-246)237-178(294)137-34-25-69-242(137)183(299)111(191)75-100-36-46-106(248)47-37-100/h36-55,86,90-99,111-138,148-150,245-252H,15-35,56-85,87-89,190-191H2,1-14H3,(H2,192,253)(H2,193,254)(H2,194,255)(H2,195,267)(H,204,210)(H,209,291)(H,211,271)(H,212,289)(H,213,292)(H,214,279)(H,215,256)(H,216,268)(H,217,269)(H,218,277)(H,219,280)(H,220,272)(H,221,285)(H,222,297)(H,223,290)(H,224,273)(H,225,270)(H,226,284)(H,227,274)(H,228,281)(H,229,283)(H,230,275)(H,231,276)(H,232,278)(H,233,293)(H,234,295)(H,235,286)(H,236,282)(H,237,294)(H,238,288)(H,239,287)(H,240,296)(H,257,258)(H,259,260)(H,261,262)(H,263,264)(H,265,266)(H4,196,197,205)(H4,198,199,206)(H4,200,201,207)(H4,202,203,208)/t93-,94-,95-,96-,97-,98-,99+,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,135-,136-,137-,138-,148-,149-,150-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal Institute of Technology of Zurich
Curated by PDSP Ki Database
| |
J Biol Chem 275: 36043-8 (2000)
Article DOI: 10.1074/jbc.M000626200
BindingDB Entry DOI: 10.7270/Q2PV6HX1 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50123490
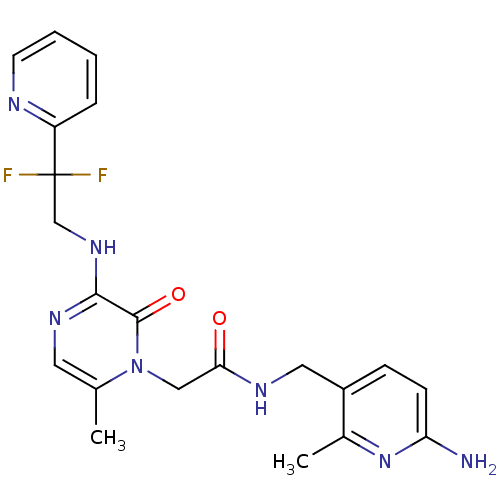
(CHEMBL143418 | N-(6-Amino-2-methyl-pyridin-3-ylmet...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccn2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C21H23F2N7O2/c1-13-9-27-19(28-12-21(22,23)16-5-3-4-8-25-16)20(32)30(13)11-18(31)26-10-15-6-7-17(24)29-14(15)2/h3-9H,10-12H2,1-2H3,(H2,24,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin (IIa) |
J Med Chem 46: 461-73 (2003)
Article DOI: 10.1021/jm020311f
BindingDB Entry DOI: 10.7270/Q2W958J5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142620
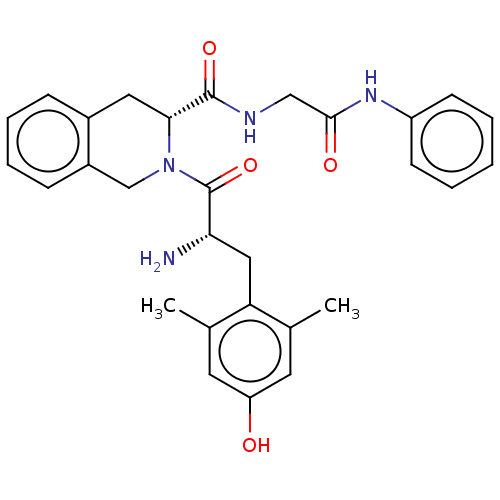
(CHEMBL3758712)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)NCC(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C29H32N4O4/c1-18-12-23(34)13-19(2)24(18)15-25(30)29(37)33-17-21-9-7-6-8-20(21)14-26(33)28(36)31-16-27(35)32-22-10-4-3-5-11-22/h3-13,25-26,34H,14-17,30H2,1-2H3,(H,31,36)(H,32,35)/t25-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity at mu opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045775
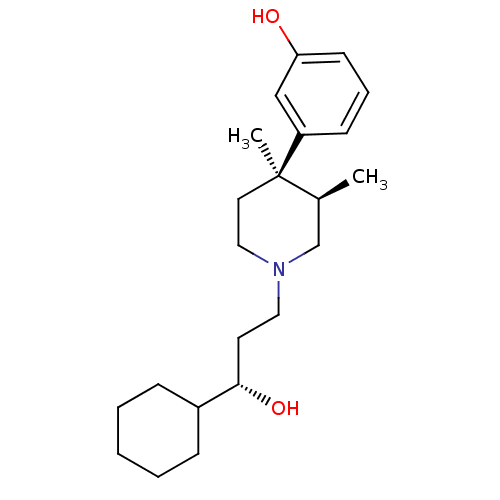
((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...)Show SMILES C[C@H]1CN(CC[C@H](O)C2CCCCC2)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C22H35NO2/c1-17-16-23(13-11-21(25)18-7-4-3-5-8-18)14-12-22(17,2)19-9-6-10-20(24)15-19/h6,9-10,15,17-18,21,24-25H,3-5,7-8,11-14,16H2,1-2H3/t17-,21-,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50551314

(CHEMBL4751305)Show SMILES [H][C@]12Oc3c4c(C[C@@]5([H])N(C)CC[C@]14C51C[C@]([H])([C@@](C)(O)CCc4ccccc4)[C@]2(O)C=C1)ccc3O |r,c:36,TLB:3:4:12.11.9:14,33:5:12.11.9:14,4:13:16.15:31.32,12:13:16.15:31.32,THB:18:16:13.1:31.32| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes measured after 45 mins by scintillation counting method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112145
BindingDB Entry DOI: 10.7270/Q2W66QD3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50303630

(17-(Cyclopropylmethyl)-N-phenylmorphinan-3-amine |...)Show SMILES C(C1CC1)N1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(Nc2ccccc2)cc31 |r| Show InChI InChI=1S/C26H32N2/c1-2-6-21(7-3-1)27-22-12-11-20-16-25-23-8-4-5-13-26(23,24(20)17-22)14-15-28(25)18-19-9-10-19/h1-3,6-7,11-12,17,19,23,25,27H,4-5,8-10,13-16,18H2/t23-,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135806
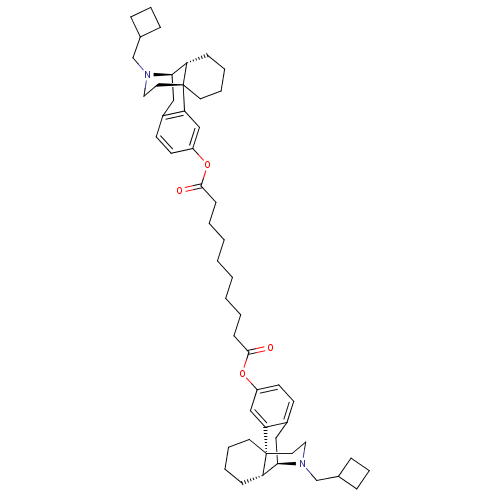
(CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...)Show SMILES O=C(CCCCCCCCC(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C52H72N2O4/c55-49(57-41-23-21-39-31-47-43-17-7-9-25-51(43,45(39)33-41)27-29-53(47)35-37-13-11-14-37)19-5-3-1-2-4-6-20-50(56)58-42-24-22-40-32-48-44-18-8-10-26-52(44,46(40)34-42)28-30-54(48)36-38-15-12-16-38/h21-24,33-34,37-38,43-44,47-48H,1-20,25-32,35-36H2/t43-,44-,47+,48+,51+,52+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50142694
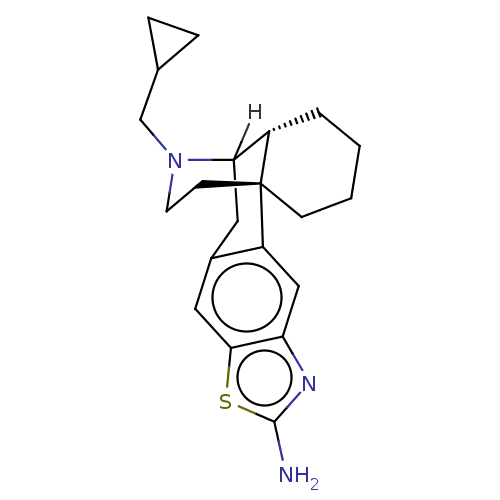
(CHEMBL3759660)Show SMILES [H]C12Cc3cc4sc(N)nc4cc3[C@]3(CCCC[C@@]13[H])CCN2CC1CC1 |r,TLB:23:22:3.12.2:18| Show InChI InChI=1S/C21H27N3S/c22-20-23-17-11-16-14(10-19(17)25-20)9-18-15-3-1-2-6-21(15,16)7-8-24(18)12-13-4-5-13/h10-11,13,15,18H,1-9,12H2,(H2,22,23)/t15-,18?,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50594655
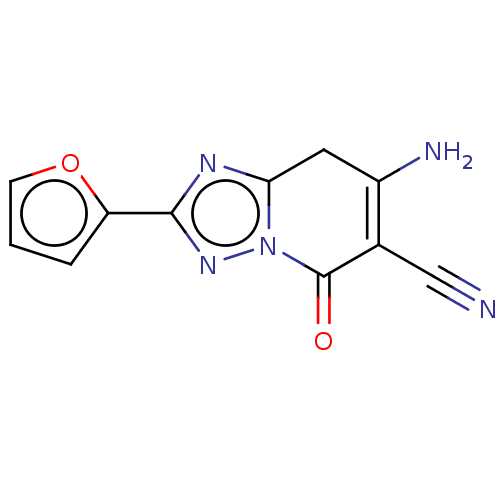
(CHEMBL5177144) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113907
BindingDB Entry DOI: 10.7270/Q20K2DJC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
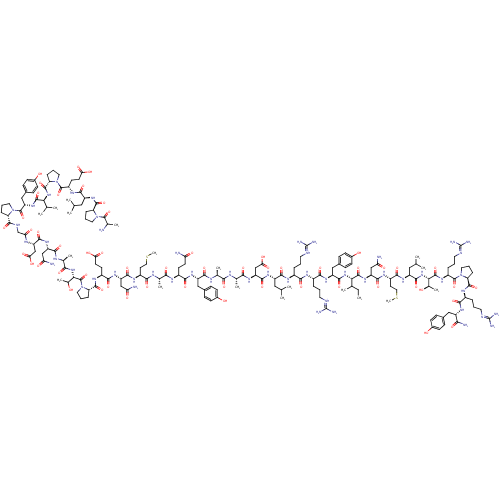
(Homo sapiens (Human)) | BDBM85679
(CAS_59763-91-6 | PP, human)Show SMILES [#6]-[#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7+]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7+])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#16]-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6](-[#6])-[#7])-[#6](-[#6])-[#6])-[#6](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#7])=O Show InChI InChI=1S/C184H285N53O54S2/c1-20-91(10)143(174(285)227-124(83-136(189)247)163(274)213-113(62-73-293-19)157(268)220-119(76-89(6)7)166(277)231-144(97(16)238)175(286)217-114(33-24-66-202-184(197)198)177(288)235-69-27-36-130(235)169(280)214-109(32-23-65-201-183(195)196)153(264)218-116(146(190)257)77-99-39-47-103(240)48-40-99)230-167(278)121(79-101-43-51-105(242)52-44-101)223-154(265)108(31-22-64-200-182(193)194)210-152(263)107(30-21-63-199-181(191)192)211-160(271)117(74-87(2)3)221-165(276)126(85-141(255)256)219-149(260)94(13)204-147(258)93(12)206-158(269)120(78-100-41-49-104(241)50-42-100)222-155(266)110(55-58-133(186)244)209-148(259)95(14)205-151(262)112(61-72-292-18)212-162(273)123(82-135(188)246)224-156(267)111(56-59-138(249)250)215-170(281)131-37-29-71-237(131)180(291)145(98(17)239)232-150(261)96(15)207-159(270)122(81-134(187)245)225-164(275)125(84-140(253)254)208-137(248)86-203-168(279)128-34-25-68-234(128)179(290)127(80-102-45-53-106(243)54-46-102)228-173(284)142(90(8)9)229-172(283)132-38-28-70-236(132)178(289)115(57-60-139(251)252)216-161(272)118(75-88(4)5)226-171(282)129-35-26-67-233(129)176(287)92(11)185/h39-54,87-98,107-132,142-145,238-243H,20-38,55-86,185H2,1-19H3,(H2,186,244)(H2,187,245)(H2,188,246)(H2,189,247)(H2,190,257)(H,203,279)(H,204,258)(H,205,262)(H,206,269)(H,207,270)(H,208,248)(H,209,259)(H,210,263)(H,211,271)(H,212,273)(H,213,274)(H,214,280)(H,215,281)(H,216,272)(H,217,286)(H,218,264)(H,219,260)(H,220,268)(H,221,276)(H,222,266)(H,223,265)(H,224,267)(H,225,275)(H,226,282)(H,227,285)(H,228,284)(H,229,283)(H,230,278)(H,231,277)(H,232,261)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H4,191,192,199)(H4,193,194,200)(H4,195,196,201)(H4,197,198,202)/p+2/t91?,92?,93-,94-,95-,96-,97+,98?,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,142-,143-,144-,145-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal Institute of Technology of Zurich
Curated by PDSP Ki Database
| |
J Biol Chem 275: 36043-8 (2000)
Article DOI: 10.1074/jbc.M000626200
BindingDB Entry DOI: 10.7270/Q2PV6HX1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50135800

((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CC3)c2c1 Show InChI InChI=1S/C20H27NO/c22-16-7-6-15-11-19-17-3-1-2-8-20(17,18(15)12-16)9-10-21(19)13-14-4-5-14/h6-7,12,14,17,19,22H,1-5,8-11,13H2/t17-,19+,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50240829
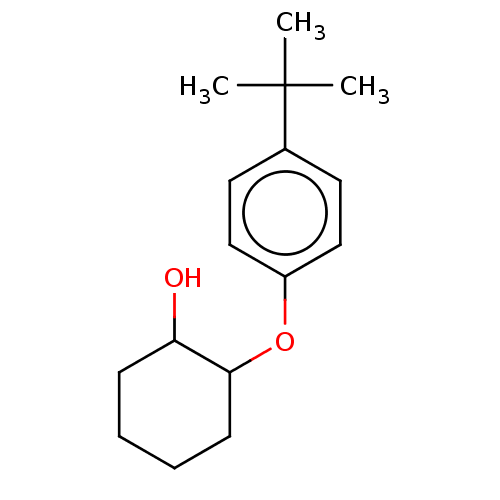
(CHEMBL3559801)Show InChI InChI=1S/C16H24O2/c1-16(2,3)12-8-10-13(11-9-12)18-15-7-5-4-6-14(15)17/h8-11,14-15,17H,4-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leipzig University
Curated by ChEMBL
| Assay Description
Effect on pancreatic polypeptide-mediated displacement of 125I-pancreatic polypeptide from human C-terminal eYFP-tagged NY4 receptor expressed in HEK... |
J Med Chem 60: 7605-7612 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00976
BindingDB Entry DOI: 10.7270/Q22B915R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50219919
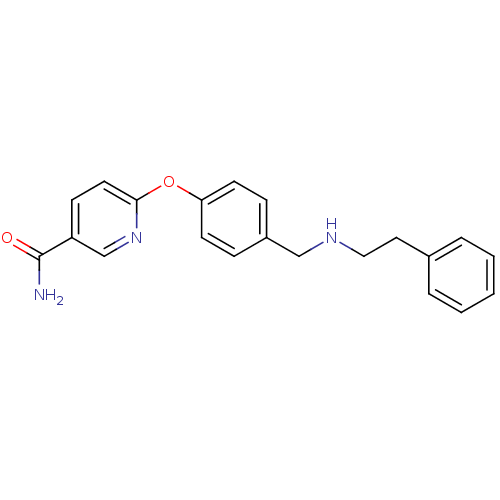
(6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...)Show InChI InChI=1S/C21H21N3O2/c22-21(25)18-8-11-20(24-15-18)26-19-9-6-17(7-10-19)14-23-13-12-16-4-2-1-3-5-16/h1-11,15,23H,12-14H2,(H2,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 5349-52 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.008
BindingDB Entry DOI: 10.7270/Q2668CX7 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50183152

(CHEMBL206580 | N-(4-(trifluoromethyl)benzyl)-N-iso...)Show InChI InChI=1S/C17H25F3N2/c1-13(2)11-22(16-7-9-21-10-8-16)12-14-3-5-15(6-4-14)17(18,19)20/h3-6,13,16,21H,7-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from SERT |
Bioorg Med Chem Lett 16: 2714-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.008
BindingDB Entry DOI: 10.7270/Q2SF2VR6 |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM50122293
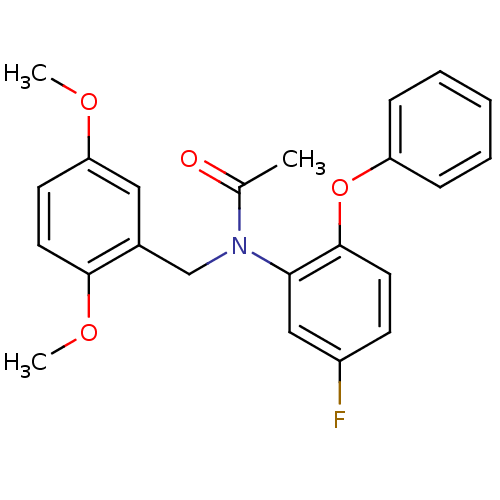
(CHEMBL401000 | CHEMBL63064 | N-(2,5-Dimethoxy-benz...)Show SMILES COc1ccc(OC)c(CN(C(C)=O)c2cc(F)ccc2Oc2ccccc2)c1 Show InChI InChI=1S/C23H22FNO4/c1-16(26)25(15-17-13-20(27-2)10-12-22(17)28-3)21-14-18(24)9-11-23(21)29-19-7-5-4-6-8-19/h4-14H,15H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0726 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from rat PBR receptor |
J Med Chem 52: 688-99 (2009)
Article DOI: 10.1021/jm8011855
BindingDB Entry DOI: 10.7270/Q2HX1CJP |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135808
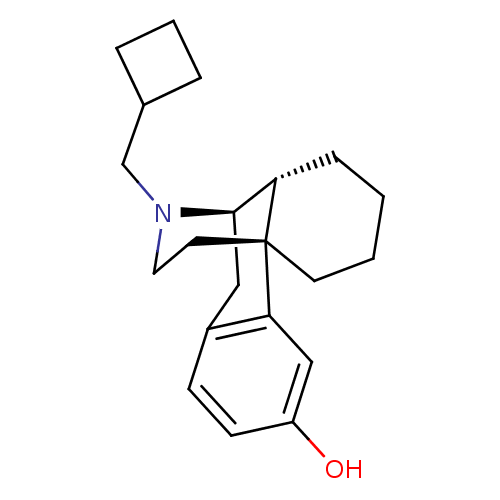
((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 |r,TLB:16:15:4.21.5:7| Show InChI InChI=1S/C21H29NO/c23-17-8-7-16-12-20-18-6-1-2-9-21(18,19(16)13-17)10-11-22(20)14-15-4-3-5-15/h7-8,13,15,18,20,23H,1-6,9-12,14H2/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor (unknown origin) |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50594654
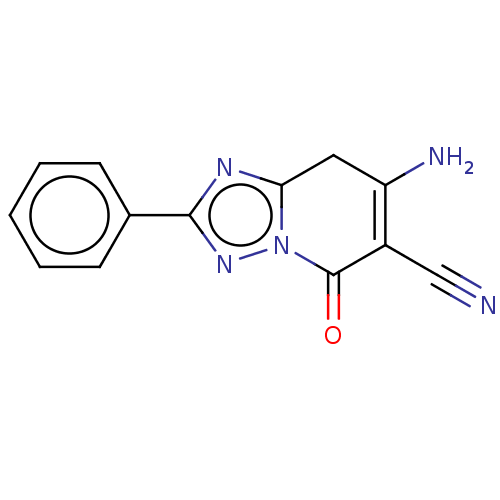
(CHEMBL5171044) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113907
BindingDB Entry DOI: 10.7270/Q20K2DJC |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135797
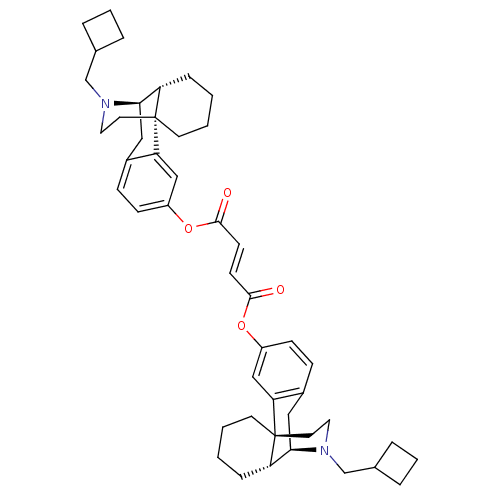
(CHEMBL146756 | di[17-cyclobutylmethyl-(1R,9R,10R)-...)Show SMILES O=C(Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1)\C=C\C(=O)Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 Show InChI InChI=1S/C46H58N2O4/c49-43(51-35-15-13-33-25-41-37-11-1-3-19-45(37,39(33)27-35)21-23-47(41)29-31-7-5-8-31)17-18-44(50)52-36-16-14-34-26-42-38-12-2-4-20-46(38,40(34)28-36)22-24-48(42)30-32-9-6-10-32/h13-18,27-28,31-32,37-38,41-42H,1-12,19-26,29-30H2/b18-17+/t37-,38-,41+,42+,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cell membrane |
Eur J Med Chem 108: 211-28 (2016)
Article DOI: 10.1016/j.ejmech.2015.11.028
BindingDB Entry DOI: 10.7270/Q2R2137W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50135808

((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...)Show SMILES Oc1ccc2C[C@@H]3[C@@H]4CCCC[C@]4(CCN3CC3CCC3)c2c1 |r,TLB:16:15:4.21.5:7| Show InChI InChI=1S/C21H29NO/c23-17-8-7-16-12-20-18-6-1-2-9-21(18,19(16)13-17)10-11-22(20)14-15-4-3-5-15/h7-8,13,15,18,20,23H,1-6,9-12,14H2/t18-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50303630

(17-(Cyclopropylmethyl)-N-phenylmorphinan-3-amine |...)Show SMILES C(C1CC1)N1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(Nc2ccccc2)cc31 |r| Show InChI InChI=1S/C26H32N2/c1-2-6-21(7-3-1)27-22-12-11-20-16-25-23-8-4-5-13-26(23,24(20)17-22)14-15-28(25)18-19-9-10-19/h1-3,6-7,11-12,17,19,23,25,27H,4-5,8-10,13-16,18H2/t23-,25+,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells |
J Med Chem 53: 402-18 (2010)
Article DOI: 10.1021/jm9013482
BindingDB Entry DOI: 10.7270/Q2668D84 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50258612
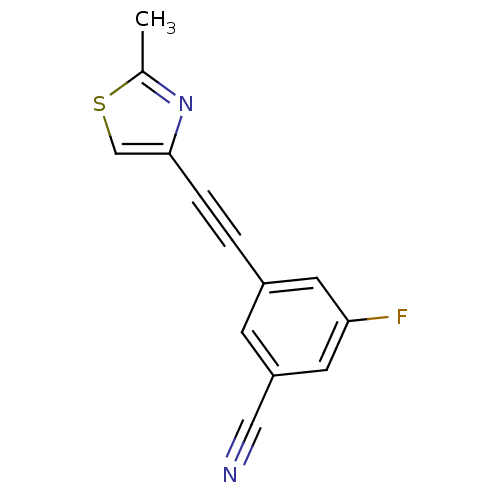
(3-Fluoro-5-cyano-1-(2-methylthiazol-4-ylethynyl)be...)Show InChI InChI=1S/C13H7FN2S/c1-9-16-13(8-17-9)3-2-10-4-11(7-15)6-12(14)5-10/h4-6,8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]MPEP from mGluR5 in rat brain |
J Med Chem 54: 901-8 (2012)
Article DOI: 10.1021/jm101430m
BindingDB Entry DOI: 10.7270/Q26Q1Z7T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50359955
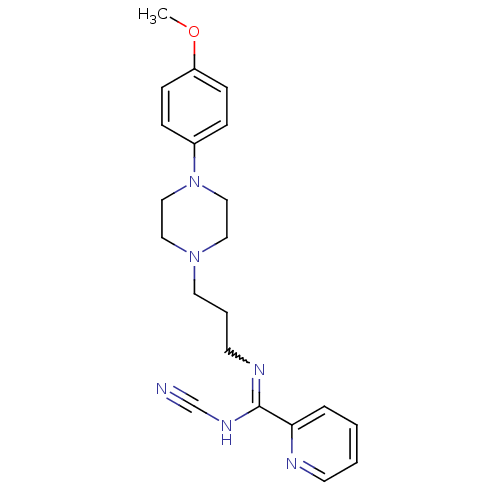
(CHEMBL1927089)Show SMILES COc1ccc(cc1)N1CCN(CCCN=C(NC#N)c2ccccn2)CC1 |w:15.15| Show InChI InChI=1S/C21H26N6O/c1-28-19-8-6-18(7-9-19)27-15-13-26(14-16-27)12-4-11-24-21(25-17-22)20-5-2-3-10-23-20/h2-3,5-10H,4,11-16H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [3H]-8-OH-DPAT from Sprague-Dawley rat brain cortex serotonin 5-HT1A receptor after 30 mins by liquid scintillation counting |
Eur J Med Chem 47: 520-9 (2012)
Article DOI: 10.1016/j.ejmech.2011.11.023
BindingDB Entry DOI: 10.7270/Q29S1RFN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data