

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417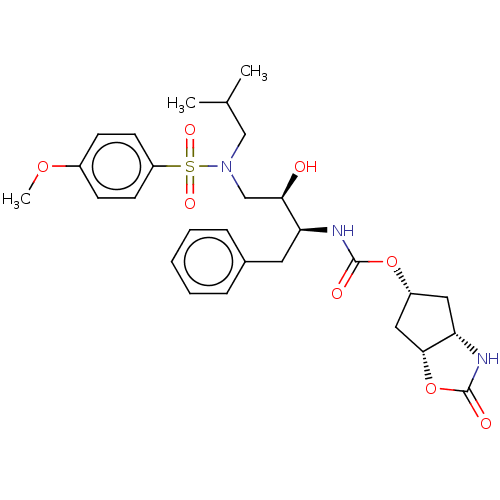 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520752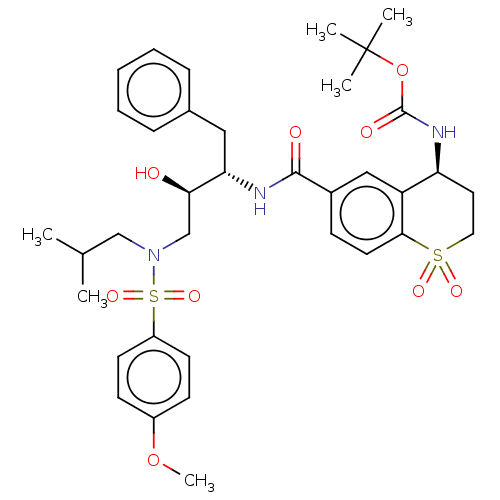 (CHEMBL4474261) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50095105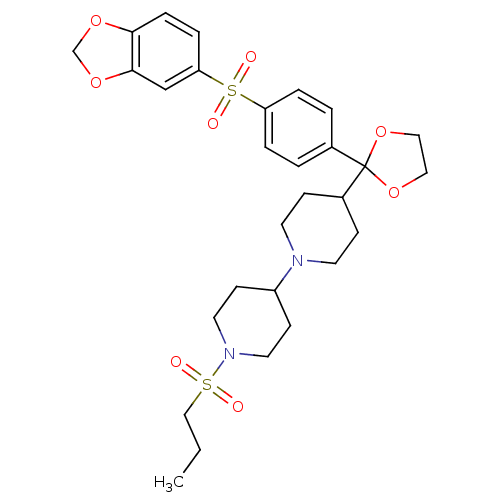 (4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469413 (CHEMBL4286231) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469407 (CHEMBL4286714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469410 (CHEMBL4277886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469405 (CHEMBL4292006) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50560609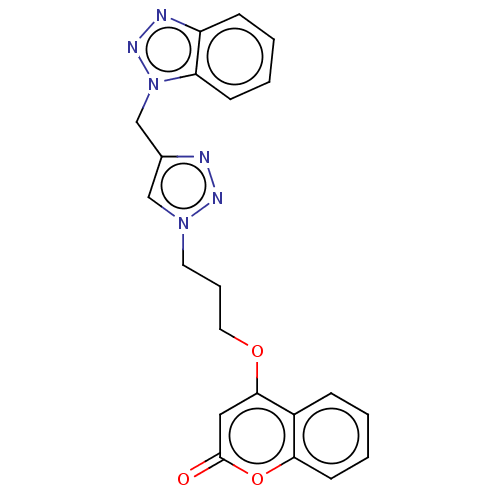 (CHEMBL4749763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127477 BindingDB Entry DOI: 10.7270/Q2W099MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809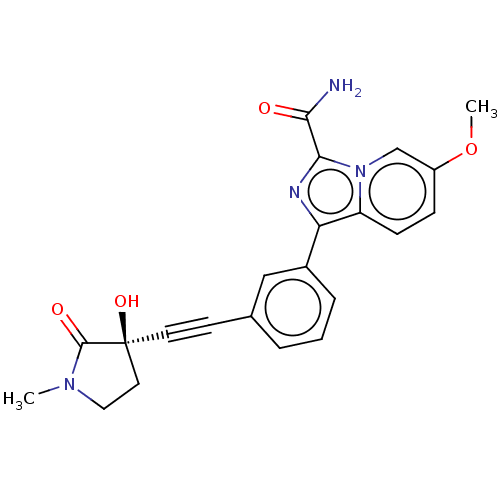 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520751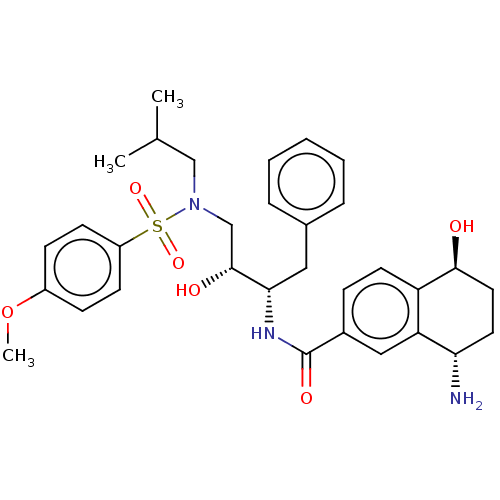 (CHEMBL4473891) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457816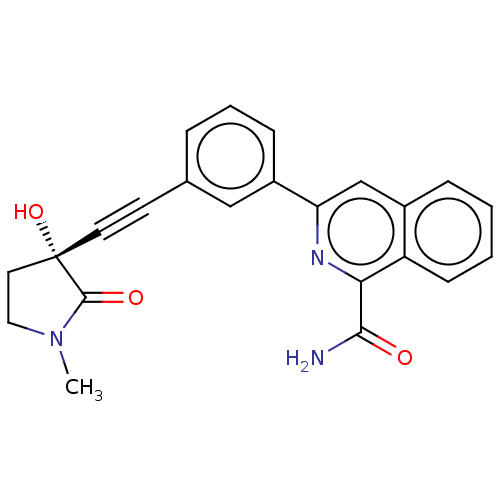 (CHEMBL4215425) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM50457820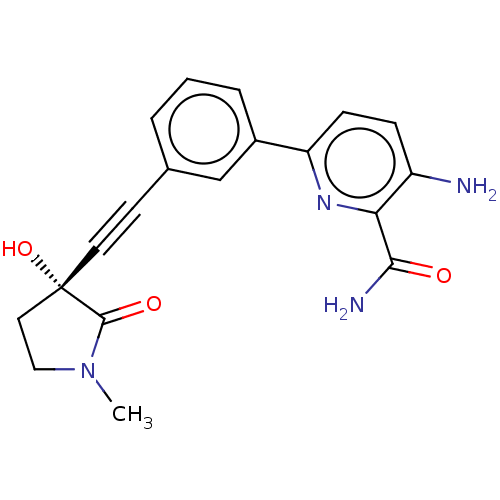 (CHEMBL4216289) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422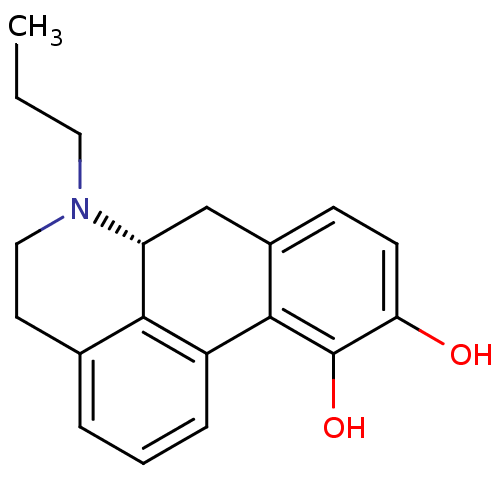 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from high-affinity state of human dopamine D2L receptor transfected in HEK293 cells after 2 hrs by scintillation counti... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from high-affinity state of human dopamine D2L receptor transfected in HEK293 cells after 2 hrs by scintillation counti... | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469412 (CHEMBL4277970) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312763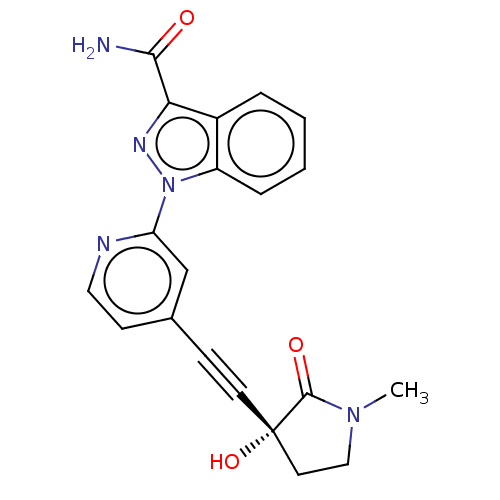 (1-[4-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165935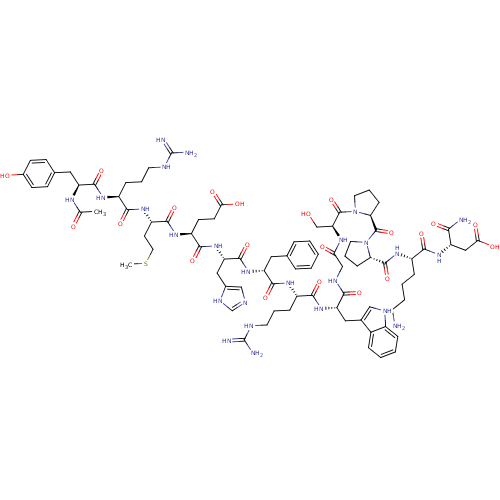 (Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Rattus norvegicus) | BDBM50165931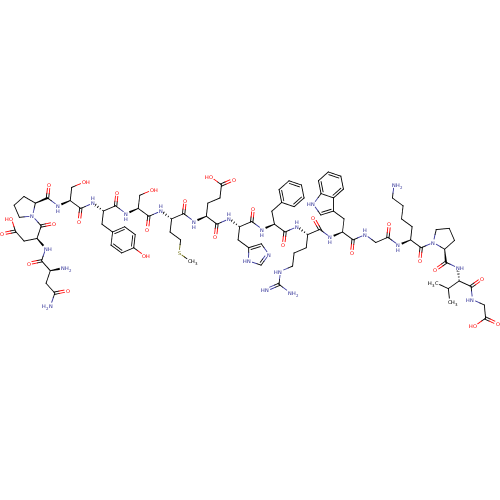 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity for rat melanocortin-4 receptor | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165931 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520749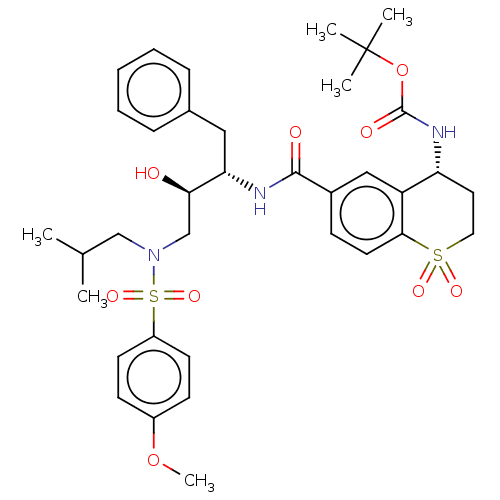 (CHEMBL4459250) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50243232 (CHEMBL486232 | GNF-PF-5434 | N-((S)-4-methyl-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF) Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S by fluorescence assay | Bioorg Med Chem 17: 1064-70 (2009) Article DOI: 10.1016/j.bmc.2008.02.002 BindingDB Entry DOI: 10.7270/Q20R9P6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007422 ((+)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Amsterdam Curated by ChEMBL | Assay Description Binding affinity to high-affinity state of D2L receptor (unknown origin) expressed in CHO cell membranes | J Med Chem 57: 391-410 (2014) Article DOI: 10.1021/jm401384w BindingDB Entry DOI: 10.7270/Q2WS8VQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50165935 (Ac-YRMEHdFRWGSPPKD-NH2 | CHEMBL414718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312785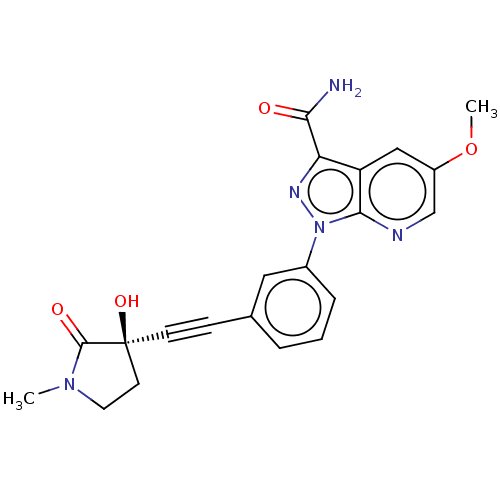 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013943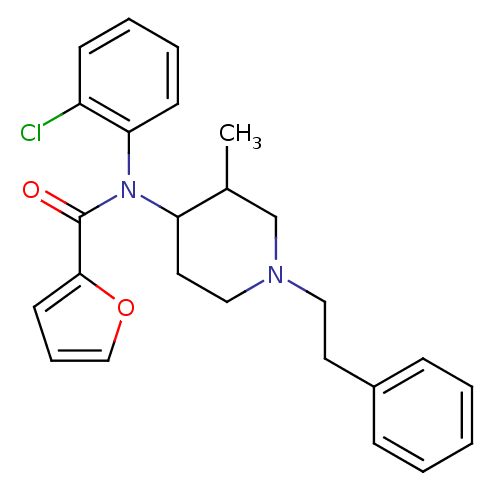 (CHEMBL327270 | Furan-2-carboxylic acid (2-chloro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103771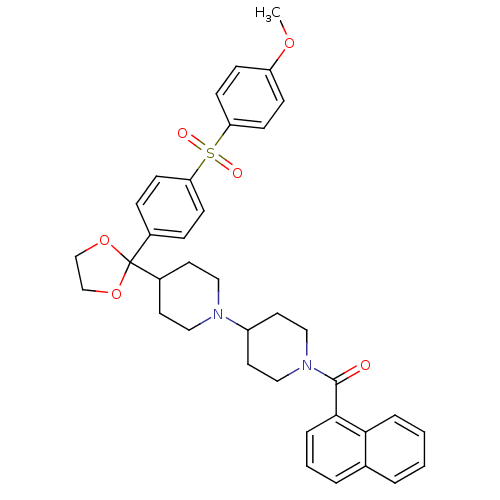 ((4-{2-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-[1,3]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50165929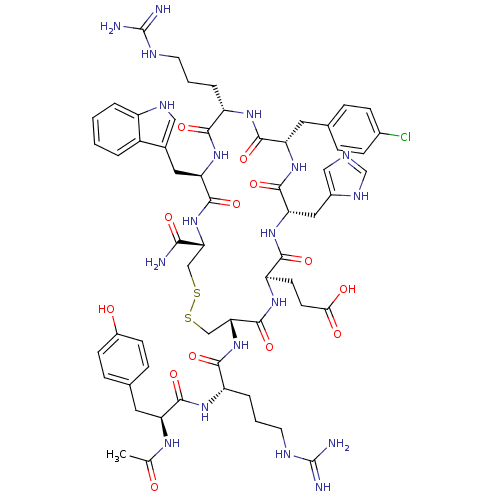 (Ac-YR[CEH(pCl-dF)RWC]-NH2 | CHEMBL415661) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-4 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469418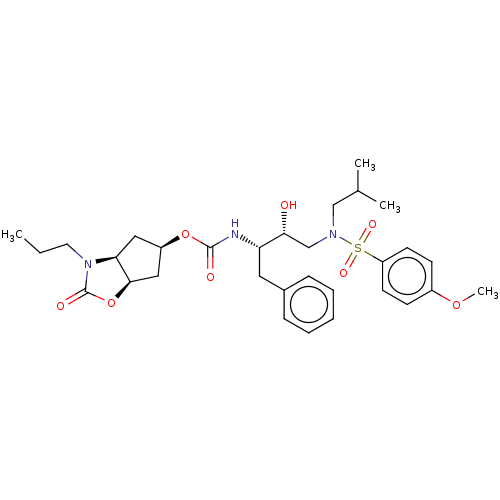 (CHEMBL4283819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50520742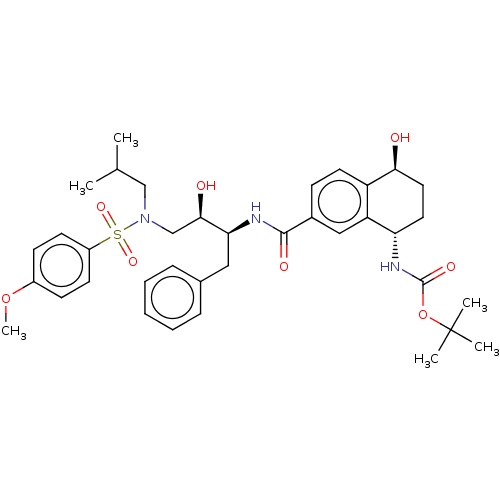 (CHEMBL4526105) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using fluorogenic substrate by continuous fluorometric assay | Eur J Med Chem 160: 171-182 (2018) Article DOI: 10.1016/j.ejmech.2018.09.046 BindingDB Entry DOI: 10.7270/Q2320098 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013945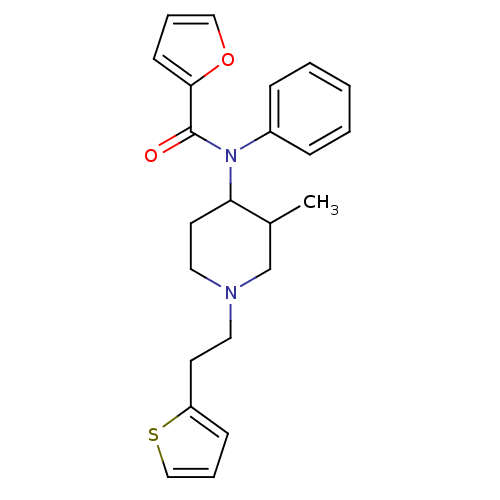 (CHEMBL431047 | Furan-2-carboxylic acid [3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469409 (CHEMBL4278306) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469406 (CHEMBL4277539) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312765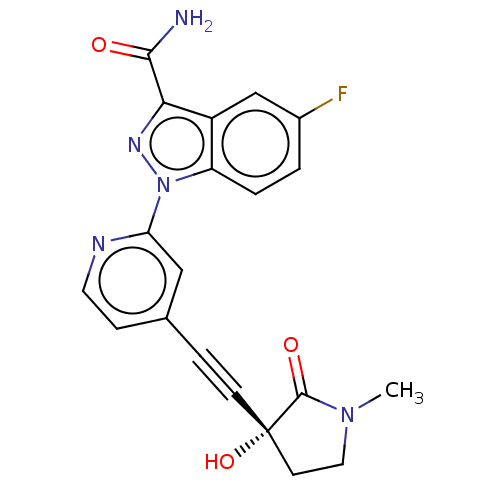 (5-fluoro-1-[4-[2-[(3R)-3-hy- droxy-1-methyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 5 (Homo sapiens (Human)) | BDBM50165931 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-5 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312788 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30369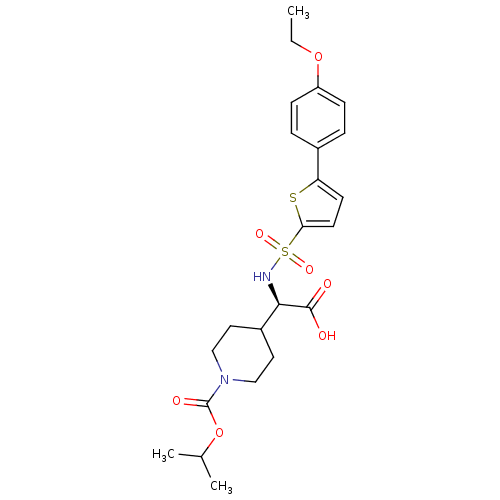 (piperidinyl glycine derivative, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934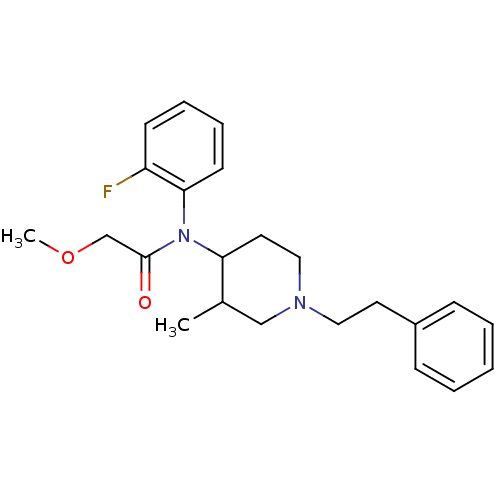 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469408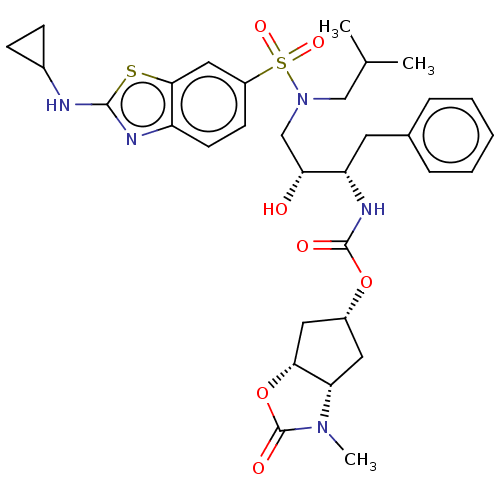 (CHEMBL4278989) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21137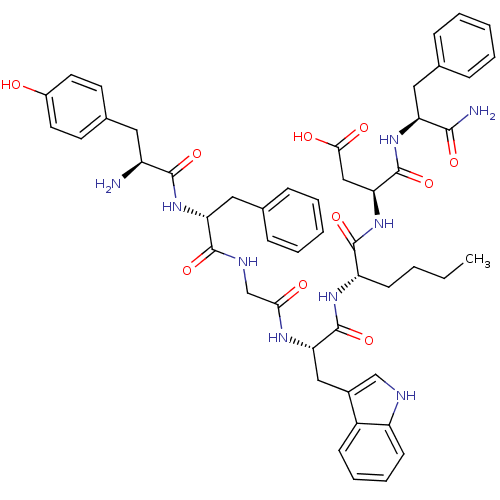 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -55.4 | n/a | n/a | 0.900 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103774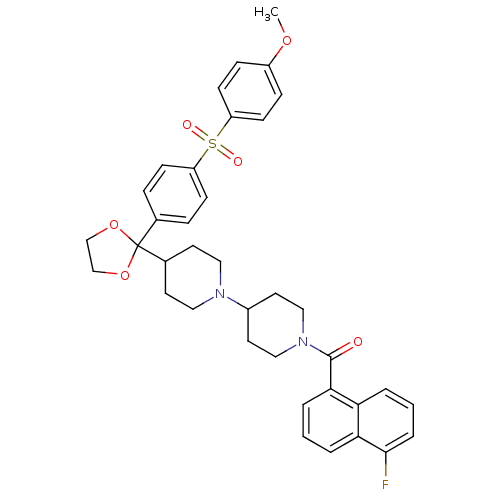 ((5-Fluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50165932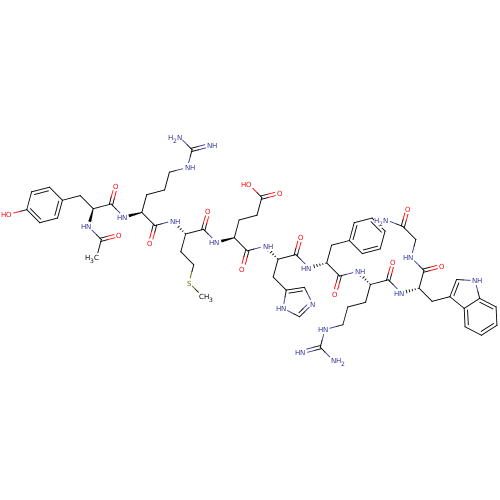 (Ac-YRMEHdFRWG-NH2 | CHEMBL266879) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]NDP-alpha-MSH binding to melanocortin-1 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM94503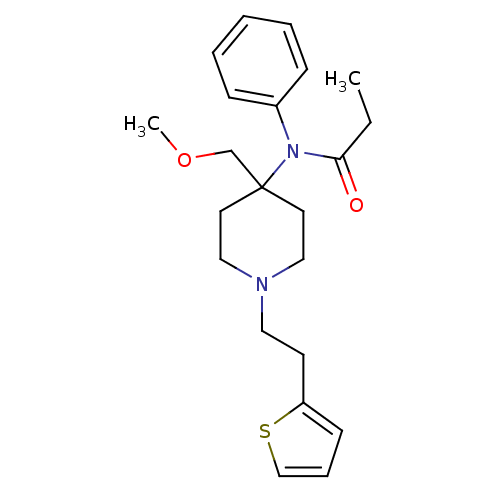 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21135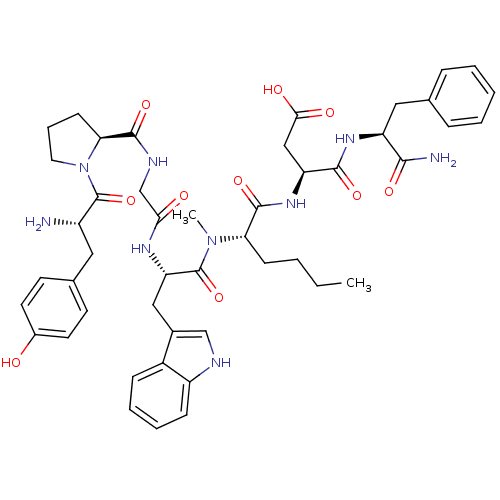 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21138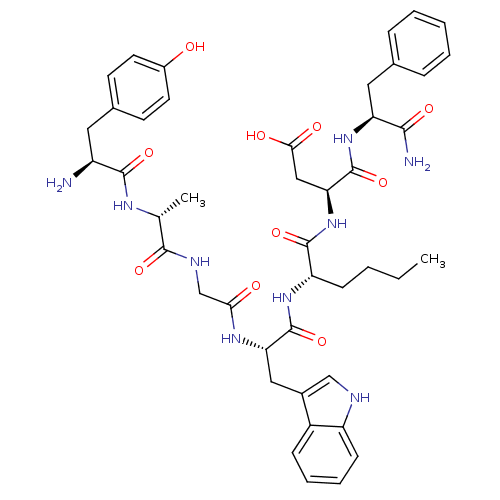 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -55.1 | n/a | n/a | 5.90 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50103772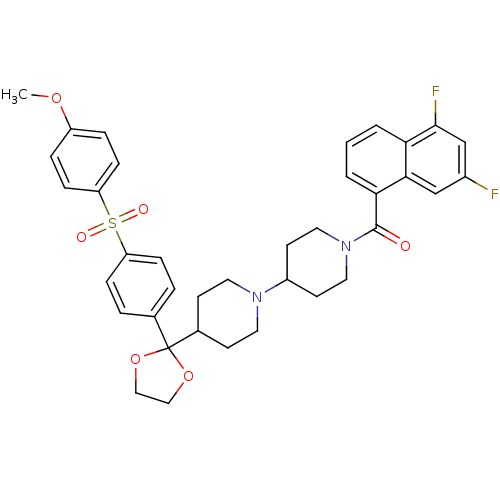 ((5,7-Difluoro-naphthalen-1-yl)-(4-{2-[4-(4-methoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 11: 2311-4 (2001) BindingDB Entry DOI: 10.7270/Q28S4P7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50183555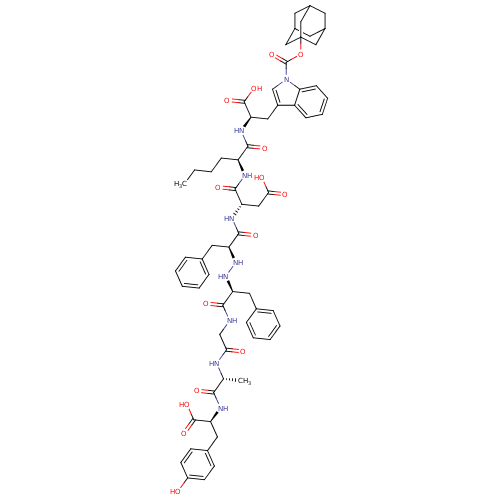 (CHEMBL408832 | H-Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cells | J Med Chem 49: 1773-80 (2006) Article DOI: 10.1021/jm050851n BindingDB Entry DOI: 10.7270/Q2FT8MVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50165931 (CHEMBL415165 | NDP-SYSMEHFRWGKPVG) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-NDP-alpha-MSH binding to melanocortin-3 receptor expressed in HEK293 cells | J Med Chem 48: 3095-8 (2005) Article DOI: 10.1021/jm0501432 BindingDB Entry DOI: 10.7270/Q2251HQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 14879 total ) | Next | Last >> |