 Found 1319 hits with Last Name = 'zheng' and Initial = 'h'
Found 1319 hits with Last Name = 'zheng' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129
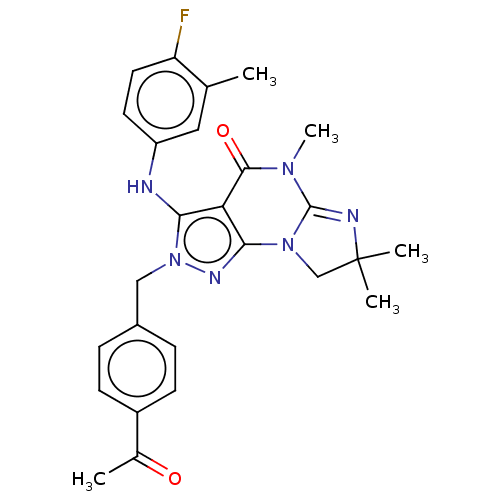
(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM497267
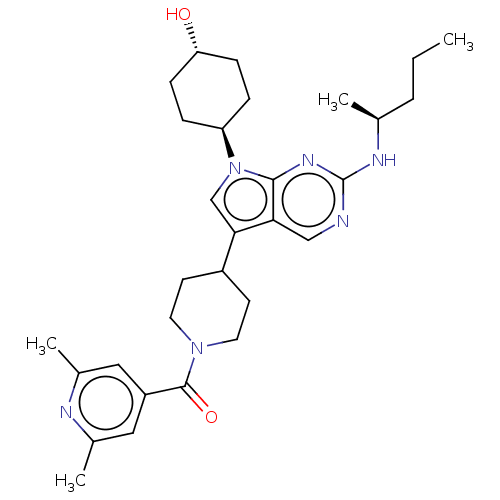
((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...)Show SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)C1CCN(CC1)C(=O)c1cc(C)nc(C)c1 |r,wU:13.12,wD:3.3,16.16,(-8.93,-2.23,;-7.59,-1.46,;-6.26,-2.23,;-4.93,-1.46,;-4.93,.08,;-3.59,-2.23,;-2.26,-1.46,;-2.26,.08,;-.93,.85,;.41,.08,;1.87,.56,;2.78,-.69,;1.87,-1.94,;2.35,-3.4,;3.85,-3.72,;4.33,-5.19,;3.3,-6.33,;3.78,-7.79,;1.79,-6.01,;1.32,-4.55,;.41,-1.46,;-.93,-2.23,;2.35,2.02,;3.85,2.34,;4.33,3.81,;3.3,4.95,;1.79,4.63,;1.32,3.16,;3.78,6.41,;3.04,7.77,;5.26,6.02,;6.35,7.1,;7.84,6.71,;8.93,7.79,;8.24,5.22,;7.15,4.13,;7.55,2.64,;5.66,4.53,)| Show InChI InChI=1S/C30H42N6O2/c1-5-6-19(2)33-30-31-17-26-27(18-36(28(26)34-30)24-7-9-25(37)10-8-24)22-11-13-35(14-12-22)29(38)23-15-20(3)32-21(4)16-23/h15-19,22,24-25,37H,5-14H2,1-4H3,(H,31,33,34)/t19-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ATP competitive inhibition of MERTK (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113534
BindingDB Entry DOI: 10.7270/Q2M90DGJ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126
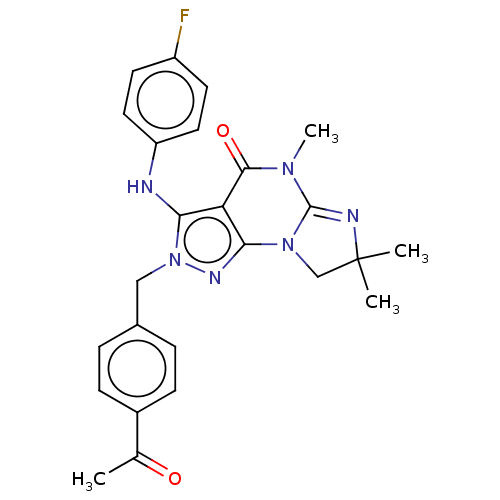
(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128
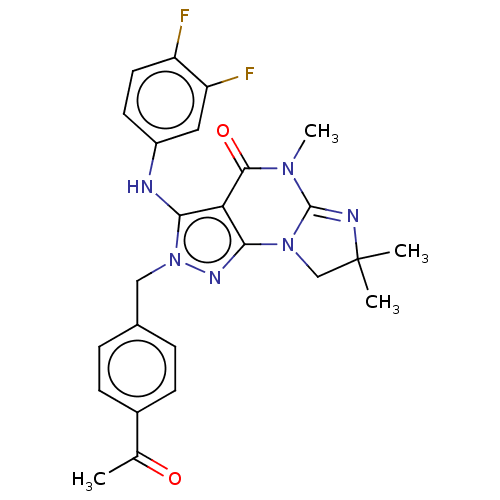
(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168129

(US9073936, 4 | US9598426, 4)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(C)c3)c2C1=O |t:2| Show InChI InChI=1S/C26H27FN6O2/c1-15-12-19(10-11-20(15)27)28-22-21-23(32-14-26(3,4)29-25(32)31(5)24(21)35)30-33(22)13-17-6-8-18(9-7-17)16(2)34/h6-12,28H,13-14H2,1-5H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127
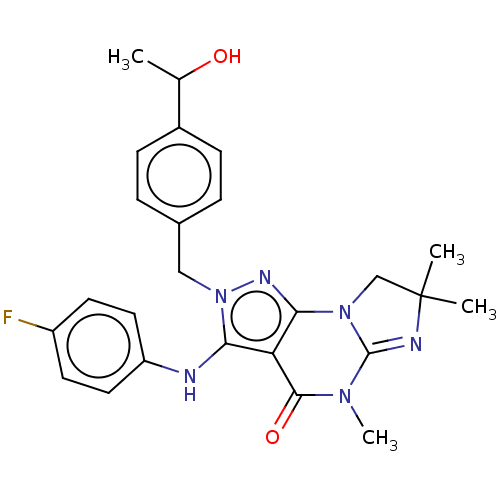
(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Assay: The following phosphodiesterase enzymes may be used: 3′,5′-cyclic-nucleotide-specific bovine brain phosphodiesterase (Sigma, St. L... |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50088373
(CHEBI:46295 | Vardenafil | cid_110634)Show SMILES CCCc1nc(C)c2n1[nH]c(nc2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168126

(US9073936, 1 | US9598426, 1)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)cc3)c2C1=O |t:2| Show InChI InChI=1S/C25H25FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,27H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50510071
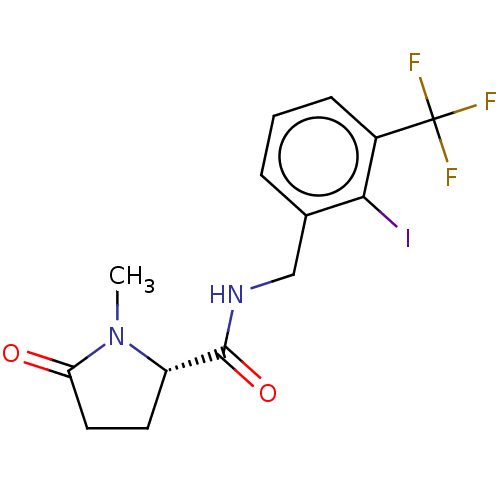
(CHEMBL4541082)Show SMILES CN1[C@@H](CCC1=O)C(=O)NCc1cccc(c1I)C(F)(F)F |r| Show InChI InChI=1S/C14H14F3IN2O2/c1-20-10(5-6-11(20)21)13(22)19-7-8-3-2-4-9(12(8)18)14(15,16)17/h2-4,10H,5-7H2,1H3,(H,19,22)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... |
Bioorg Med Chem Lett 29: 1476-1480 (2019)
Article DOI: 10.1016/j.bmcl.2019.04.018
BindingDB Entry DOI: 10.7270/Q2C250RG |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50510072
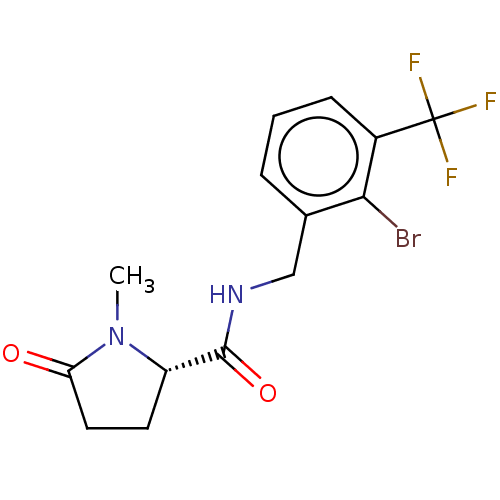
(CHEMBL4435339)Show SMILES CN1[C@@H](CCC1=O)C(=O)NCc1cccc(c1Br)C(F)(F)F |r| Show InChI InChI=1S/C14H14BrF3N2O2/c1-20-10(5-6-11(20)21)13(22)19-7-8-3-2-4-9(12(8)15)14(16,17)18/h2-4,10H,5-7H2,1H3,(H,19,22)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... |
Bioorg Med Chem Lett 29: 1476-1480 (2019)
Article DOI: 10.1016/j.bmcl.2019.04.018
BindingDB Entry DOI: 10.7270/Q2C250RG |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416603
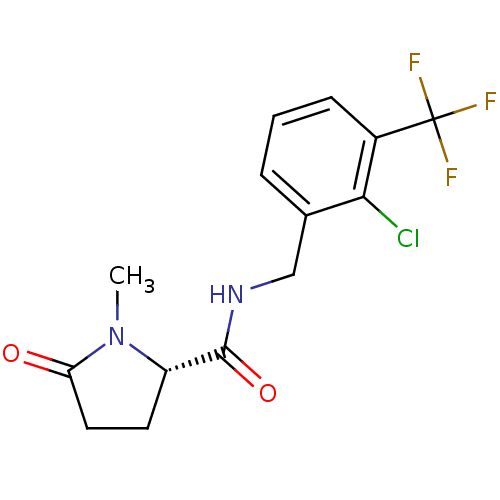
(CHEMBL1222883)Show SMILES CN1[C@@H](CCC1=O)C(=O)NCc1cccc(c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C14H14ClF3N2O2/c1-20-10(5-6-11(20)21)13(22)19-7-8-3-2-4-9(12(8)15)14(16,17)18/h2-4,10H,5-7H2,1H3,(H,19,22)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... |
Bioorg Med Chem Lett 29: 1476-1480 (2019)
Article DOI: 10.1016/j.bmcl.2019.04.018
BindingDB Entry DOI: 10.7270/Q2C250RG |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50125136

(2-[2,3']Bipyridinyl-6'-yl-1-(2,3-dihydro-benzofura...)Show SMILES C1Cc2cc(ccc2O1)C1N(CCc2c1[nH]c1ccccc21)c1ccc(cn1)-c1ccccn1 Show InChI InChI=1S/C29H24N4O/c1-2-7-25-22(5-1)23-12-15-33(27-11-9-21(18-31-27)24-6-3-4-14-30-24)29(28(23)32-25)20-8-10-26-19(17-20)13-16-34-26/h1-11,14,17-18,29,32H,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human corpus cavernosum PDE5 |
Eur J Med Chem 150: 30-38 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.039
BindingDB Entry DOI: 10.7270/Q2222XCS |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50125136

(2-[2,3']Bipyridinyl-6'-yl-1-(2,3-dihydro-benzofura...)Show SMILES C1Cc2cc(ccc2O1)C1N(CCc2c1[nH]c1ccccc21)c1ccc(cn1)-c1ccccn1 Show InChI InChI=1S/C29H24N4O/c1-2-7-25-22(5-1)23-12-15-33(27-11-9-21(18-31-27)24-6-3-4-14-30-24)29(28(23)32-25)20-8-10-26-19(17-20)13-16-34-26/h1-11,14,17-18,29,32H,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5-HT2A receptor in human frontal cortex after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 1953-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.045
BindingDB Entry DOI: 10.7270/Q2S183VK |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | -48.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| Assay Description
Materials: All chemicals are available from Sigma-Aldrich (St. Louis, Mo.) except for IMAP reagents (reaction buffer, binding buffer, FL-GMP and IMAP... |
US Patent US9073936 (2015)
BindingDB Entry DOI: 10.7270/Q2348J5T |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168128

(US9073936, 3 | US9598426, 3)Show SMILES CN1C2=NC(C)(C)CN2c2nn(Cc3ccc(cc3)C(C)=O)c(Nc3ccc(F)c(F)c3)c2C1=O |t:2| Show InChI InChI=1S/C25H24F2N6O2/c1-14(34)16-7-5-15(6-8-16)12-33-21(28-17-9-10-18(26)19(27)11-17)20-22(30-33)32-13-25(2,3)29-24(32)31(4)23(20)35/h5-11,28H,12-13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM168127

(US9073936, 2 | US9598426, 2)Show SMILES CC(O)c1ccc(Cn2nc3N4CC(C)(C)N=C4N(C)C(=O)c3c2Nc2ccc(F)cc2)cc1 |c:16| Show InChI InChI=1S/C25H27FN6O2/c1-15(33)17-7-5-16(6-8-17)13-32-21(27-19-11-9-18(26)10-12-19)20-22(29-32)31-14-25(2,3)28-24(31)30(4)23(20)34/h5-12,15,27,33H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INTRA-CELLULAR THERAPIES, INC.
US Patent
| |
US Patent US9598426 (2017)
BindingDB Entry DOI: 10.7270/Q29S1T2J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50036629
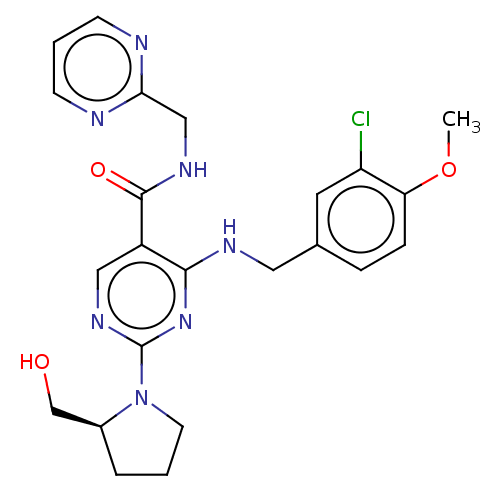
(Avanafil | CHEBI:66876 | Stendra | TA-1790)Show SMILES COc1ccc(CNc2nc(ncc2C(=O)NCc2ncccn2)N2CCC[C@H]2CO)cc1Cl |r| Show InChI InChI=1S/C23H26ClN7O3/c1-34-19-6-5-15(10-18(19)24)11-27-21-17(22(33)28-13-20-25-7-3-8-26-20)12-29-23(30-21)31-9-2-4-16(31)14-32/h3,5-8,10,12,16,32H,2,4,9,11,13-14H2,1H3,(H,28,33)(H,27,29,30)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308541
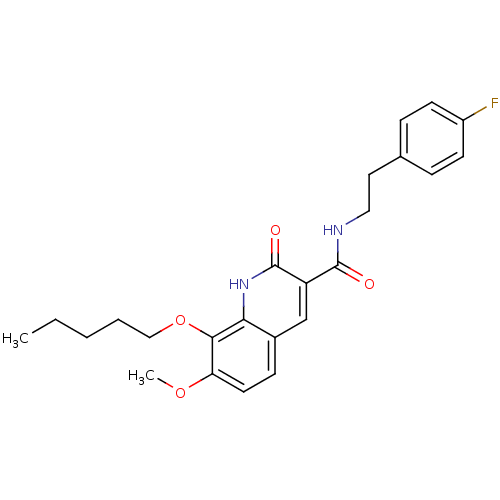
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27FN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308537
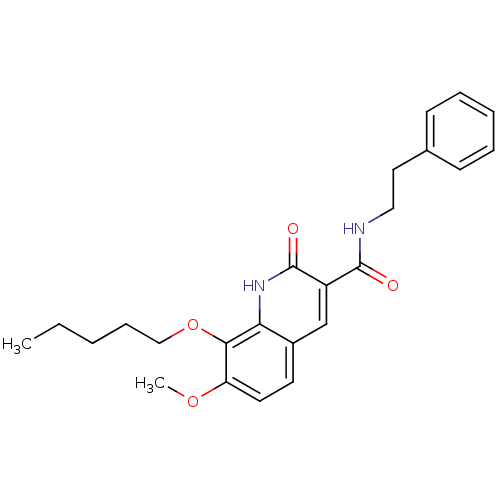
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H28N2O4/c1-3-4-8-15-30-22-20(29-2)12-11-18-16-19(24(28)26-21(18)22)23(27)25-14-13-17-9-6-5-7-10-17/h5-7,9-12,16H,3-4,8,13-15H2,1-2H3,(H,25,27)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50467496
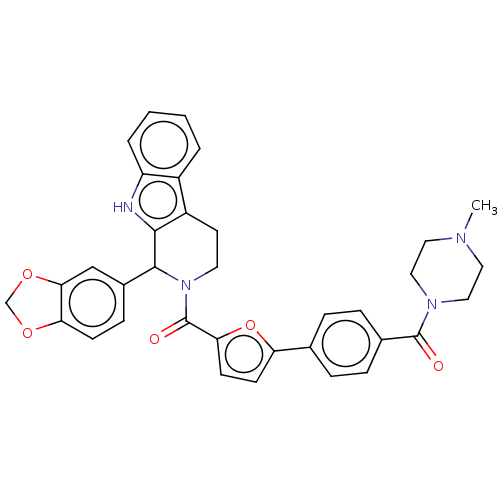
(CHEMBL4277253)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1CCc2c([nH]c3ccccc23)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C35H32N4O5/c1-37-16-18-38(19-17-37)34(40)23-8-6-22(7-9-23)28-12-13-30(44-28)35(41)39-15-14-26-25-4-2-3-5-27(25)36-32(26)33(39)24-10-11-29-31(20-24)43-21-42-29/h2-13,20,33,36H,14-19,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118253
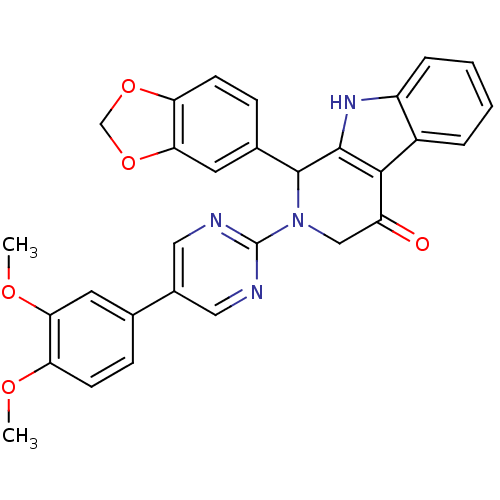
(1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1CC(=O)c2c([nH]c3ccccc23)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H24N4O5/c1-36-23-9-7-17(11-25(23)37-2)19-13-31-30(32-14-19)34-15-22(35)27-20-5-3-4-6-21(20)33-28(27)29(34)18-8-10-24-26(12-18)39-16-38-24/h3-14,29,33H,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118253

(1-Benzo[1,3]dioxol-5-yl-2-[5-(3,4-dimethoxy-phenyl...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1CC(=O)c2c([nH]c3ccccc23)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H24N4O5/c1-36-23-9-7-17(11-25(23)37-2)19-13-31-30(32-14-19)34-15-22(35)27-20-5-3-4-6-21(20)33-28(27)29(34)18-8-10-24-26(12-18)39-16-38-24/h3-14,29,33H,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of human corpus cavernosum PDE5 |
Eur J Med Chem 150: 30-38 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.039
BindingDB Entry DOI: 10.7270/Q2222XCS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308538
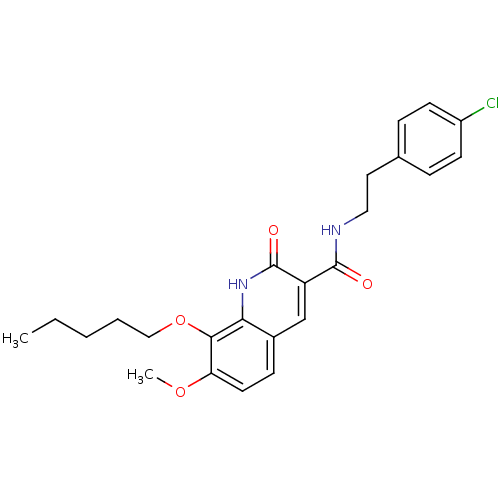
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(Cl)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C24H27ClN2O4/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(24(29)27-21(17)22)23(28)26-13-12-16-6-9-18(25)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308539
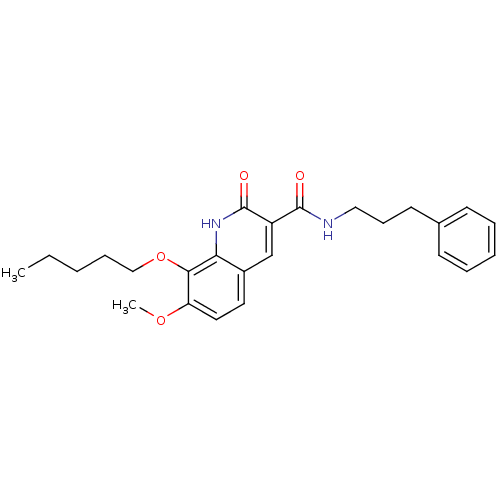
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCCc3ccccc3)c(=O)[nH]c12 Show InChI InChI=1S/C25H30N2O4/c1-3-4-8-16-31-23-21(30-2)14-13-19-17-20(25(29)27-22(19)23)24(28)26-15-9-12-18-10-6-5-7-11-18/h5-7,10-11,13-14,17H,3-4,8-9,12,15-16H2,1-2H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]raclopride from D2 receptor in human corpus striatum after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 1953-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.045
BindingDB Entry DOI: 10.7270/Q2S183VK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from 5-HT2C receptor in human frontal cortex after 30 mins by scintillation counting |
Bioorg Med Chem Lett 23: 1953-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.045
BindingDB Entry DOI: 10.7270/Q2S183VK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM497267

((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...)Show SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)C1CCN(CC1)C(=O)c1cc(C)nc(C)c1 |r,wU:13.12,wD:3.3,16.16,(-8.93,-2.23,;-7.59,-1.46,;-6.26,-2.23,;-4.93,-1.46,;-4.93,.08,;-3.59,-2.23,;-2.26,-1.46,;-2.26,.08,;-.93,.85,;.41,.08,;1.87,.56,;2.78,-.69,;1.87,-1.94,;2.35,-3.4,;3.85,-3.72,;4.33,-5.19,;3.3,-6.33,;3.78,-7.79,;1.79,-6.01,;1.32,-4.55,;.41,-1.46,;-.93,-2.23,;2.35,2.02,;3.85,2.34,;4.33,3.81,;3.3,4.95,;1.79,4.63,;1.32,3.16,;3.78,6.41,;3.04,7.77,;5.26,6.02,;6.35,7.1,;7.84,6.71,;8.93,7.79,;8.24,5.22,;7.15,4.13,;7.55,2.64,;5.66,4.53,)| Show InChI InChI=1S/C30H42N6O2/c1-5-6-19(2)33-30-31-17-26-27(18-36(28(26)34-30)24-7-9-25(37)10-8-24)22-11-13-35(14-12-22)29(38)23-15-20(3)32-21(4)16-23/h15-19,22,24-25,37H,5-14H2,1-4H3,(H,31,33,34)/t19-,24-,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ATP competitive inhibition of TYRO3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113534
BindingDB Entry DOI: 10.7270/Q2M90DGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308546
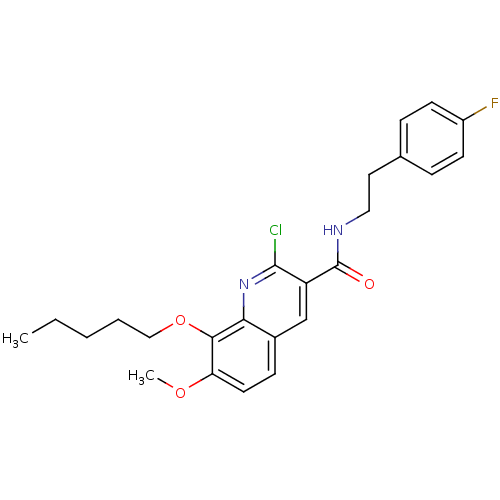
(2-Chloro-7-methoxy-8-pentyloxy-1-quinoline-3-carbo...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(F)cc3)c(Cl)nc12 Show InChI InChI=1S/C24H26ClFN2O3/c1-3-4-5-14-31-22-20(30-2)11-8-17-15-19(23(25)28-21(17)22)24(29)27-13-12-16-6-9-18(26)10-7-16/h6-11,15H,3-5,12-14H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50308540
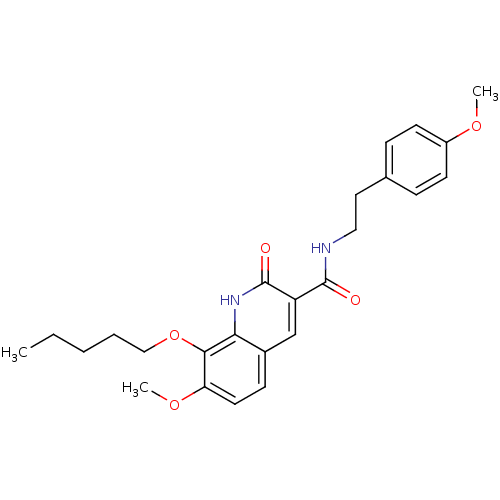
(7-Methoxy-2-oxo-8-pentyloxy-1,2-dihydroquinoline-3...)Show SMILES CCCCCOc1c(OC)ccc2cc(C(=O)NCCc3ccc(OC)cc3)c(=O)[nH]c12 Show InChI InChI=1S/C25H30N2O5/c1-4-5-6-15-32-23-21(31-3)12-9-18-16-20(25(29)27-22(18)23)24(28)26-14-13-17-7-10-19(30-2)11-8-17/h7-12,16H,4-6,13-15H2,1-3H3,(H,26,28)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB2 receptor |
Bioorg Med Chem 18: 2099-106 (2010)
Article DOI: 10.1016/j.bmc.2010.02.011
BindingDB Entry DOI: 10.7270/Q2CN741B |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM497267

((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...)Show SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)C1CCN(CC1)C(=O)c1cc(C)nc(C)c1 |r,wU:13.12,wD:3.3,16.16,(-8.93,-2.23,;-7.59,-1.46,;-6.26,-2.23,;-4.93,-1.46,;-4.93,.08,;-3.59,-2.23,;-2.26,-1.46,;-2.26,.08,;-.93,.85,;.41,.08,;1.87,.56,;2.78,-.69,;1.87,-1.94,;2.35,-3.4,;3.85,-3.72,;4.33,-5.19,;3.3,-6.33,;3.78,-7.79,;1.79,-6.01,;1.32,-4.55,;.41,-1.46,;-.93,-2.23,;2.35,2.02,;3.85,2.34,;4.33,3.81,;3.3,4.95,;1.79,4.63,;1.32,3.16,;3.78,6.41,;3.04,7.77,;5.26,6.02,;6.35,7.1,;7.84,6.71,;8.93,7.79,;8.24,5.22,;7.15,4.13,;7.55,2.64,;5.66,4.53,)| Show InChI InChI=1S/C30H42N6O2/c1-5-6-19(2)33-30-31-17-26-27(18-36(28(26)34-30)24-7-9-25(37)10-8-24)22-11-13-35(14-12-22)29(38)23-15-20(3)32-21(4)16-23/h15-19,22,24-25,37H,5-14H2,1-4H3,(H,31,33,34)/t19-,24-,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ATP competitive inhibition of FLT3 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113534
BindingDB Entry DOI: 10.7270/Q2M90DGJ |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50510070
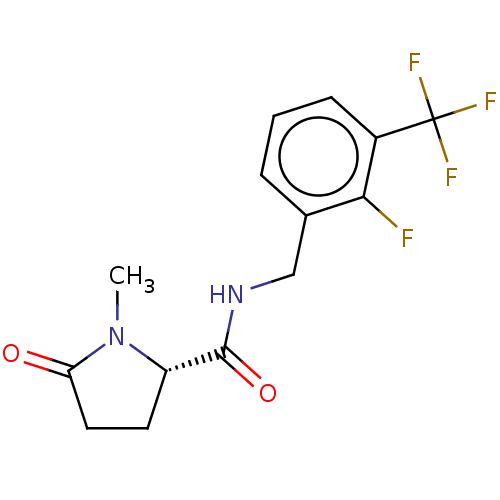
(CHEMBL2218191)Show SMILES CN1[C@@H](CCC1=O)C(=O)NCc1cccc(c1F)C(F)(F)F Show InChI InChI=1S/C14H14F4N2O2/c1-20-10(5-6-11(20)21)13(22)19-7-8-3-2-4-9(12(8)15)14(16,17)18/h2-4,10H,5-7H2,1H3,(H,19,22)/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... |
Bioorg Med Chem Lett 29: 1476-1480 (2019)
Article DOI: 10.1016/j.bmcl.2019.04.018
BindingDB Entry DOI: 10.7270/Q2C250RG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50150119
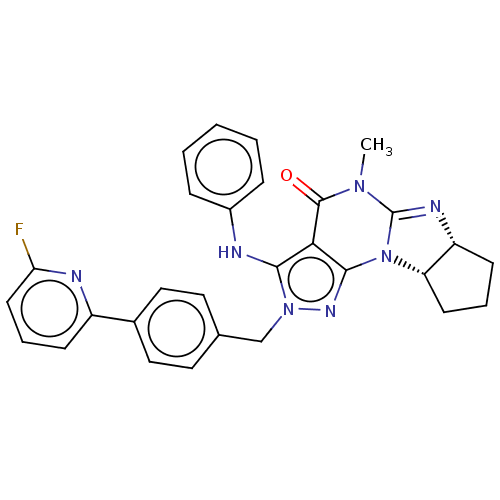
(CHEMBL3769414)Show SMILES [H][C@@]12CCC[C@]1([H])N1C(=N2)N(C)C(=O)c2c(Nc3ccccc3)n(Cc3ccc(cc3)-c3cccc(F)n3)nc12 |r,c:9| Show InChI InChI=1S/C29H26FN7O/c1-35-28(38)25-26(31-20-7-3-2-4-8-20)36(34-27(25)37-23-11-5-10-22(23)33-29(35)37)17-18-13-15-19(16-14-18)21-9-6-12-24(30)32-21/h2-4,6-9,12-16,22-23,31H,5,10-11,17H2,1H3/t22-,23+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full lenght human PDE4A using fluorescent labeled cAMP as substrate after 15 mins by IMAP assay |
J Med Chem 59: 1149-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01751
BindingDB Entry DOI: 10.7270/Q2X068W7 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50150241
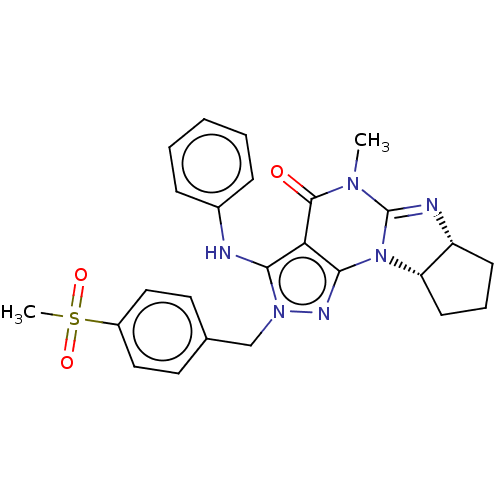
(CHEMBL3770585)Show SMILES [H][C@@]12CCC[C@]1([H])N1C(=N2)N(C)C(=O)c2c1nn(Cc1ccc(cc1)S(C)(=O)=O)c2Nc1ccccc1 |r,c:9| Show InChI InChI=1S/C25H26N6O3S/c1-29-24(32)21-22(26-17-7-4-3-5-8-17)30(15-16-11-13-18(14-12-16)35(2,33)34)28-23(21)31-20-10-6-9-19(20)27-25(29)31/h3-5,7-8,11-14,19-20,26H,6,9-10,15H2,1-2H3/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full lenght human PDE4A using fluorescent labeled cAMP as substrate after 15 mins by IMAP assay |
J Med Chem 59: 1149-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01751
BindingDB Entry DOI: 10.7270/Q2X068W7 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50016469
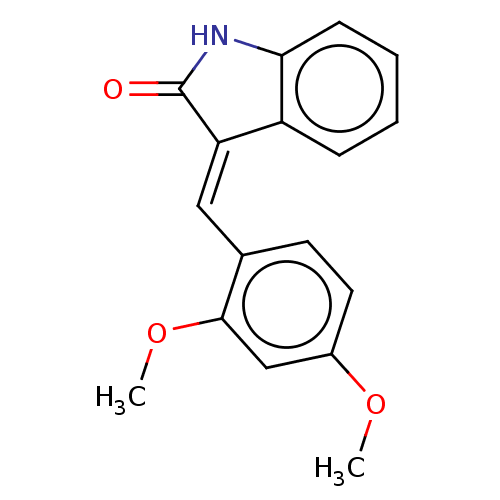
(CHEMBL514709)Show InChI InChI=1S/C17H15NO3/c1-20-12-8-7-11(16(10-12)21-2)9-14-13-5-3-4-6-15(13)18-17(14)19/h3-10H,1-2H3,(H,18,19)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM497267

((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...)Show SMILES CCC[C@H](C)Nc1ncc2c(cn([C@H]3CC[C@H](O)CC3)c2n1)C1CCN(CC1)C(=O)c1cc(C)nc(C)c1 |r,wU:13.12,wD:3.3,16.16,(-8.93,-2.23,;-7.59,-1.46,;-6.26,-2.23,;-4.93,-1.46,;-4.93,.08,;-3.59,-2.23,;-2.26,-1.46,;-2.26,.08,;-.93,.85,;.41,.08,;1.87,.56,;2.78,-.69,;1.87,-1.94,;2.35,-3.4,;3.85,-3.72,;4.33,-5.19,;3.3,-6.33,;3.78,-7.79,;1.79,-6.01,;1.32,-4.55,;.41,-1.46,;-.93,-2.23,;2.35,2.02,;3.85,2.34,;4.33,3.81,;3.3,4.95,;1.79,4.63,;1.32,3.16,;3.78,6.41,;3.04,7.77,;5.26,6.02,;6.35,7.1,;7.84,6.71,;8.93,7.79,;8.24,5.22,;7.15,4.13,;7.55,2.64,;5.66,4.53,)| Show InChI InChI=1S/C30H42N6O2/c1-5-6-19(2)33-30-31-17-26-27(18-36(28(26)34-30)24-7-9-25(37)10-8-24)22-11-13-35(14-12-22)29(38)23-15-20(3)32-21(4)16-23/h15-19,22,24-25,37H,5-14H2,1-4H3,(H,31,33,34)/t19-,24-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ATP competitive inhibition of AXL (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113534
BindingDB Entry DOI: 10.7270/Q2M90DGJ |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50016480
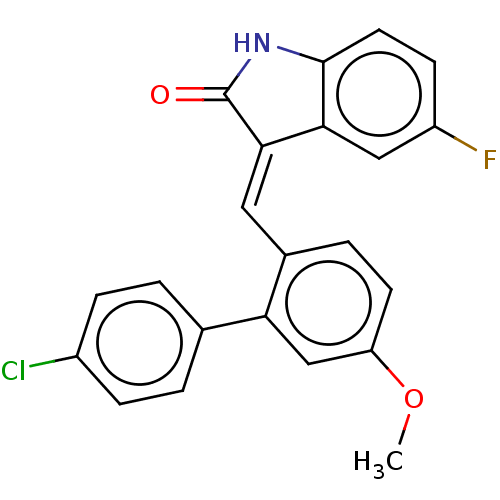
(CHEMBL3265104)Show SMILES COc1ccc(\C=C2\C(=O)Nc3ccc(F)cc23)c(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H15ClFNO2/c1-27-17-8-4-14(18(12-17)13-2-5-15(23)6-3-13)10-20-19-11-16(24)7-9-21(19)25-22(20)26/h2-12H,1H3,(H,25,26)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50016470
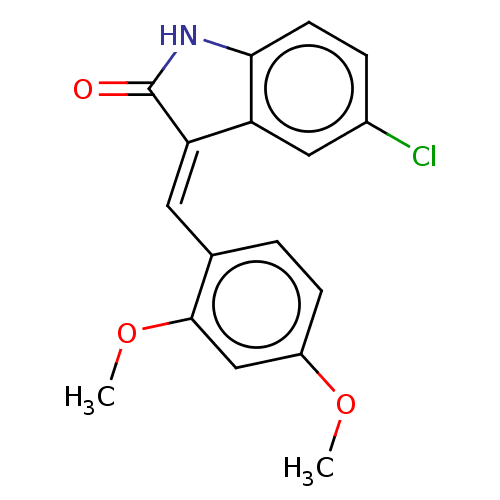
(CHEMBL3265094)Show InChI InChI=1S/C17H14ClNO3/c1-21-12-5-3-10(16(9-12)22-2)7-14-13-8-11(18)4-6-15(13)19-17(14)20/h3-9H,1-2H3,(H,19,20)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50016478
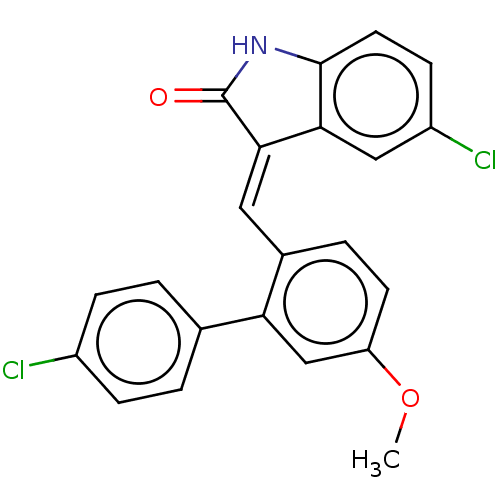
(CHEMBL3265103)Show SMILES COc1ccc(\C=C2\C(=O)Nc3ccc(Cl)cc23)c(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H15Cl2NO2/c1-27-17-8-4-14(18(12-17)13-2-5-15(23)6-3-13)10-20-19-11-16(24)7-9-21(19)25-22(20)26/h2-12H,1H3,(H,25,26)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50016476
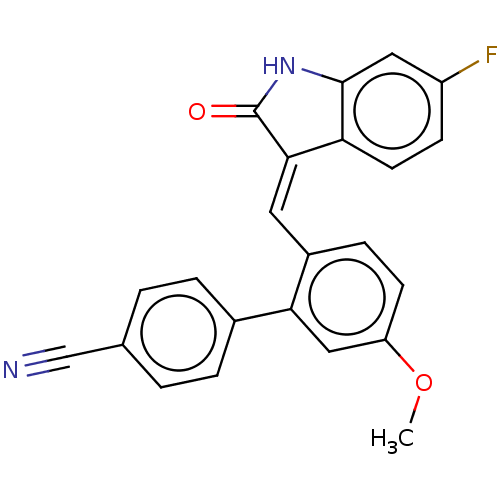
(CHEMBL3265101)Show SMILES COc1ccc(\C=C2\C(=O)Nc3cc(F)ccc23)c(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H15FN2O2/c1-28-18-8-6-16(20(12-18)15-4-2-14(13-25)3-5-15)10-21-19-9-7-17(24)11-22(19)26-23(21)27/h2-12H,1H3,(H,26,27)/b21-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Science and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to MDM2 (1 to 118) (unknown origin) after 30 mins by fluorescence polarization assay |
Eur J Med Chem 81: 277-88 (2014)
Article DOI: 10.1016/j.ejmech.2014.05.027
BindingDB Entry DOI: 10.7270/Q2736SGW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data