

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50225899 ((+/-)-4-[(N-(2-methoxy-5-trifluoromethoxybenzyl)am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]substance P from NK1 human receptor expressed in IM9 cells | Bioorg Med Chem Lett 17: 6887-90 (2007) Article DOI: 10.1016/j.bmcl.2007.10.010 BindingDB Entry DOI: 10.7270/Q2GQ6XGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]substance P from NK1 human receptor expressed in IM9 cells | Bioorg Med Chem Lett 17: 6887-90 (2007) Article DOI: 10.1016/j.bmcl.2007.10.010 BindingDB Entry DOI: 10.7270/Q2GQ6XGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50225898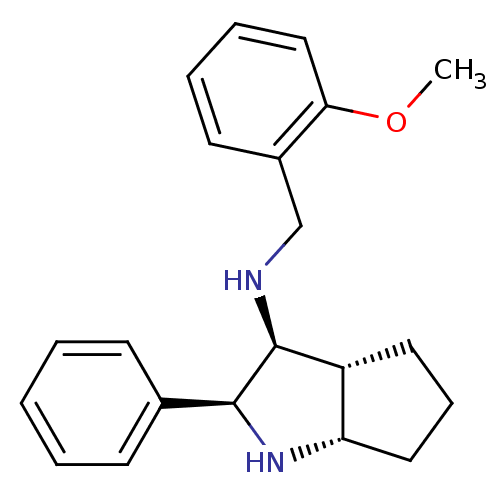 ((2-Methoxy-benzyl)-((2S,3S,3aR,6aS)-2-phenyl-octah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]substance P from NK1 human receptor expressed in IM9 cells | Bioorg Med Chem Lett 17: 6887-90 (2007) Article DOI: 10.1016/j.bmcl.2007.10.010 BindingDB Entry DOI: 10.7270/Q2GQ6XGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020299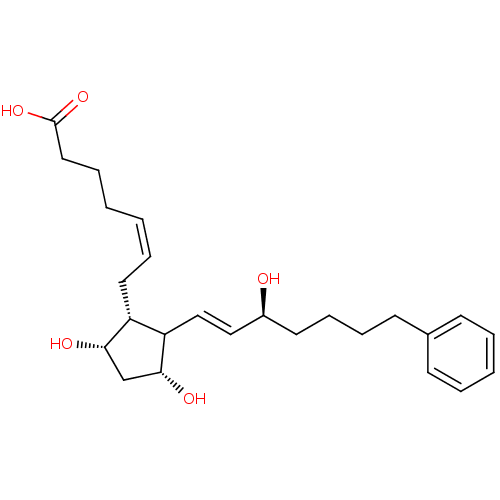 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50035622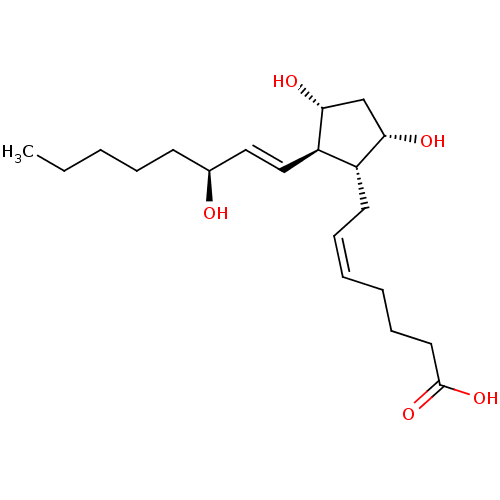 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase | Bioorg Med Chem 15: 7803-8 (2007) Article DOI: 10.1016/j.bmc.2007.08.043 BindingDB Entry DOI: 10.7270/Q2F190JK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50405735 (CHEMBL2114230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes... | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020299 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020302 ((S-isomer)7-{2-[5-(4-Azido-phenyl)-3-hydroxy-pent-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020303 ((R-isomer)7-{2-[5-(4-Azido-3-iodo-phenyl)-3-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308527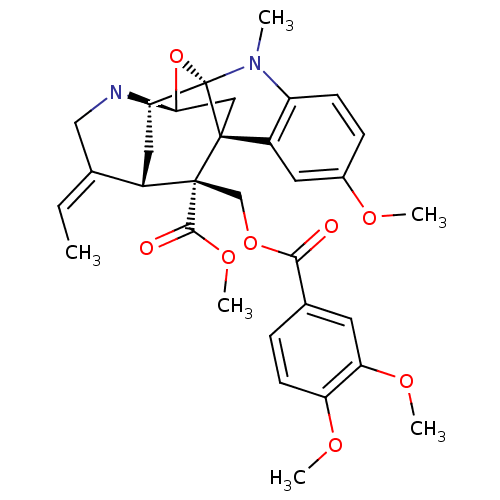 (10-methoxy-N(1)-methylburnamine-17-O-veratrate | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50405735 (CHEMBL2114230) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50012278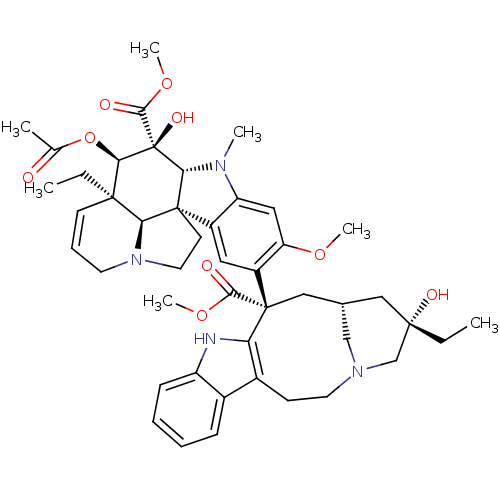 ((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308529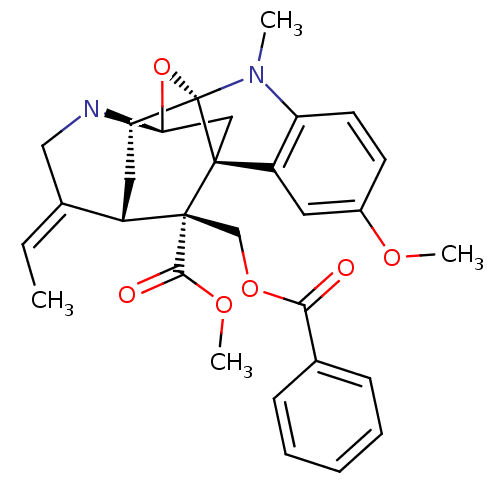 (CHEMBL589220 | Methyl (14E)-19-[(benzoyloxy)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308533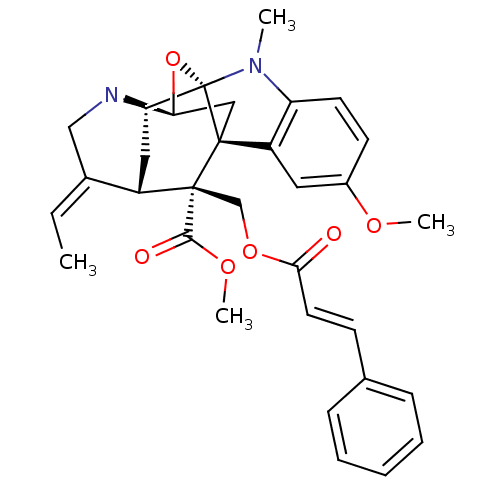 (CHEMBL589254 | Methyl (14E)-14-ethylidene-6-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483304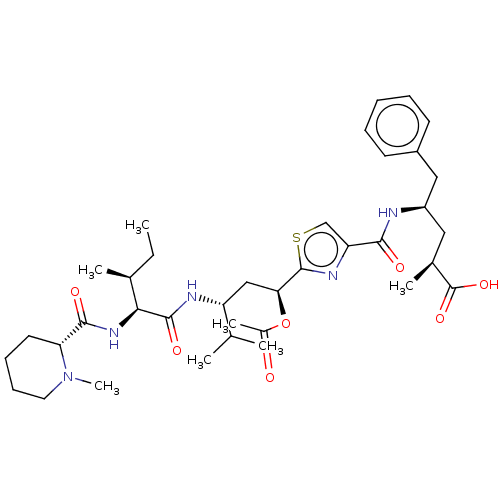 (11-Epi-Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50012278 ((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483306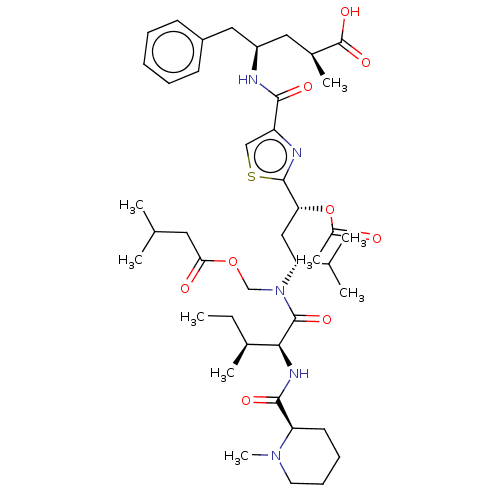 (CHEBI:80036 | Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483305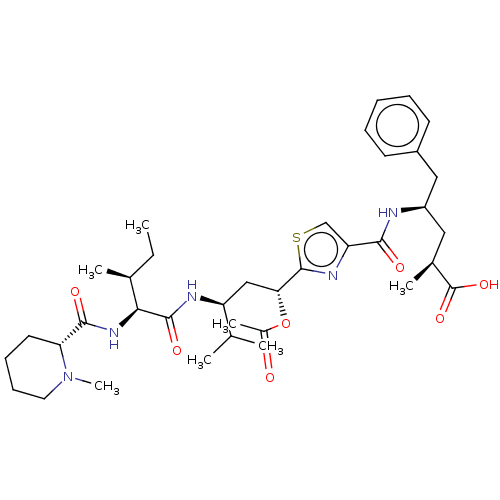 (13-Epi-Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020301 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308528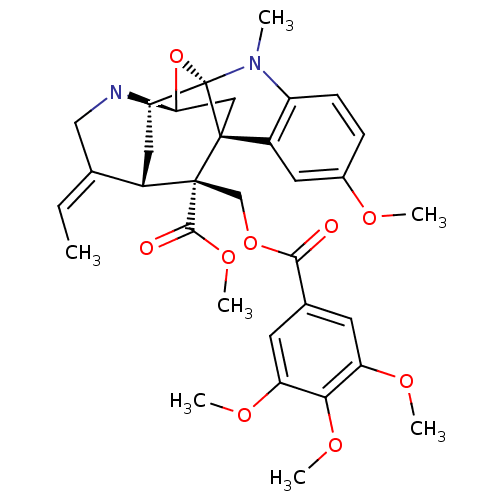 (Alstiphyllanine D | CHEMBL540945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308532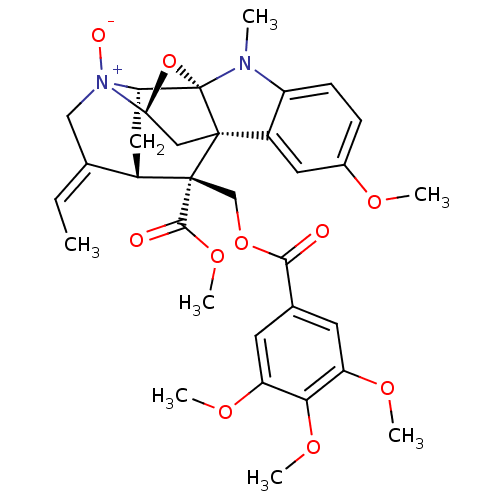 ((14E)-14-ethylidene-6-methoxy-19-(methoxycarbonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308527 (10-methoxy-N(1)-methylburnamine-17-O-veratrate | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50201171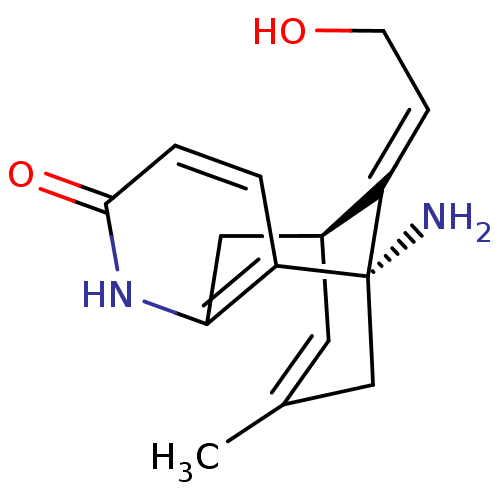 ((1S,9R)-1-amino-13-[2-hydroxy-eth-(E)-ylidene]-11-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte acetylcholinesterase | Bioorg Med Chem 15: 1703-7 (2007) Article DOI: 10.1016/j.bmc.2006.12.005 BindingDB Entry DOI: 10.7270/Q2X929XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308531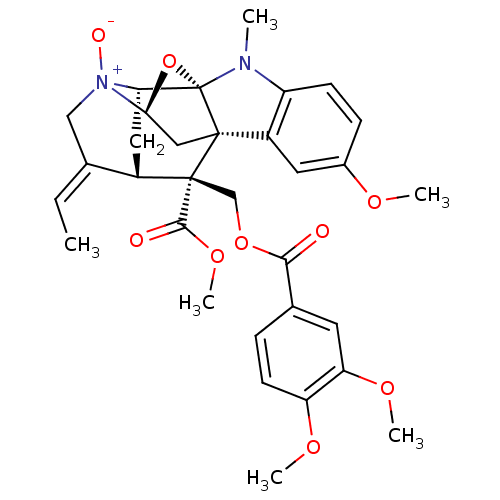 ((14E)-19-{[(3,4-dimethoxyphenyl)carbonyloxy]methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308533 (CHEMBL589254 | Methyl (14E)-14-ethylidene-6-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308528 (Alstiphyllanine D | CHEMBL540945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020306 ((S-isomer)7-{2-[5-(4-Azido-2-hydroxy-phenyl)-3-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483307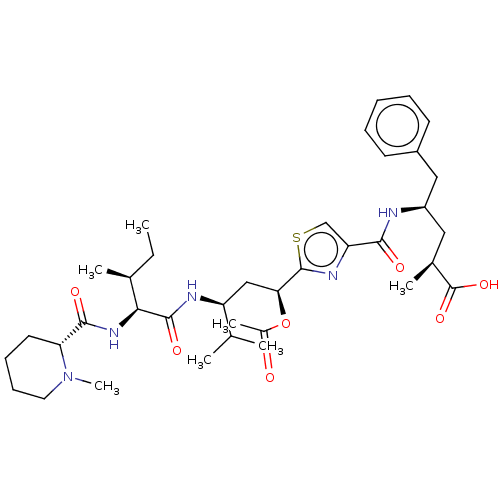 (CHEMBL1643767) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020302 ((S-isomer)7-{2-[5-(4-Azido-phenyl)-3-hydroxy-pent-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes... | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308530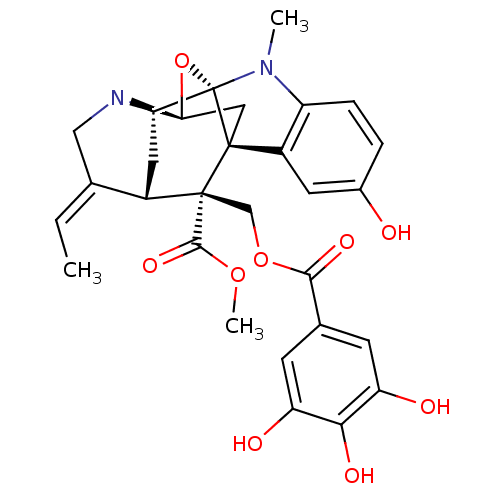 (CHEMBL602442 | Methyl (14E)-14-ethylidene-6-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020303 ((R-isomer)7-{2-[5-(4-Azido-3-iodo-phenyl)-3-hydrox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes... | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50201170 (CHEMBL241838 | carinatumins B (2)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte acetylcholinesterase | Bioorg Med Chem 15: 1703-7 (2007) Article DOI: 10.1016/j.bmc.2006.12.005 BindingDB Entry DOI: 10.7270/Q2X929XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin alpha-1A chain (Sus scrofa (Pig)) | BDBM50148881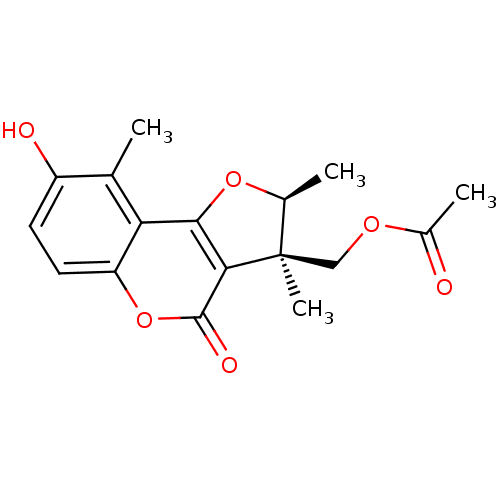 (Acetic acid (2S,3R)-8-hydroxy-2,3,9-trimethyl-4-ox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of the polymerization of tubulin | Bioorg Med Chem Lett 14: 3665-8 (2004) Article DOI: 10.1016/j.bmcl.2004.05.015 BindingDB Entry DOI: 10.7270/Q2Z037MD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50160802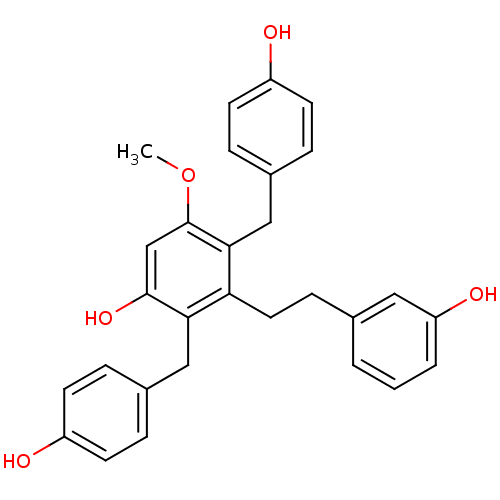 (2,4-Bis-(4-hydroxy-benzyl)-3-[2-(3-hydroxy-phenyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50160801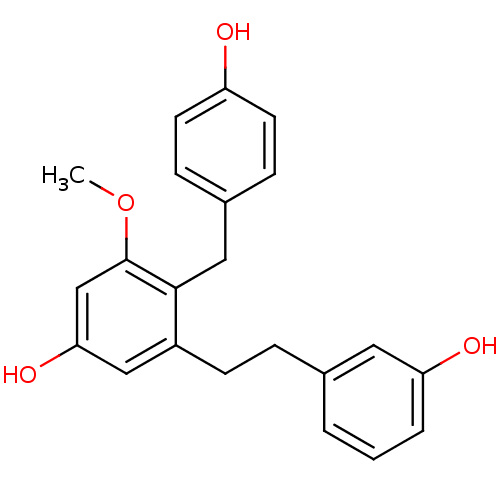 (4-(4-Hydroxy-benzyl)-3-[2-(3-hydroxy-phenyl)-ethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308532 ((14E)-14-ethylidene-6-methoxy-19-(methoxycarbonyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020305 ((R-isomer)7-{2-[4-(3-Azido-5-hydroxy-phenoxy)-3-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50308535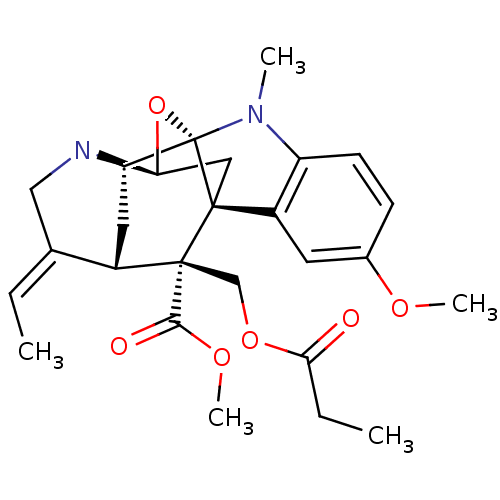 (CHEMBL601712 | methyl (14E)-14-ethylidene-6-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT2 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50308529 (CHEMBL589220 | Methyl (14E)-19-[(benzoyloxy)methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of alpha-D-[U-14C]glucopyranoside uptake at human SGLT1 expressed in african green monkey COS1 cells after 30 mins by liquid scintillation... | Bioorg Med Chem 18: 2152-8 (2010) Article DOI: 10.1016/j.bmc.2010.01.077 BindingDB Entry DOI: 10.7270/Q2HD7VSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50316419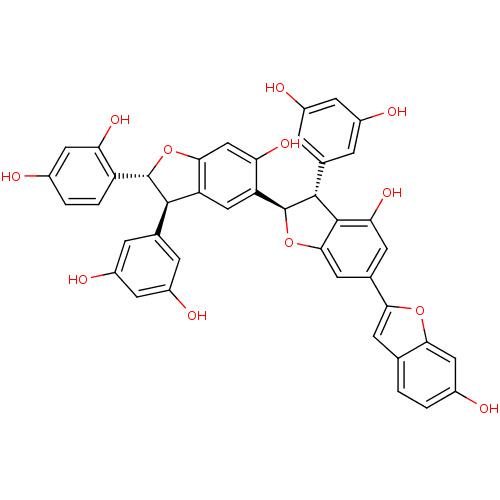 (CHEMBL1097908 | Gneyulin B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of SGLT2 transfected in african green monkey COS1 cells assessed as alpha-D-[U-14C]glucopyranoside uptake after 30 mins by scintillation c... | J Nat Prod 73: 763-7 (2010) Article DOI: 10.1021/np9007987 BindingDB Entry DOI: 10.7270/Q2BP02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50223709 (CHEMBL240094 | cryptadine B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase | Bioorg Med Chem 15: 7803-8 (2007) Article DOI: 10.1016/j.bmc.2007.08.043 BindingDB Entry DOI: 10.7270/Q2F190JK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271482 (CHEMBL455781 | lycoparin C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase from bovine erythrocyte | Bioorg Med Chem 16: 6167-71 (2008) Article DOI: 10.1016/j.bmc.2008.04.044 BindingDB Entry DOI: 10.7270/Q28S4PQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50316418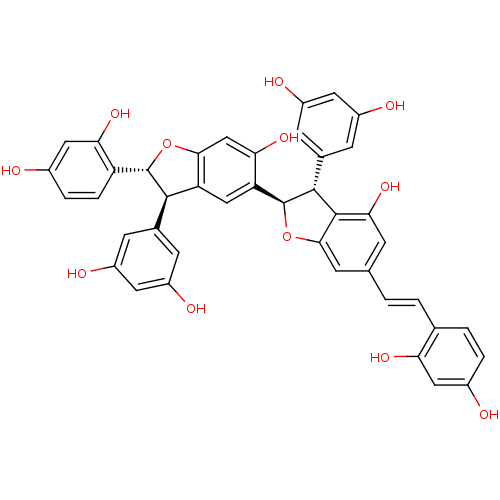 (CHEMBL1097907 | Gneyulin A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of SGLT2 transfected in african green monkey COS1 cells assessed as alpha-D-[U-14C]glucopyranoside uptake after 30 mins by scintillation c... | J Nat Prod 73: 763-7 (2010) Article DOI: 10.1021/np9007987 BindingDB Entry DOI: 10.7270/Q2BP02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50316418 (CHEMBL1097907 | Gneyulin A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoshi University Curated by ChEMBL | Assay Description Inhibition of SGLT1 transfected in african green monkey COS1 cells assessed as alpha-D-[U-14C]glucopyranoside uptake after 30 mins by scintillation c... | J Nat Prod 73: 763-7 (2010) Article DOI: 10.1021/np9007987 BindingDB Entry DOI: 10.7270/Q2BP02XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50160804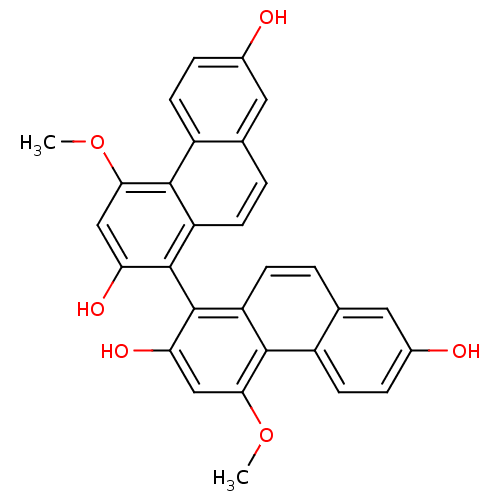 (4,4''-Dimethoxy-[1,1'']biphenanthrenyl-2,7,2'',7''...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50160803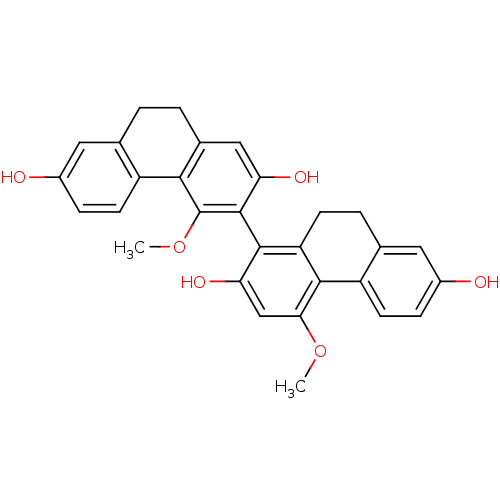 (4,4''-Dimethoxy-9,10,9'',10''-tetrahydro-[1,3'']bi...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain [1-43] (Leishmania donovani) | BDBM50160805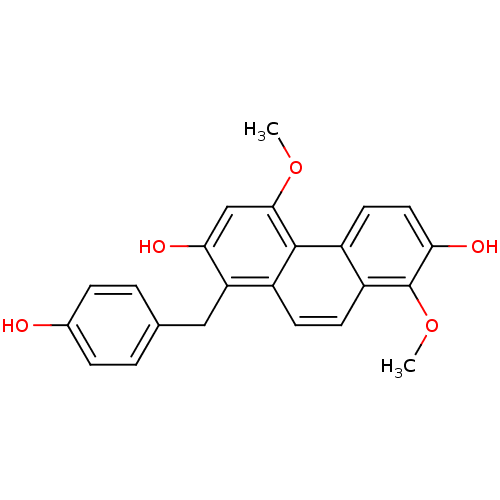 (1-(4-Hydroxy-benzyl)-4,8-dimethoxy-phenanthrene-2,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of tubulin polymerization interacting at the colchicine binding site. | Bioorg Med Chem Lett 15: 1051-4 (2005) Article DOI: 10.1016/j.bmcl.2004.12.026 BindingDB Entry DOI: 10.7270/Q22Z151S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |