 Found 214 hits with Last Name = 'schulman' and Initial = 'i'
Found 214 hits with Last Name = 'schulman' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204085

(CHEMBL3960606)Show SMILES CC(C)(O)c1cn(c(Cc2ccccc2Cl)n1)-c1ccc(cc1)-c1ccc(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C27H27ClN2O4S/c1-27(2,32)25-16-30(26(29-25)15-20-6-4-5-7-23(20)28)22-12-10-18(11-13-22)19-8-9-21(17-31)24(14-19)35(3,33)34/h4-14,16,31-32H,15,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204079
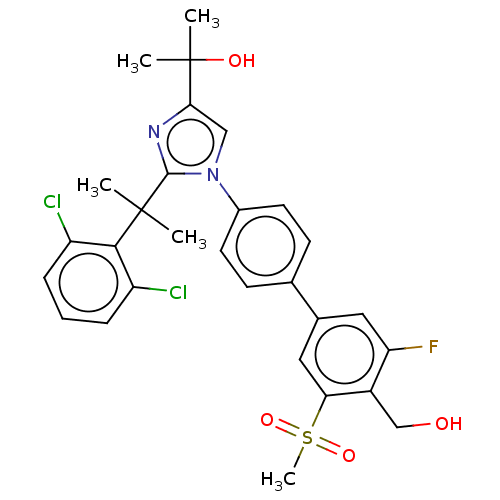
(CHEMBL3935187)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29Cl2FN2O4S/c1-28(2,26-21(30)7-6-8-22(26)31)27-33-25(29(3,4)36)15-34(27)19-11-9-17(10-12-19)18-13-23(32)20(16-35)24(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204078

(CHEMBL3926292)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29ClF2N2O4S/c1-28(2,20-8-6-7-9-21(20)30)27-33-26(29(3,4)36)15-34(27)24-11-10-17(12-23(24)32)18-13-22(31)19(16-35)25(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204077
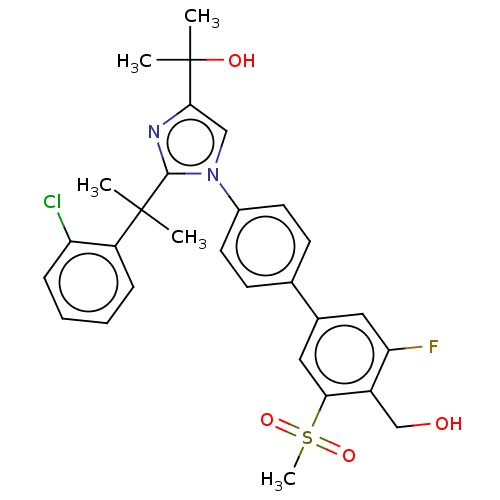
(CHEMBL3944154)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H30ClFN2O4S/c1-28(2,22-8-6-7-9-23(22)30)27-32-26(29(3,4)35)16-33(27)20-12-10-18(11-13-20)19-14-24(31)21(17-34)25(15-19)38(5,36)37/h6-16,34-35H,17H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204076

(CHEMBL3890276)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C28H28Cl2N2O3S/c1-27(2,25-22(29)10-7-11-23(25)30)26-31-24(28(3,4)33)17-32(26)20-14-12-18(13-15-20)19-8-6-9-21(16-19)36(5,34)35/h6-17,33H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204075
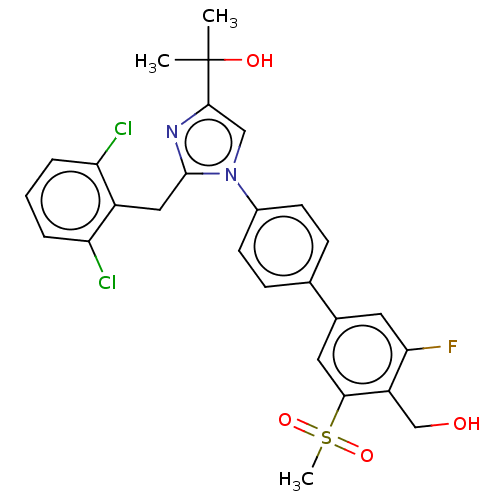
(CHEMBL3980683)Show SMILES CC(C)(O)c1cn(c(Cc2c(Cl)cccc2Cl)n1)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C27H25Cl2FN2O4S/c1-27(2,34)25-14-32(26(31-25)13-19-21(28)5-4-6-22(19)29)18-9-7-16(8-10-18)17-11-23(30)20(15-33)24(12-17)37(3,35)36/h4-12,14,33-34H,13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204072
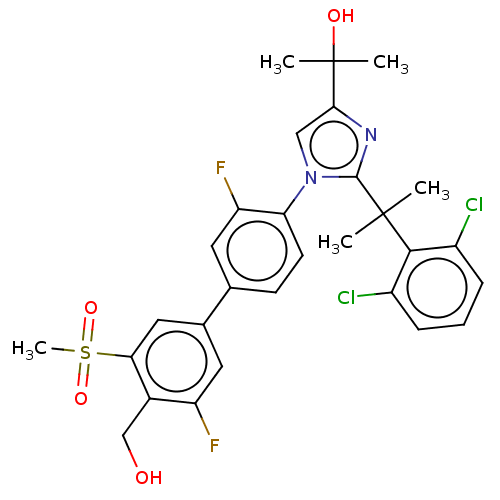
(CHEMBL3945199 | US10543183, Compound 12 | US109459...)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28Cl2F2N2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-22(23)33)17-12-21(32)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204081

(CHEMBL3913162)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1ccc(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H31ClN2O4S/c1-28(2,23-8-6-7-9-24(23)30)27-31-26(29(3,4)34)17-32(27)22-14-12-19(13-15-22)20-10-11-21(18-33)25(16-20)37(5,35)36/h6-17,33-34H,18H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204082

(CHEMBL3945820)Show SMILES CC(c1nc(cn1-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O)C(C)(C)O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H26Cl2F2N2O4S/c1-15(26-19(29)6-5-7-20(26)30)27-33-25(28(2,3)36)13-34(27)23-9-8-16(10-22(23)32)17-11-21(31)18(14-35)24(12-17)39(4,37)38/h5-13,15,35-36H,14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204083
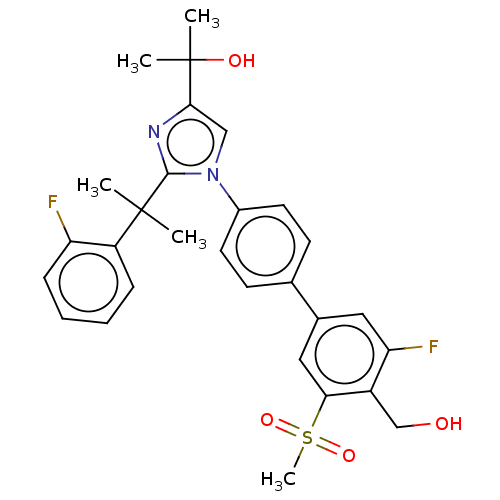
(CHEMBL3941775)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1F)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H30F2N2O4S/c1-28(2,22-8-6-7-9-23(22)30)27-32-26(29(3,4)35)16-33(27)20-12-10-18(11-13-20)19-14-24(31)21(17-34)25(15-19)38(5,36)37/h6-16,34-35H,17H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204073
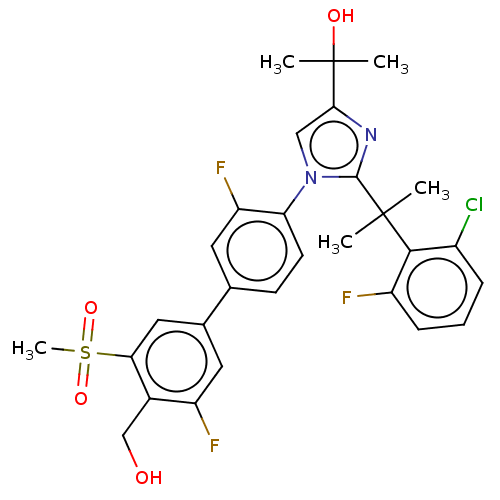
(CHEMBL3917300)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(F)cccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28ClF3N2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-22(23)33)17-12-21(32)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204071
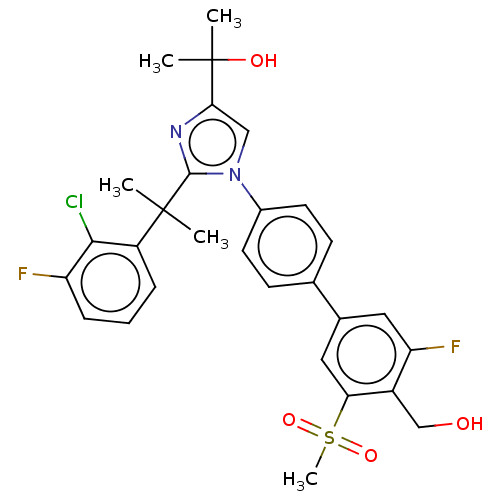
(CHEMBL3953927)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1cccc(F)c1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29ClF2N2O4S/c1-28(2,21-7-6-8-22(31)26(21)30)27-33-25(29(3,4)36)15-34(27)19-11-9-17(10-12-19)18-13-23(32)20(16-35)24(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50034775
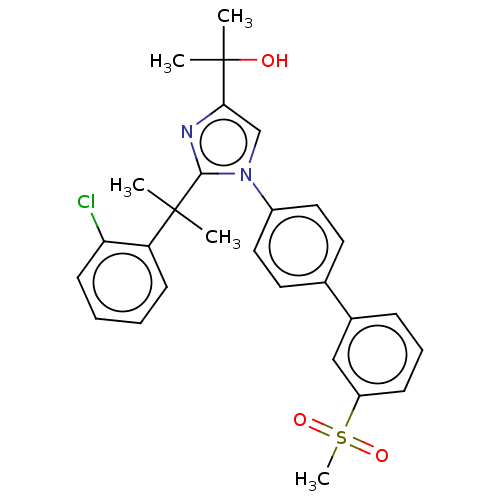
(CHEMBL3360975 | US10543183, Compound 38 | US109459...)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C28H29ClN2O3S/c1-27(2,23-11-6-7-12-24(23)29)26-30-25(28(3,4)32)18-31(26)21-15-13-19(14-16-21)20-9-8-10-22(17-20)35(5,33)34/h6-18,32H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204076

(CHEMBL3890276)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C28H28Cl2N2O3S/c1-27(2,25-22(29)10-7-11-23(25)30)26-31-24(28(3,4)33)17-32(26)20-14-12-18(13-15-20)19-8-6-9-21(16-19)36(5,34)35/h6-17,33H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204082

(CHEMBL3945820)Show SMILES CC(c1nc(cn1-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O)C(C)(C)O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C28H26Cl2F2N2O4S/c1-15(26-19(29)6-5-7-20(26)30)27-33-25(28(2,3)36)13-34(27)23-9-8-16(10-22(23)32)17-11-21(31)18(14-35)24(12-17)39(4,37)38/h5-13,15,35-36H,14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204078

(CHEMBL3926292)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29ClF2N2O4S/c1-28(2,20-8-6-7-9-21(20)30)27-33-26(29(3,4)36)15-34(27)24-11-10-17(12-23(24)32)18-13-22(31)19(16-35)25(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204072

(CHEMBL3945199 | US10543183, Compound 12 | US109459...)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28Cl2F2N2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-22(23)33)17-12-21(32)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204085

(CHEMBL3960606)Show SMILES CC(C)(O)c1cn(c(Cc2ccccc2Cl)n1)-c1ccc(cc1)-c1ccc(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C27H27ClN2O4S/c1-27(2,32)25-16-30(26(29-25)15-20-6-4-5-7-23(20)28)22-12-10-18(11-13-22)19-8-9-21(17-31)24(14-19)35(3,33)34/h4-14,16,31-32H,15,17H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204079

(CHEMBL3935187)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29Cl2FN2O4S/c1-28(2,26-21(30)7-6-8-22(26)31)27-33-25(29(3,4)36)15-34(27)19-11-9-17(10-12-19)18-13-23(32)20(16-35)24(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204080
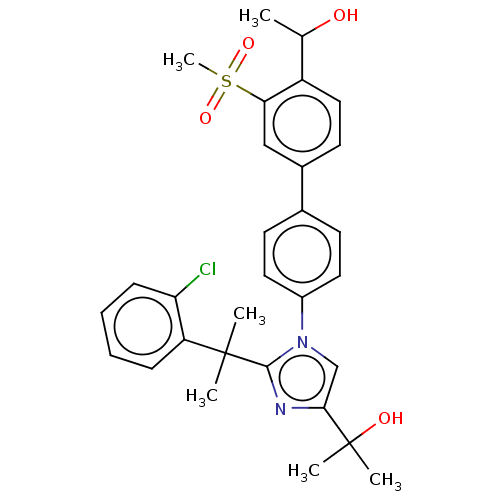
(CHEMBL3963566)Show SMILES CC(O)c1ccc(cc1S(C)(=O)=O)-c1ccc(cc1)-n1cc(nc1C(C)(C)c1ccccc1Cl)C(C)(C)O Show InChI InChI=1S/C30H33ClN2O4S/c1-19(34)23-16-13-21(17-26(23)38(6,36)37)20-11-14-22(15-12-20)33-18-27(30(4,5)35)32-28(33)29(2,3)24-9-7-8-10-25(24)31/h7-19,34-35H,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204074

(CHEMBL3910597)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1Cl)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28Cl3FN2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-21(23)32)17-12-22(33)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRbeta/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analys... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204074

(CHEMBL3910597)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(Cl)cccc1Cl)-c1ccc(cc1Cl)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28Cl3FN2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-21(23)32)17-12-22(33)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204071

(CHEMBL3953927)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1cccc(F)c1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H29ClF2N2O4S/c1-28(2,21-7-6-8-22(31)26(21)30)27-33-25(29(3,4)36)15-34(27)19-11-9-17(10-12-19)18-13-23(32)20(16-35)24(14-18)39(5,37)38/h6-15,35-36H,16H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204077

(CHEMBL3944154)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H30ClFN2O4S/c1-28(2,22-8-6-7-9-23(22)30)27-32-26(29(3,4)35)16-33(27)20-12-10-18(11-13-20)19-14-24(31)21(17-34)25(15-19)38(5,36)37/h6-16,34-35H,17H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50034775

(CHEMBL3360975 | US10543183, Compound 38 | US109459...)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C28H29ClN2O3S/c1-27(2,23-11-6-7-12-24(23)29)26-30-25(28(3,4)32)18-31(26)21-15-13-19(14-16-21)20-9-8-10-22(17-20)35(5,33)34/h6-18,32H,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204073

(CHEMBL3917300)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1c(F)cccc1Cl)-c1ccc(cc1F)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H28ClF3N2O4S/c1-28(2,26-19(30)7-6-8-20(26)31)27-34-25(29(3,4)37)14-35(27)23-10-9-16(11-22(23)33)17-12-21(32)18(15-36)24(13-17)40(5,38)39/h6-14,36-37H,15H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204081

(CHEMBL3913162)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1Cl)-c1ccc(cc1)-c1ccc(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H31ClN2O4S/c1-28(2,23-8-6-7-9-24(23)30)27-31-26(29(3,4)34)17-32(27)22-14-12-19(13-15-22)20-10-11-21(18-33)25(16-20)37(5,35)36/h6-17,33-34H,18H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204075

(CHEMBL3980683)Show SMILES CC(C)(O)c1cn(c(Cc2c(Cl)cccc2Cl)n1)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C27H25Cl2FN2O4S/c1-27(2,34)25-14-32(26(31-25)13-19-21(28)5-4-6-22(19)29)18-9-7-16(8-10-18)17-11-23(30)20(15-33)24(12-17)37(3,35)36/h4-12,14,33-34H,13,15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204083

(CHEMBL3941775)Show SMILES CC(C)(O)c1cn(c(n1)C(C)(C)c1ccccc1F)-c1ccc(cc1)-c1cc(F)c(CO)c(c1)S(C)(=O)=O Show InChI InChI=1S/C29H30F2N2O4S/c1-28(2,22-8-6-7-9-23(22)30)27-32-26(29(3,4)35)16-33(27)20-12-10-18(11-13-20)19-14-24(31)21(17-34)25(15-19)38(5,36)37/h6-16,34-35H,17H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50204080

(CHEMBL3963566)Show SMILES CC(O)c1ccc(cc1S(C)(=O)=O)-c1ccc(cc1)-n1cc(nc1C(C)(C)c1ccccc1Cl)C(C)(C)O Show InChI InChI=1S/C30H33ClN2O4S/c1-19(34)23-16-13-21(17-26(23)38(6,36)37)20-11-14-22(15-12-20)33-18-27(30(4,5)35)32-28(33)29(2,3)24-9-7-8-10-25(24)31/h7-19,34-35H,1-6H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Displacement of [3H]-24,25-epoxycholesterol from human LXRalpha/RXRalpha expressed in baculovirus infected Sf9 cells by scintillation proximity analy... |
ACS Med Chem Lett 7: 1207-1212 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00234
BindingDB Entry DOI: 10.7270/Q2V98B13 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337251
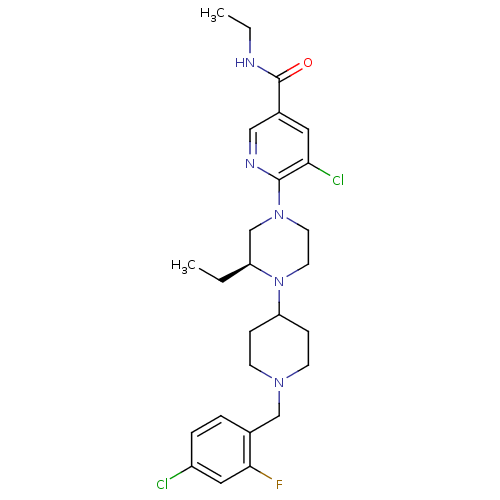
((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250
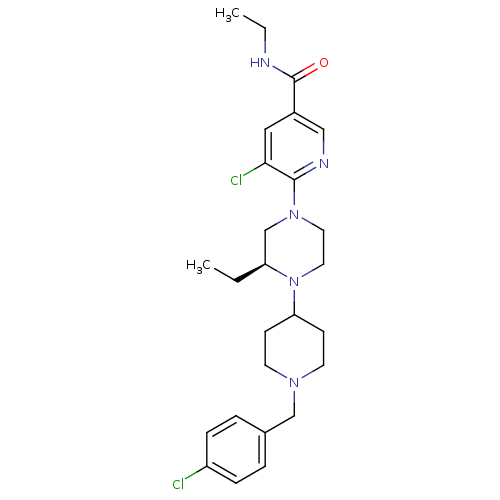
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337251

((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337251

((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337236
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-3-28-25(33)20-14-23(27)24(29-15-20)31-12-13-32(18(2)16-31)22-8-10-30(11-9-22)17-19-4-6-21(26)7-5-19/h4-7,14-15,18,22H,3,8-13,16-17H2,1-2H3,(H,28,33)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
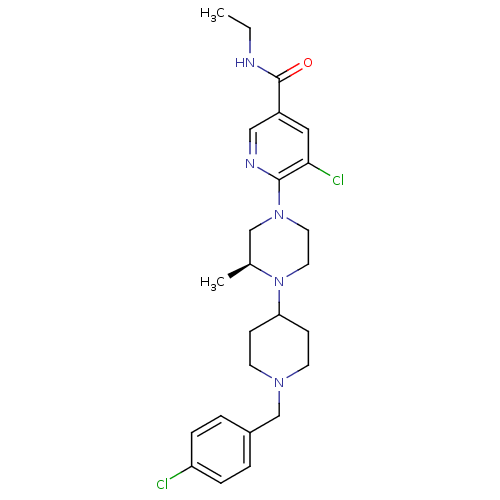
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337238
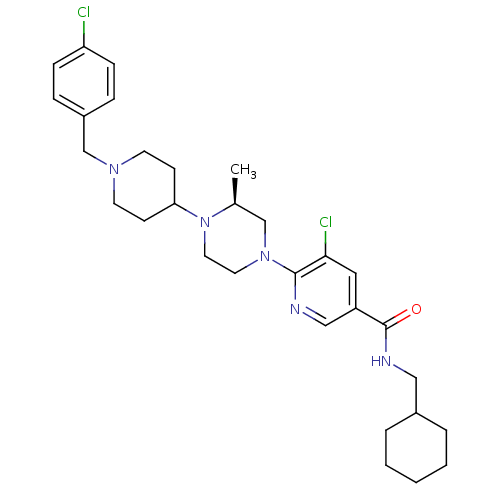
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCC1CCCCC1 |r| Show InChI InChI=1S/C30H41Cl2N5O/c1-22-20-36(29-28(32)17-25(19-33-29)30(38)34-18-23-5-3-2-4-6-23)15-16-37(22)27-11-13-35(14-12-27)21-24-7-9-26(31)10-8-24/h7-10,17,19,22-23,27H,2-6,11-16,18,20-21H2,1H3,(H,34,38)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
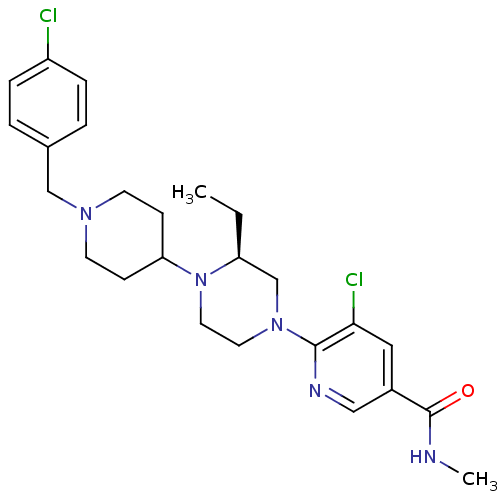
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337242
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NC |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-3-21-17-31(24-23(27)14-19(15-29-24)25(33)28-2)12-13-32(21)22-8-10-30(11-9-22)16-18-4-6-20(26)7-5-18/h4-7,14-15,21-22H,3,8-13,16-17H2,1-2H3,(H,28,33)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337212
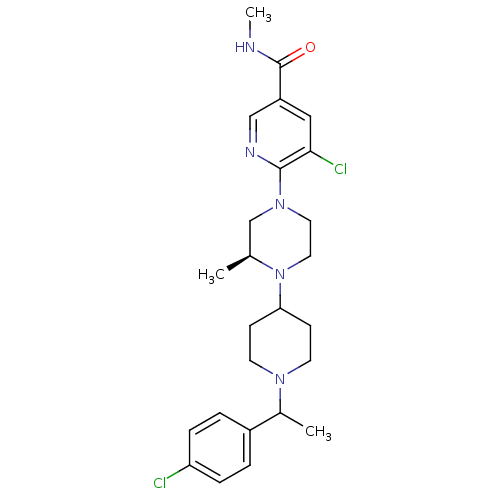
(5-chloro-6-((3S)-4-(1-(1-(4-chlorophenyl)ethyl)pip...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(C)c2ccc(Cl)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-17-16-31(24-23(27)14-20(15-29-24)25(33)28-3)12-13-32(17)22-8-10-30(11-9-22)18(2)19-4-6-21(26)7-5-19/h4-7,14-15,17-18,22H,8-13,16H2,1-3H3,(H,28,33)/t17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337212

(5-chloro-6-((3S)-4-(1-(1-(4-chlorophenyl)ethyl)pip...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(CC2)C(C)c2ccc(Cl)cc2)c(Cl)c1 |r| Show InChI InChI=1S/C25H33Cl2N5O/c1-17-16-31(24-23(27)14-20(15-29-24)25(33)28-3)12-13-32(17)22-8-10-30(11-9-22)18(2)19-4-6-21(26)7-5-19/h4-7,14-15,17-18,22H,8-13,16H2,1-3H3,(H,28,33)/t17-,18?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337237

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CC(C)NC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-18(2)30-26(34)21-14-24(28)25(29-15-21)32-12-13-33(19(3)16-32)23-8-10-31(11-9-23)17-20-4-6-22(27)7-5-20/h4-7,14-15,18-19,23H,8-13,16-17H2,1-3H3,(H,30,34)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337233
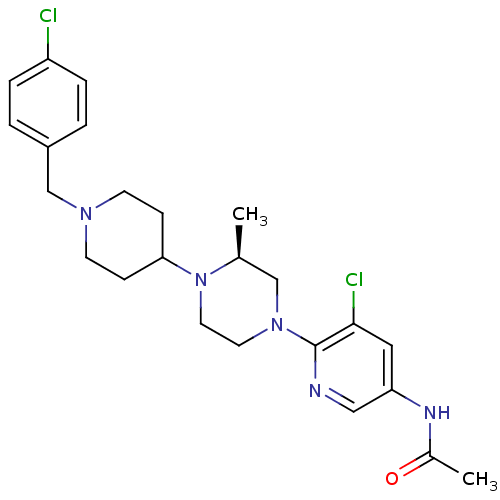
((S)-N-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(NC(C)=O)cc1Cl |r| Show InChI InChI=1S/C24H31Cl2N5O/c1-17-15-30(24-23(26)13-21(14-27-24)28-18(2)32)11-12-31(17)22-7-9-29(10-8-22)16-19-3-5-20(25)6-4-19/h3-6,13-14,17,22H,7-12,15-16H2,1-2H3,(H,28,32)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50337218

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H33Cl2F2N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-26(32)15-23(17-35-29)30(40)36-16-22-4-7-27(33)28(34)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at rat CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337219
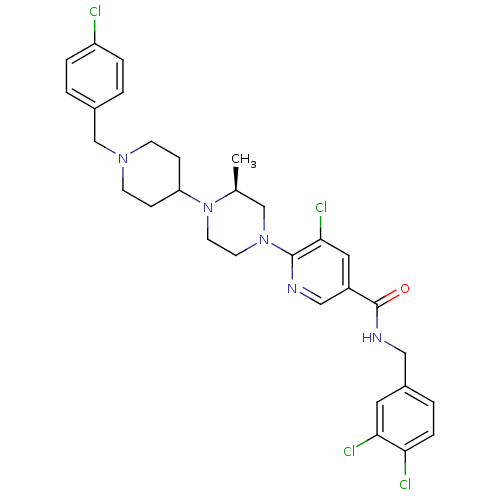
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(cc1Cl)C(=O)NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H33Cl4N5O/c1-20-18-38(12-13-39(20)25-8-10-37(11-9-25)19-21-2-5-24(31)6-3-21)29-28(34)15-23(17-35-29)30(40)36-16-22-4-7-26(32)27(33)14-22/h2-7,14-15,17,20,25H,8-13,16,18-19H2,1H3,(H,36,40)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337210
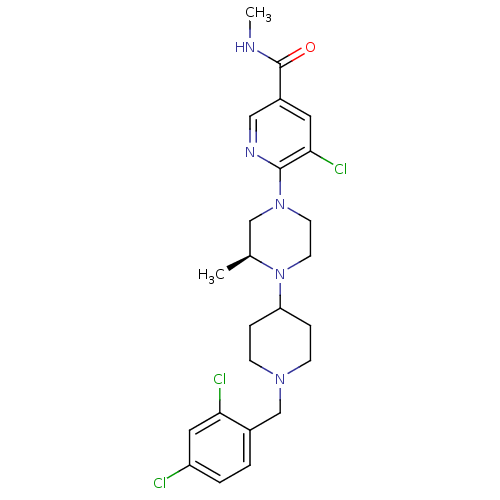
((S)-5-chloro-6-(4-(1-(2,4-dichlorobenzyl)piperidin...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3Cl)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H30Cl3N5O/c1-16-14-31(23-22(27)11-18(13-29-23)24(33)28-2)9-10-32(16)20-5-7-30(8-6-20)15-17-3-4-19(25)12-21(17)26/h3-4,11-13,16,20H,5-10,14-15H2,1-2H3,(H,28,33)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337262
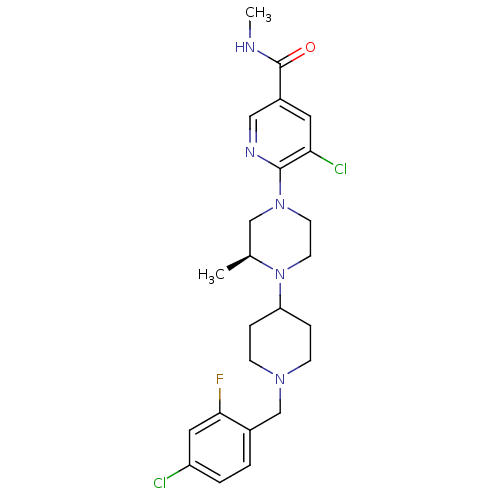
((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CNC(=O)c1cnc(N2CCN([C@@H](C)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C24H30Cl2FN5O/c1-16-14-31(23-21(26)11-18(13-29-23)24(33)28-2)9-10-32(16)20-5-7-30(8-6-20)15-17-3-4-19(25)12-22(17)27/h3-4,11-13,16,20H,5-10,14-15H2,1-2H3,(H,28,33)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
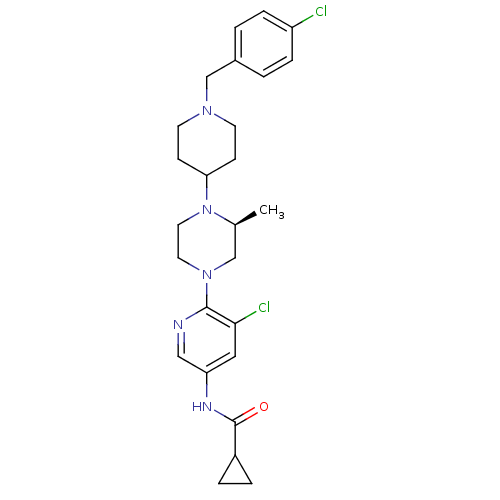
(Homo sapiens (Human)) | BDBM50337234
((S)-N-(5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-...)Show SMILES C[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1ncc(NC(=O)C2CC2)cc1Cl |r| Show InChI InChI=1S/C26H33Cl2N5O/c1-18-16-32(25-24(28)14-22(15-29-25)30-26(34)20-4-5-20)12-13-33(18)23-8-10-31(11-9-23)17-19-2-6-21(27)7-3-19/h2-3,6-7,14-15,18,20,23H,4-5,8-13,16-17H2,1H3,(H,30,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data