 Found 247 hits with Last Name = 'agans-fantuzzi' and Initial = 'j'
Found 247 hits with Last Name = 'agans-fantuzzi' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human PAR1 in HCASMC assessed as inhibition of thrombin-induced calcium efflux |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260518
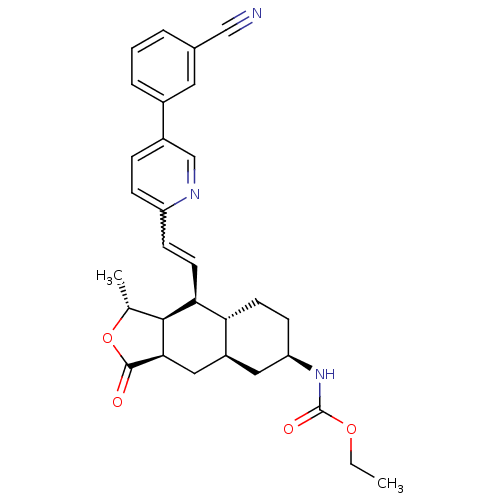
(CHEMBL442649 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(c2)C#N)C1 |r,w:21.23| Show InChI InChI=1S/C30H33N3O4/c1-3-36-30(35)33-24-10-11-25-22(14-24)15-27-28(18(2)37-29(27)34)26(25)12-9-23-8-7-21(17-32-23)20-6-4-5-19(13-20)16-31/h4-9,12-13,17-18,22,24-28H,3,10-11,14-15H2,1-2H3,(H,33,35)/t18-,22+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50173419
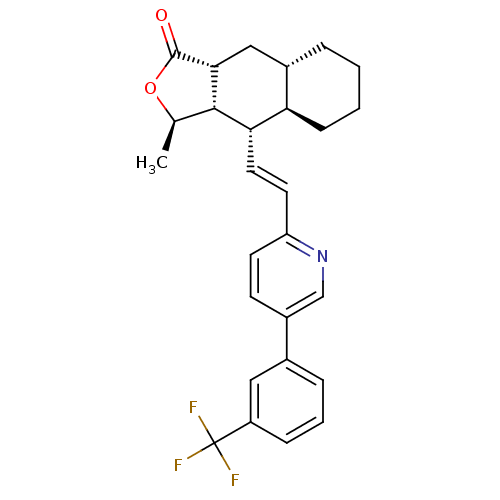
((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12 Show InChI InChI=1S/C27H28F3NO2/c1-16-25-23(22-8-3-2-5-18(22)14-24(25)26(32)33-16)12-11-21-10-9-19(15-31-21)17-6-4-7-20(13-17)27(28,29)30/h4,6-7,9-13,15-16,18,22-25H,2-3,5,8,14H2,1H3/b12-11+/t16-,18+,22-,23+,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory constant aganist Protease-activated receptor 1 |
J Med Chem 48: 5884-7 (2005)
Article DOI: 10.1021/jm0502236
BindingDB Entry DOI: 10.7270/Q2V69J5Q |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261116
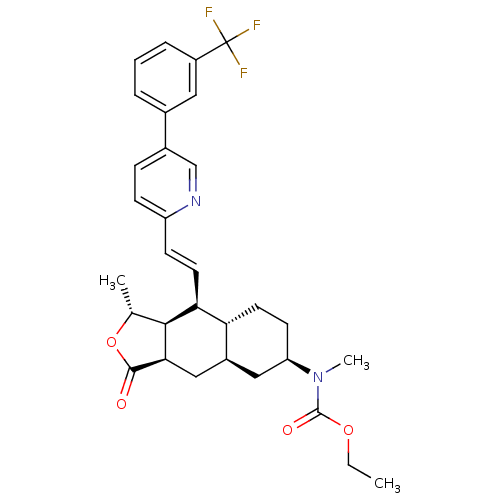
(CHEMBL453277 | ethyl methyl((1R,3aR,4aR,6R,8aR,9S,...)Show SMILES CCOC(=O)N(C)[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(c2)C(F)(F)F)C1 |r| Show InChI InChI=1S/C31H35F3N2O4/c1-4-39-30(38)36(3)24-11-13-25-21(15-24)16-27-28(18(2)40-29(27)37)26(25)12-10-23-9-8-20(17-35-23)19-6-5-7-22(14-19)31(32,33)34/h5-10,12,14,17-18,21,24-28H,4,11,13,15-16H2,1-3H3/b12-10+/t18-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261114
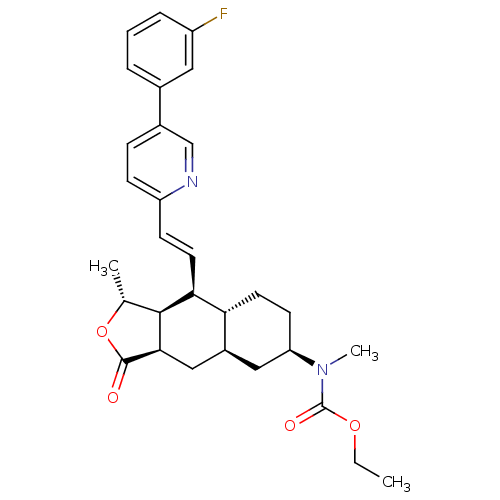
(CHEMBL500551 | Ethyl [(1R,3aR,4aR,6R,8aR,9S,9aS)-9...)Show SMILES CCOC(=O)N(C)[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C30H35FN2O4/c1-4-36-30(35)33(3)24-11-13-25-21(15-24)16-27-28(18(2)37-29(27)34)26(25)12-10-23-9-8-20(17-32-23)19-6-5-7-22(31)14-19/h5-10,12,14,17-18,21,24-28H,4,11,13,15-16H2,1-3H3/b12-10+/t18-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222015
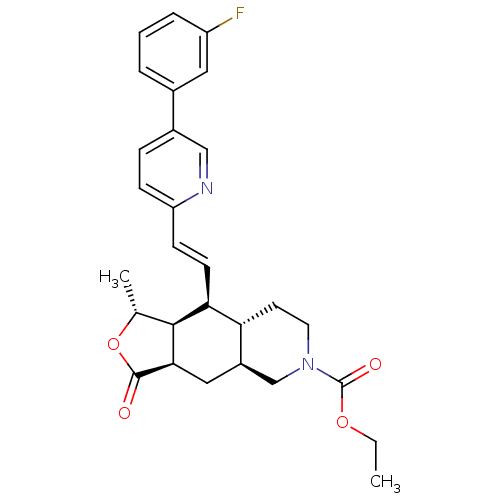
((1R,3aR,4aS,8aS,9S,9aS)-decahydro-1-methyl-3-oxo-9...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 Show InChI InChI=1S/C28H31FN2O4/c1-3-34-28(33)31-12-11-23-20(16-31)14-25-26(17(2)35-27(25)32)24(23)10-9-22-8-7-19(15-30-22)18-5-4-6-21(29)13-18/h4-10,13,15,17,20,23-26H,3,11-12,14,16H2,1-2H3/b10-9+/t17-,20-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261061
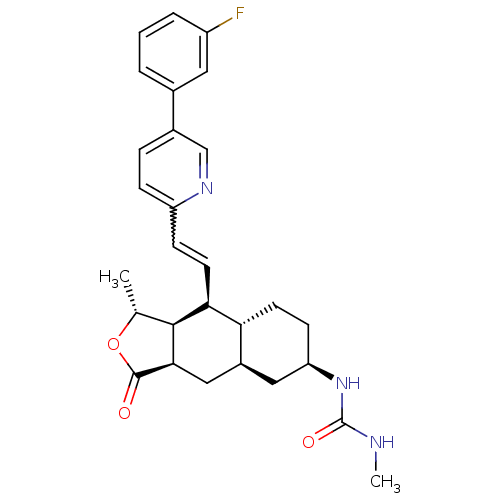
(CHEMBL493633 | N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E...)Show SMILES CNC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:20.22| Show InChI InChI=1S/C28H32FN3O3/c1-16-26-24(11-8-21-7-6-18(15-31-21)17-4-3-5-20(29)12-17)23-10-9-22(32-28(34)30-2)13-19(23)14-25(26)27(33)35-16/h3-8,11-12,15-16,19,22-26H,9-10,13-14H2,1-2H3,(H2,30,32,34)/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261062
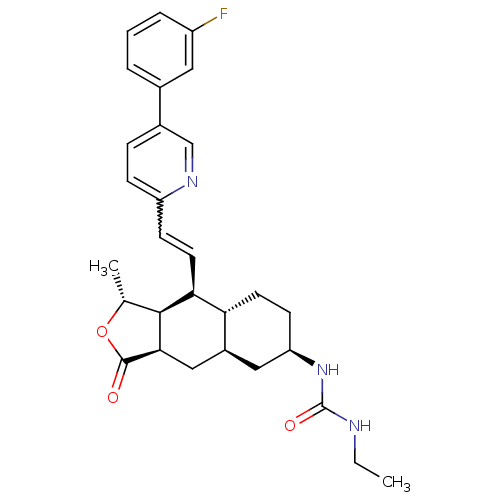
(1-ethyl-3-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(5-...)Show SMILES CCNC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H34FN3O3/c1-3-31-29(35)33-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)34)25(24)12-9-22-8-7-19(16-32-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H2,31,33,35)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260520
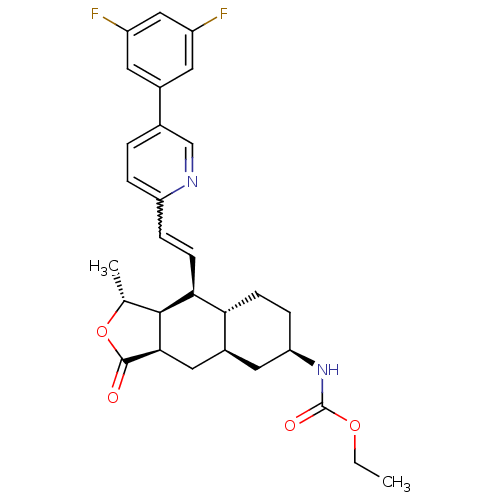
(CHEMBL458264 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cc(F)cc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H32F2N2O4/c1-3-36-29(35)33-23-7-8-24-19(12-23)13-26-27(16(2)37-28(26)34)25(24)9-6-22-5-4-17(15-32-22)18-10-20(30)14-21(31)11-18/h4-6,9-11,14-16,19,23-27H,3,7-8,12-13H2,1-2H3,(H,33,35)/t16-,19+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261117
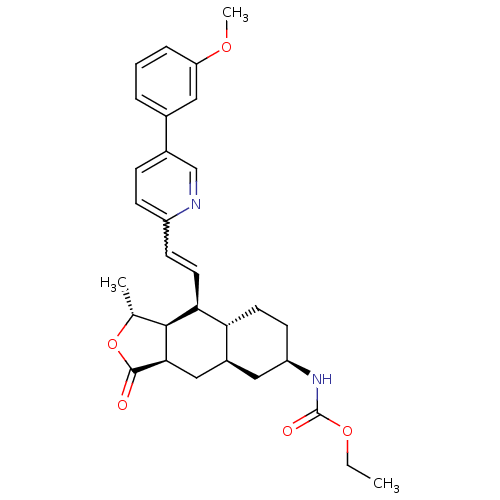
(CHEMBL446344 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(OC)c2)C1 |r,w:21.23| Show InChI InChI=1S/C30H36N2O5/c1-4-36-30(34)32-23-11-12-25-21(14-23)16-27-28(18(2)37-29(27)33)26(25)13-10-22-9-8-20(17-31-22)19-6-5-7-24(15-19)35-3/h5-10,13,15,17-18,21,23,25-28H,4,11-12,14,16H2,1-3H3,(H,32,34)/t18-,21+,23-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261111
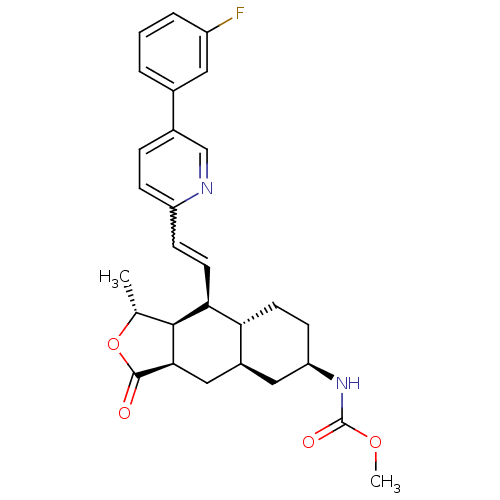
(CHEMBL493983 | methyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9...)Show SMILES COC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:20.22| Show InChI InChI=1S/C28H31FN2O4/c1-16-26-24(11-8-21-7-6-18(15-30-21)17-4-3-5-20(29)12-17)23-10-9-22(31-28(33)34-2)13-19(23)14-25(26)27(32)35-16/h3-8,11-12,15-16,19,22-26H,9-10,13-14H2,1-2H3,(H,31,33)/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261060
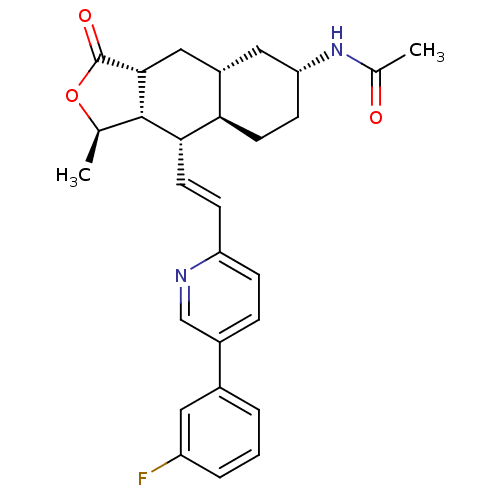
(CHEMBL493632 | N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)NC(C)=O |r| Show InChI InChI=1S/C28H31FN2O3/c1-16-27-25(11-8-22-7-6-19(15-30-22)18-4-3-5-21(29)12-18)24-10-9-23(31-17(2)32)13-20(24)14-26(27)28(33)34-16/h3-8,11-12,15-16,20,23-27H,9-10,13-14H2,1-2H3,(H,31,32)/b11-8+/t16-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261017
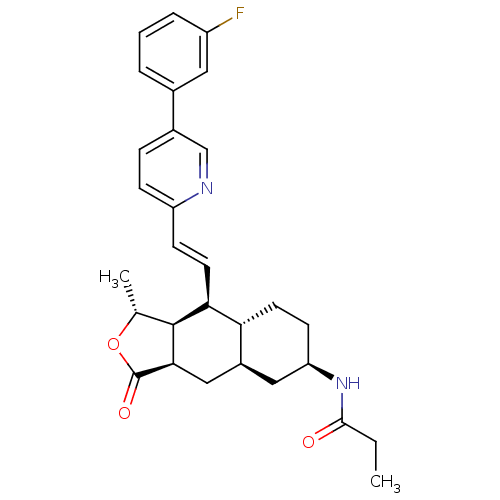
(CHEMBL493792 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E...)Show SMILES CCC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 |r| Show InChI InChI=1S/C29H33FN2O3/c1-3-27(33)32-23-10-11-24-20(14-23)15-26-28(17(2)35-29(26)34)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-26,28H,3,10-11,14-15H2,1-2H3,(H,32,33)/b12-9+/t17-,20+,23-,24-,25+,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261018
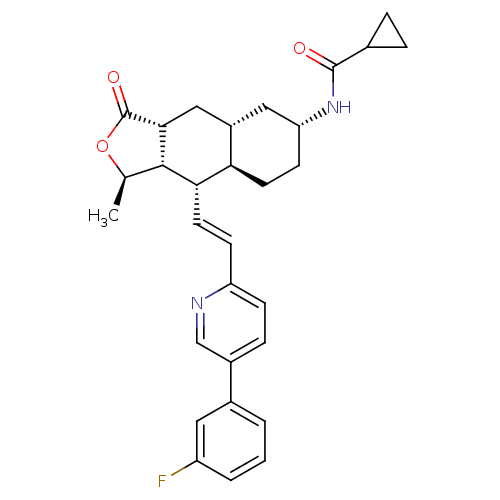
(CHEMBL494618 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33FN2O3/c1-17-28-26(12-9-23-8-7-20(16-32-23)19-3-2-4-22(31)13-19)25-11-10-24(33-29(34)18-5-6-18)14-21(25)15-27(28)30(35)36-17/h2-4,7-9,12-13,16-18,21,24-28H,5-6,10-11,14-15H2,1H3,(H,33,34)/b12-9+/t17-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261108
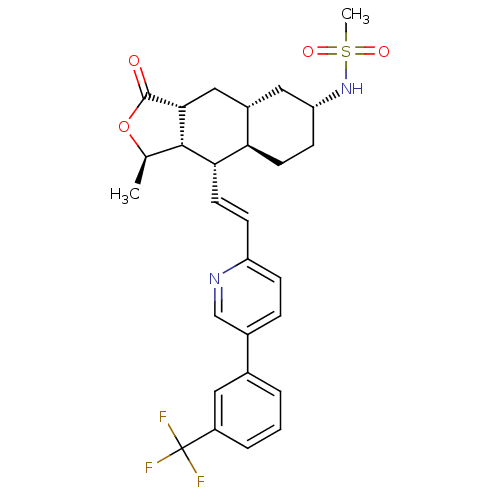
(CHEMBL447996 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-1-met...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12)NS(C)(=O)=O |r| Show InChI InChI=1S/C28H31F3N2O4S/c1-16-26-24(23-10-9-22(33-38(2,35)36)13-19(23)14-25(26)27(34)37-16)11-8-21-7-6-18(15-32-21)17-4-3-5-20(12-17)28(29,30)31/h3-8,11-12,15-16,19,22-26,33H,9-10,13-14H2,1-2H3/b11-8+/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260519
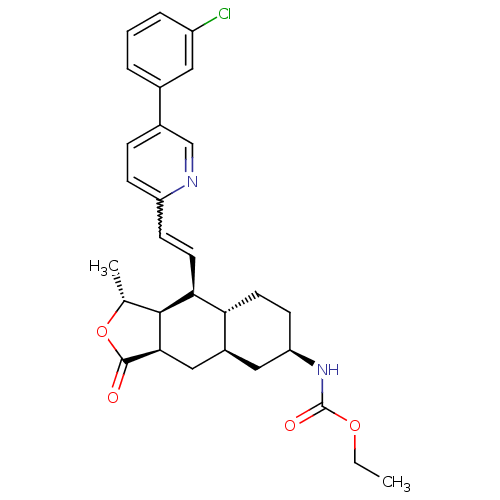
(CHEMBL521531 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(Cl)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33ClN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261107
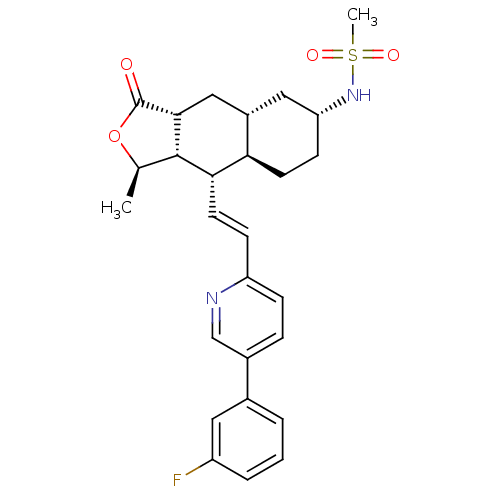
(CHEMBL493975 | N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(E...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)NS(C)(=O)=O |r| Show InChI InChI=1S/C27H31FN2O4S/c1-16-26-24(11-8-21-7-6-18(15-29-21)17-4-3-5-20(28)12-17)23-10-9-22(30-35(2,32)33)13-19(23)14-25(26)27(31)34-16/h3-8,11-12,15-16,19,22-26,30H,9-10,13-14H2,1-2H3/b11-8+/t16-,19+,22-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50202073
((3R,3aS,4S,4aR,7R,8aR,9aR)-decahydro-7-hydroxy-3-m...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@H](O)CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12 |r| Show InChI InChI=1S/C27H28F3NO3/c1-15-25-23(22-10-8-21(32)12-18(22)13-24(25)26(33)34-15)9-7-20-6-5-17(14-31-20)16-3-2-4-19(11-16)27(28,29)30/h2-7,9,11,14-15,18,21-25,32H,8,10,12-13H2,1H3/b9-7+/t15-,18+,21-,22-,23+,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 129-38 (2007)
Article DOI: 10.1021/jm061043e
BindingDB Entry DOI: 10.7270/Q22V2FS9 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260521
(CHEMBL507697 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(C)c2F)C1 |r,w:21.23| Show InChI InChI=1S/C30H35FN2O4/c1-4-36-30(35)33-22-11-12-23-20(14-22)15-26-27(18(3)37-29(26)34)25(23)13-10-21-9-8-19(16-32-21)24-7-5-6-17(2)28(24)31/h5-10,13,16,18,20,22-23,25-27H,4,11-12,14-15H2,1-3H3,(H,33,35)/t18-,20+,22-,23-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260516
(CHEMBL495422 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2ccccc2F)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-21-11-12-22-19(14-21)15-25-27(17(2)36-28(25)33)24(22)13-10-20-9-8-18(16-31-20)23-6-4-5-7-26(23)30/h4-10,13,16-17,19,21-22,24-25,27H,3,11-12,14-15H2,1-2H3,(H,32,34)/t17-,19+,21-,22-,24+,25-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50173417
((3R,3aS,4S,4aR,8aS,9aR)-4-(2-(6-ethylpyridin-2-yl)...)Show SMILES CCc1cccc(\C=C\[C@@H]2[C@@H]3[C@@H](C)OC(=O)[C@@H]3C[C@@H]3CCCC[C@@H]23)n1 Show InChI InChI=1S/C22H29NO2/c1-3-16-8-6-9-17(23-16)11-12-19-18-10-5-4-7-15(18)13-20-21(19)14(2)25-22(20)24/h6,8-9,11-12,14-15,18-21H,3-5,7,10,13H2,1-2H3/b12-11+/t14-,15+,18-,19+,20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory constant aganist Protease-activated receptor 1 |
J Med Chem 48: 5884-7 (2005)
Article DOI: 10.1021/jm0502236
BindingDB Entry DOI: 10.7270/Q2V69J5Q |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261109
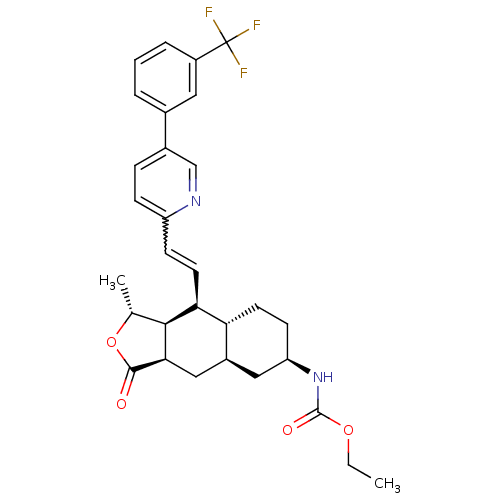
(CHEMBL449425 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-1-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(c2)C(F)(F)F)C1 |r,w:21.23| Show InChI InChI=1S/C30H33F3N2O4/c1-3-38-29(37)35-23-10-11-24-20(14-23)15-26-27(17(2)39-28(26)36)25(24)12-9-22-8-7-19(16-34-22)18-5-4-6-21(13-18)30(31,32)33/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,35,37)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261110

(CHEMBL493982 | Ethyl [(3aR,4aR,8aR,9aS)-9(S)-[(E)-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:21.23| Show InChI InChI=1S/C29H33FN2O4/c1-3-35-29(34)32-23-10-11-24-20(14-23)15-26-27(17(2)36-28(26)33)25(24)12-9-22-8-7-19(16-31-22)18-5-4-6-21(30)13-18/h4-9,12-13,16-17,20,23-27H,3,10-11,14-15H2,1-2H3,(H,32,34)/t17-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human PAR1 in HCASMC assessed as inhibition of thrombin-induced thymidine incorporation |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261112

(CHEMBL494816 | propyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9...)Show SMILES CCCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(F)c2)C1 |r,w:22.24| Show InChI InChI=1S/C30H35FN2O4/c1-3-13-36-30(35)33-24-10-11-25-21(15-24)16-27-28(18(2)37-29(27)34)26(25)12-9-23-8-7-20(17-32-23)19-5-4-6-22(31)14-19/h4-9,12,14,17-18,21,24-28H,3,10-11,13,15-16H2,1-2H3,(H,33,35)/t18-,21+,24-,25-,26+,27-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261015
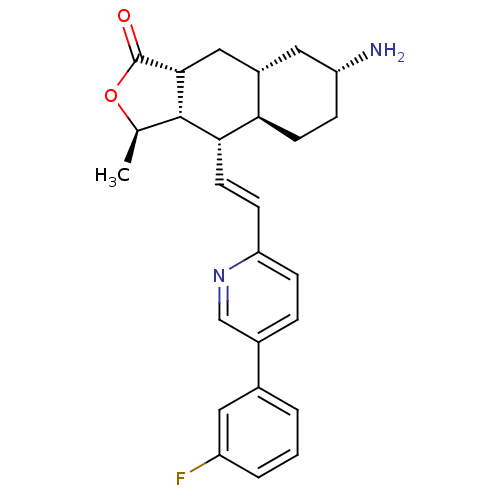
((3R,3aS,4S,4aR,7R,8aR,9aR,E)-7-amino-4-(2-(5-(3-fl...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@H](N)CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12 |r| Show InChI InChI=1S/C26H29FN2O2/c1-15-25-23(22-9-6-20(28)12-18(22)13-24(25)26(30)31-15)10-8-21-7-5-17(14-29-21)16-3-2-4-19(27)11-16/h2-5,7-8,10-11,14-15,18,20,22-25H,6,9,12-13,28H2,1H3/b10-8+/t15-,18+,20-,22-,23+,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261016
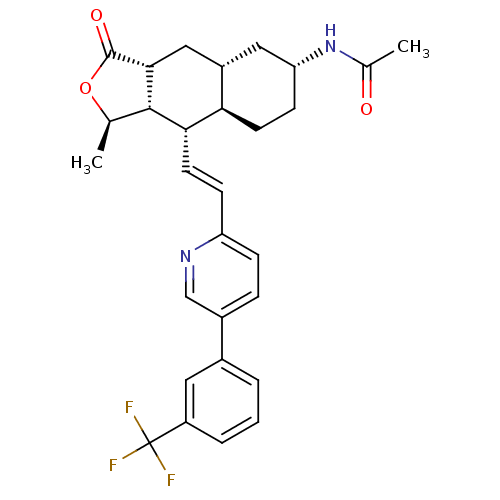
(CHEMBL447913 | N-((1R,3aR,4aR,6R,8aR,9S,9aS)-1-met...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12)NC(C)=O |r| Show InChI InChI=1S/C29H31F3N2O3/c1-16-27-25(24-10-9-23(34-17(2)35)13-20(24)14-26(27)28(36)37-16)11-8-22-7-6-19(15-33-22)18-4-3-5-21(12-18)29(30,31)32/h3-8,11-12,15-16,20,23-27H,9-10,13-14H2,1-2H3,(H,34,35)/b11-8+/t16-,20+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261113
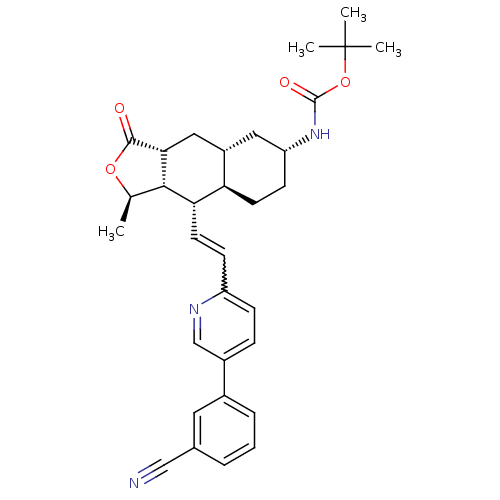
(CHEMBL455020 | tert-butyl (1R,3aR,4aR,6R,8aR,9S,9a...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3C[C@@H](CC[C@H]3[C@H](C=Cc3ccc(cn3)-c3cccc(c3)C#N)[C@H]12)NC(=O)OC(C)(C)C |r,w:15.16| Show InChI InChI=1S/C32H37N3O4/c1-19-29-27(13-10-24-9-8-22(18-34-24)21-7-5-6-20(14-21)17-33)26-12-11-25(35-31(37)39-32(2,3)4)15-23(26)16-28(29)30(36)38-19/h5-10,13-14,18-19,23,25-29H,11-12,15-16H2,1-4H3,(H,35,37)/t19-,23+,25-,26-,27+,28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50261115
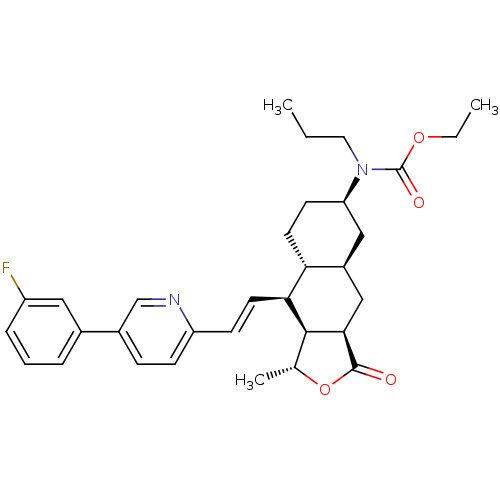
(CHEMBL506808 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCCN([C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1)C(=O)OCC |r| Show InChI InChI=1S/C32H39FN2O4/c1-4-15-35(32(37)38-5-2)26-12-14-27-23(17-26)18-29-30(20(3)39-31(29)36)28(27)13-11-25-10-9-22(19-34-25)21-7-6-8-24(33)16-21/h6-11,13,16,19-20,23,26-30H,4-5,12,14-15,17-18H2,1-3H3/b13-11+/t20-,23+,26-,27-,28+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50260517
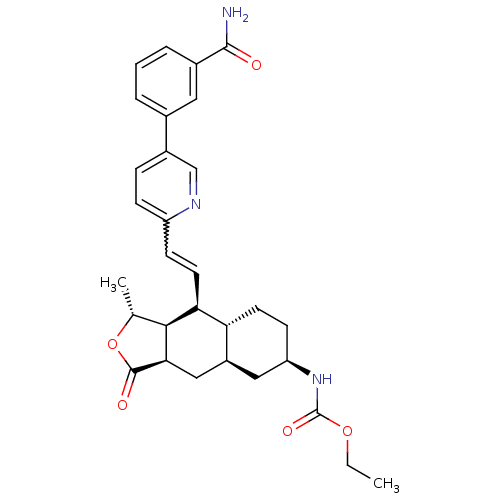
(CHEMBL460583 | ethyl (1R,3aR,4aR,6R,8aR,9S,9aS)-9-...)Show SMILES CCOC(=O)N[C@@H]1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2C=Cc2ccc(cn2)-c2cccc(c2)C(N)=O)C1 |r,w:21.23| Show InChI InChI=1S/C30H35N3O5/c1-3-37-30(36)33-23-10-11-24-21(14-23)15-26-27(17(2)38-29(26)35)25(24)12-9-22-8-7-20(16-32-22)18-5-4-6-19(13-18)28(31)34/h4-9,12-13,16-17,21,23-27H,3,10-11,14-15H2,1-2H3,(H2,31,34)(H,33,36)/t17-,21+,23-,24-,25+,26-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelets |
J Med Chem 51: 3061-4 (2008)
Article DOI: 10.1021/jm800180e
BindingDB Entry DOI: 10.7270/Q28W3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50215908
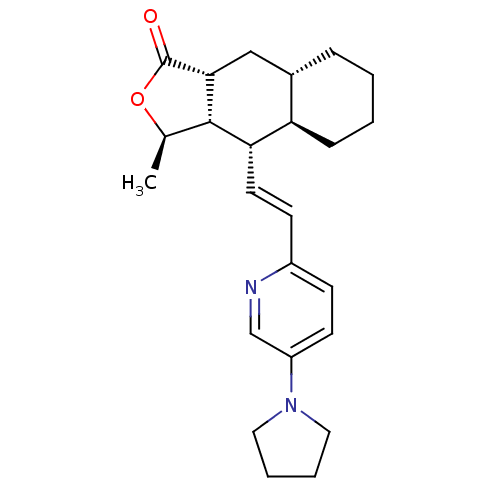
((3R,3aS,4S,4aR,8aS,9aR,E)-3-methyl-4-(2-(5-(pyrrol...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)N3CCCC3)[C@H]12 Show InChI InChI=1S/C24H32N2O2/c1-16-23-21(20-7-3-2-6-17(20)14-22(23)24(27)28-16)11-9-18-8-10-19(15-25-18)26-12-4-5-13-26/h8-11,15-17,20-23H,2-7,12-14H2,1H3/b11-9+/t16-,17+,20-,21+,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 487 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
Bioorg Med Chem Lett 17: 4509-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.002
BindingDB Entry DOI: 10.7270/Q2RN37J6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50215910
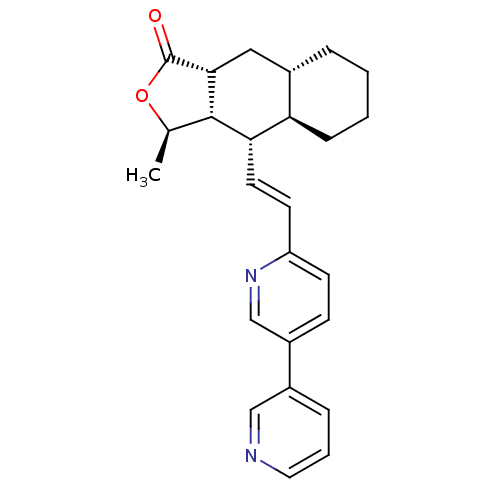
((3R,3aS,4S,4aR,8aS,9aR,E)-3-methyl-4-(2-(5-(pyridi...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccnc3)[C@H]12 Show InChI InChI=1S/C25H28N2O2/c1-16-24-22(21-7-3-2-5-17(21)13-23(24)25(28)29-16)11-10-20-9-8-19(15-27-20)18-6-4-12-26-14-18/h4,6,8-12,14-17,21-24H,2-3,5,7,13H2,1H3/b11-10+/t16-,17+,21-,22+,23-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to cannabinoid CB2 receptor |
Bioorg Med Chem Lett 17: 4509-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.002
BindingDB Entry DOI: 10.7270/Q2RN37J6 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50380022

(CHEMBL2012509)Show SMILES CC[C@@H]1[C@@H](C)C[C@]2(O)[C@@H]([C@@H](C)OC2=O)[C@H]1\C=C\c1ccc(cn1)-c1ccsc1 |r| Show InChI InChI=1S/C23H27NO3S/c1-4-19-14(2)11-23(26)21(15(3)27-22(23)25)20(19)8-7-18-6-5-16(12-24-18)17-9-10-28-13-17/h5-10,12-15,19-21,26H,4,11H2,1-3H3/b8-7+/t14-,15+,19+,20-,21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 after 1 hr by TopCount scintillation counting |
Bioorg Med Chem Lett 22: 2544-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.138
BindingDB Entry DOI: 10.7270/Q20G3M58 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50179567
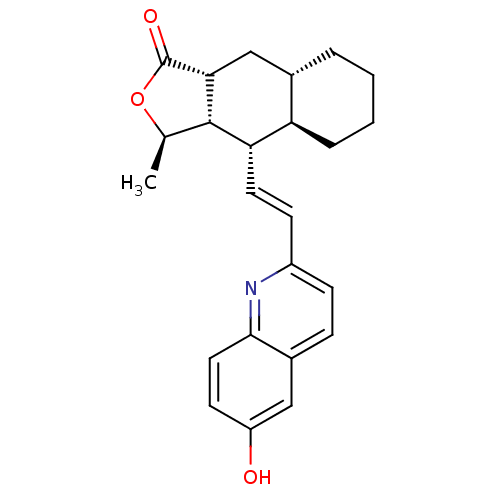
((+)-(3R,3aS,4S,4aR,8aS,9aR)-4-(2-(6-hydroxyquinoli...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc4cc(O)ccc4n3)[C@H]12 Show InChI InChI=1S/C24H27NO3/c1-14-23-20(19-5-3-2-4-15(19)13-21(23)24(27)28-14)10-8-17-7-6-16-12-18(26)9-11-22(16)25-17/h6-12,14-15,19-21,23,26H,2-5,13H2,1H3/b10-8+/t14-,15+,19-,20+,21-,23+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
Bioorg Med Chem Lett 16: 1544-8 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.042
BindingDB Entry DOI: 10.7270/Q2KH0MXM |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50380014
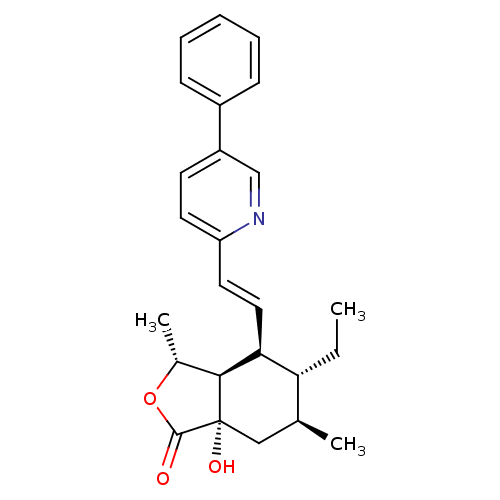
(CHEMBL2012500)Show SMILES CC[C@@H]1[C@@H](C)C[C@]2(O)[C@@H]([C@@H](C)OC2=O)[C@H]1\C=C\c1ccc(cn1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29NO3/c1-4-21-16(2)14-25(28)23(17(3)29-24(25)27)22(21)13-12-20-11-10-19(15-26-20)18-8-6-5-7-9-18/h5-13,15-17,21-23,28H,4,14H2,1-3H3/b13-12+/t16-,17+,21+,22-,23-,25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 after 1 hr by TopCount scintillation counting |
Bioorg Med Chem Lett 22: 2544-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.138
BindingDB Entry DOI: 10.7270/Q20G3M58 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212443

((3aR,4R,9aS,E)-7,8-difluoro-4-(2-(5-(3-fluoropheny...)Show SMILES Fc1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3Cc3c(F)c(F)ccc23)nc1 Show InChI InChI=1S/C25H18F3NO2/c26-16-3-1-2-14(10-16)15-4-5-17(29-12-15)6-7-19-18-8-9-23(27)24(28)20(18)11-21-22(19)13-31-25(21)30/h1-10,12,19,21-22H,11,13H2/b7-6+/t19-,21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212440
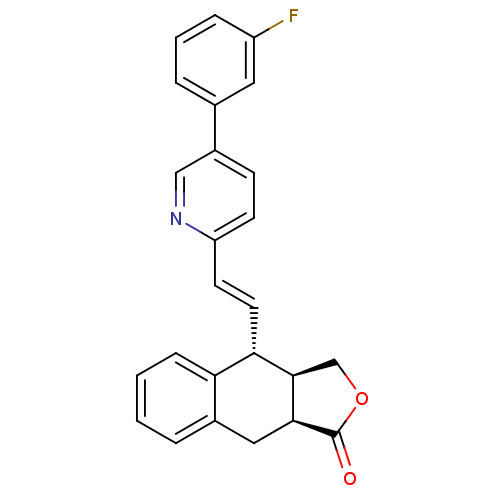
((3aR,4R,9aS,E)-4-(2-(5-(3-fluorophenyl)pyridin-2-y...)Show SMILES Fc1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3Cc3ccccc23)nc1 Show InChI InChI=1S/C25H20FNO2/c26-19-6-3-5-16(12-19)18-8-9-20(27-14-18)10-11-22-21-7-2-1-4-17(21)13-23-24(22)15-29-25(23)28/h1-12,14,22-24H,13,15H2/b11-10+/t22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212436
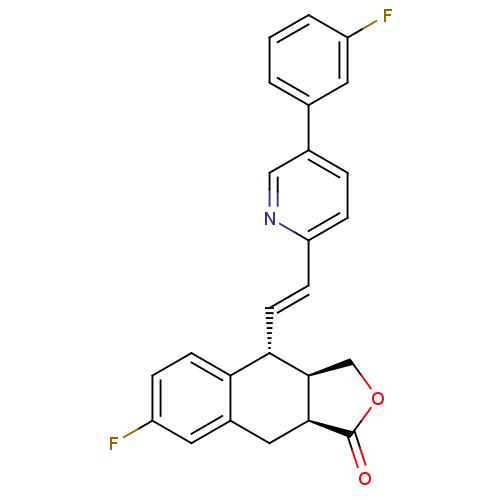
((3aR,4R,9aS,E)-7-fluoro-4-(2-(5-(3-fluorophenyl)py...)Show SMILES Fc1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3Cc3cc(F)ccc23)nc1 Show InChI InChI=1S/C25H19F2NO2/c26-18-3-1-2-15(10-18)16-4-6-20(28-13-16)7-9-22-21-8-5-19(27)11-17(21)12-23-24(22)14-30-25(23)29/h1-11,13,22-24H,12,14H2/b9-7+/t22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222017
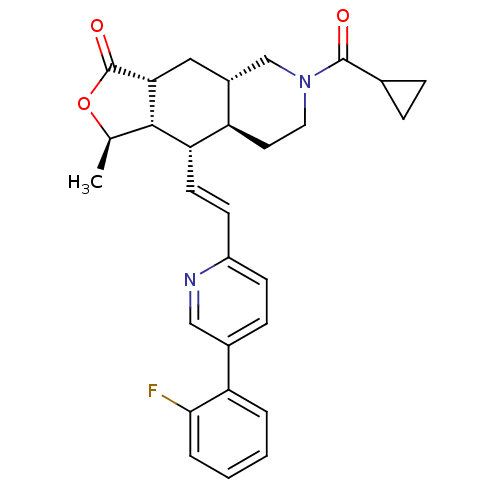
(1R,3aR,4aS,8aS,9S,9aS)-6-(cyclopropylcarbonyl)-9-[...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CN(CC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3ccccc3F)[C@H]12)C(=O)C1CC1 Show InChI InChI=1S/C29H31FN2O3/c1-17-27-24(11-10-21-9-8-19(15-31-21)23-4-2-3-5-26(23)30)22-12-13-32(28(33)18-6-7-18)16-20(22)14-25(27)29(34)35-17/h2-5,8-11,15,17-18,20,22,24-25,27H,6-7,12-14,16H2,1H3/b11-10+/t17-,20-,22-,24+,25-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222041
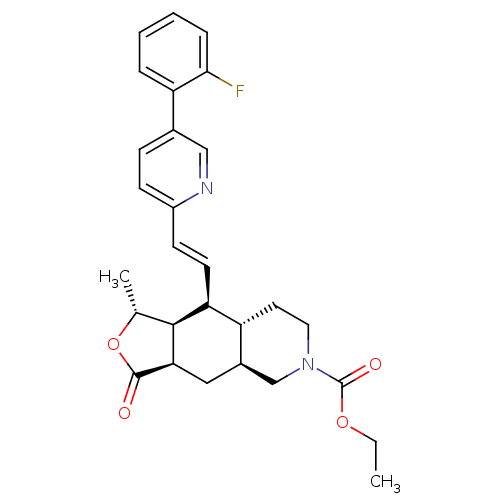
((1R,3aR,4aS,8aS,9S,9aS)-ethyl decahydro-1-methyl-3...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2ccccc2F)C1 Show InChI InChI=1S/C28H31FN2O4/c1-3-34-28(33)31-13-12-21-19(16-31)14-24-26(17(2)35-27(24)32)23(21)11-10-20-9-8-18(15-30-20)22-6-4-5-7-25(22)29/h4-11,15,17,19,21,23-24,26H,3,12-14,16H2,1-2H3/b11-10+/t17-,19-,21-,23+,24-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50380018
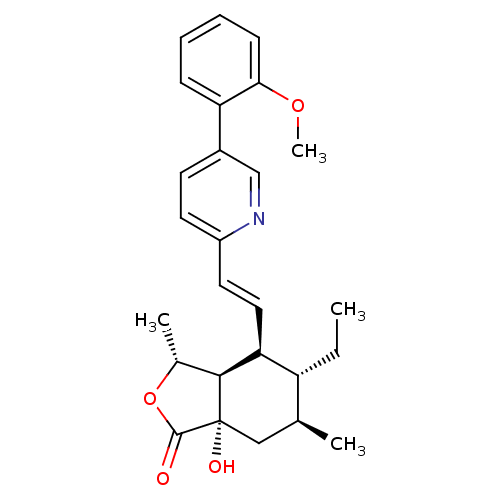
(CHEMBL2012505)Show SMILES CC[C@@H]1[C@@H](C)C[C@]2(O)[C@@H]([C@@H](C)OC2=O)[C@H]1\C=C\c1ccc(cn1)-c1ccccc1OC |r| Show InChI InChI=1S/C26H31NO4/c1-5-20-16(2)14-26(29)24(17(3)31-25(26)28)22(20)13-12-19-11-10-18(15-27-19)21-8-6-7-9-23(21)30-4/h6-13,15-17,20,22,24,29H,5,14H2,1-4H3/b13-12+/t16-,17+,20+,22-,24-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 after 1 hr by TopCount scintillation counting |
Bioorg Med Chem Lett 22: 2544-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.138
BindingDB Entry DOI: 10.7270/Q20G3M58 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50380023
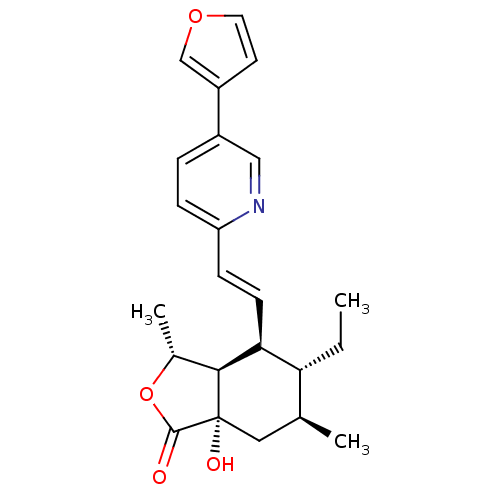
(CHEMBL2012510)Show SMILES CC[C@@H]1[C@@H](C)C[C@]2(O)[C@@H]([C@@H](C)OC2=O)[C@H]1\C=C\c1ccc(cn1)-c1ccoc1 |r| Show InChI InChI=1S/C23H27NO4/c1-4-19-14(2)11-23(26)21(15(3)28-22(23)25)20(19)8-7-18-6-5-16(12-24-18)17-9-10-27-13-17/h5-10,12-15,19-21,26H,4,11H2,1-3H3/b8-7+/t14-,15+,19+,20-,21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 after 1 hr by TopCount scintillation counting |
Bioorg Med Chem Lett 22: 2544-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.138
BindingDB Entry DOI: 10.7270/Q20G3M58 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50380004

(CHEMBL2012489)Show SMILES CC[C@@H]1[C@@H](C)C[C@@H]2[C@@H]([C@@H](C)OC2=O)[C@H]1\C=C\c1ccc(cn1)-c1ccccc1 |r| Show InChI InChI=1S/C25H29NO2/c1-4-21-16(2)14-23-24(17(3)28-25(23)27)22(21)13-12-20-11-10-19(15-26-20)18-8-6-5-7-9-18/h5-13,15-17,21-24H,4,14H2,1-3H3/b13-12+/t16-,17+,21+,22-,23+,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 after 1 hr by TopCount scintillation counting |
Bioorg Med Chem Lett 22: 2544-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.138
BindingDB Entry DOI: 10.7270/Q20G3M58 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212453
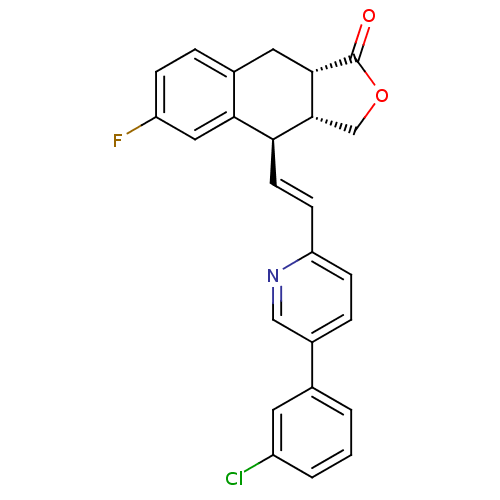
((3aR,4R,9aS,E)-4-(2-(5-(3-chlorophenyl)pyridin-2-y...)Show SMILES Fc1ccc2C[C@H]3[C@H](COC3=O)[C@@H](\C=C\c3ccc(cn3)-c3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C25H19ClFNO2/c26-18-3-1-2-15(10-18)17-5-7-20(28-13-17)8-9-21-22-12-19(27)6-4-16(22)11-23-24(21)14-30-25(23)29/h1-10,12-13,21,23-24H,11,14H2/b9-8+/t21-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212430
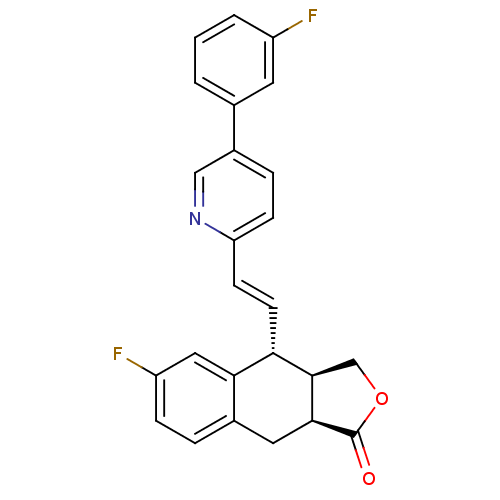
((3aR,4R,9aS,E)-6-fluoro-4-(2-(5-(3-fluorophenyl)py...)Show SMILES Fc1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3Cc3ccc(F)cc23)nc1 Show InChI InChI=1S/C25H19F2NO2/c26-18-3-1-2-15(10-18)17-5-7-20(28-13-17)8-9-21-22-12-19(27)6-4-16(22)11-23-24(21)14-30-25(23)29/h1-10,12-13,21,23-24H,11,14H2/b9-8+/t21-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50212429

((3aR,4R,9aS,E)-4-(2-(5-(3-(trifluoromethyl)phenyl)...)Show SMILES FC(F)(F)c1cccc(c1)-c1ccc(\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3Cc3ccccc23)nc1 Show InChI InChI=1S/C26H20F3NO2/c27-26(28,29)19-6-3-5-16(12-19)18-8-9-20(30-14-18)10-11-22-21-7-2-1-4-17(21)13-23-24(22)15-32-25(23)31/h1-12,14,22-24H,13,15H2/b11-10+/t22-,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 |
Bioorg Med Chem Lett 17: 3647-51 (2007)
Article DOI: 10.1016/j.bmcl.2007.04.061
BindingDB Entry DOI: 10.7270/Q2JH3KV4 |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50173437
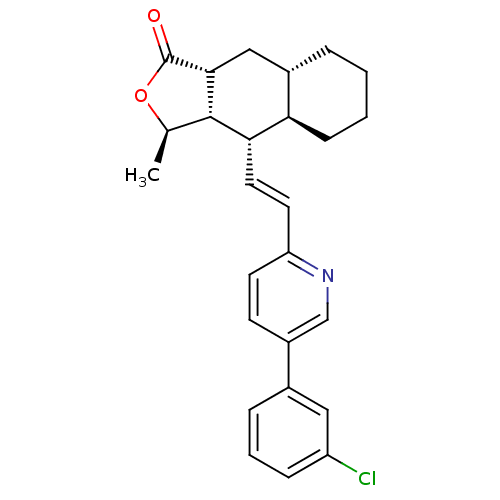
((3R,3aS,4S,4aR,8aS,9aR)-4-{(E)-2-[5-(3-Chloro-phen...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(Cl)c3)[C@H]12 Show InChI InChI=1S/C26H28ClNO2/c1-16-25-23(22-8-3-2-5-18(22)14-24(25)26(29)30-16)12-11-21-10-9-19(15-28-21)17-6-4-7-20(27)13-17/h4,6-7,9-13,15-16,18,22-25H,2-3,5,8,14H2,1H3/b12-11+/t16-,18+,22-,23+,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration aganist human protease activated receptor 1 using [3H]-haTRAP as radioligand |
J Med Chem 48: 5884-7 (2005)
Article DOI: 10.1021/jm0502236
BindingDB Entry DOI: 10.7270/Q2V69J5Q |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222015

((1R,3aR,4aS,8aS,9S,9aS)-decahydro-1-methyl-3-oxo-9...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(F)c2)C1 Show InChI InChI=1S/C28H31FN2O4/c1-3-34-28(33)31-12-11-23-20(16-31)14-25-26(17(2)35-27(25)32)24(23)10-9-22-8-7-19(15-30-22)18-5-4-6-21(29)13-18/h4-10,13,15,17,20,23-26H,3,11-12,14,16H2,1-2H3/b10-9+/t17-,20-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50222030

((1R,3aR,4aS,8aS,9S,9aS)-decahydro-1-methyl-3-oxo-9...)Show SMILES CCOC(=O)N1CC[C@@H]2[C@H](C[C@@H]3[C@@H]([C@@H](C)OC3=O)[C@H]2\C=C\c2ccc(cn2)-c2cccc(c2)C(F)(F)F)C1 Show InChI InChI=1S/C29H31F3N2O4/c1-3-37-28(36)34-12-11-23-20(16-34)14-25-26(17(2)38-27(25)35)24(23)10-9-22-8-7-19(15-33-22)18-5-4-6-21(13-18)29(30,31)32/h4-10,13,15,17,20,23-26H,3,11-12,14,16H2,1-2H3/b10-9+/t17-,20-,23-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from PAR1 in human platelet membrane |
J Med Chem 50: 5147-60 (2007)
Article DOI: 10.1021/jm070704k
BindingDB Entry DOI: 10.7270/Q2SN08PK |
More data for this
Ligand-Target Pair | |
Proteinase-activated receptor 1
(Homo sapiens (Human)) | BDBM50173419

((3R,3aS,4S,4aR,8aS,9aR)-3-Methyl-4-{(E)-2-[5-(3-tr...)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\c3ccc(cn3)-c3cccc(c3)C(F)(F)F)[C@H]12 Show InChI InChI=1S/C27H28F3NO2/c1-16-25-23(22-8-3-2-5-18(22)14-24(25)26(32)33-16)12-11-21-10-9-19(15-31-21)17-6-4-7-20(13-17)27(28,29)30/h4,6-7,9-13,15-16,18,22-25H,2-3,5,8,14H2,1H3/b12-11+/t16-,18+,22-,23+,24-,25+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]haTRAP from human PAR1 receptor in platelet membrane |
Bioorg Med Chem Lett 17: 4509-13 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.002
BindingDB Entry DOI: 10.7270/Q2RN37J6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data