

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50067536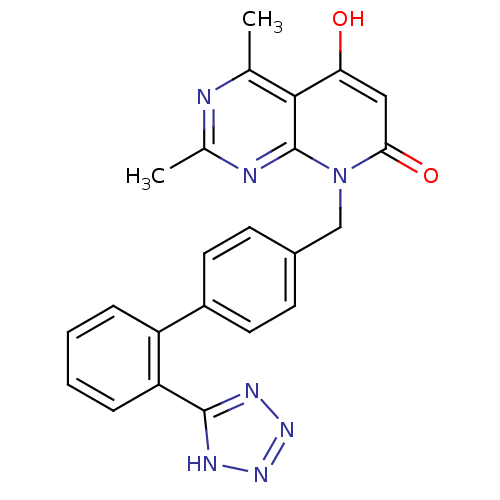 (5-Hydroxy-2,4-dimethyl-8-[2'-(1H-tetrazol-5-yl)-bi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471877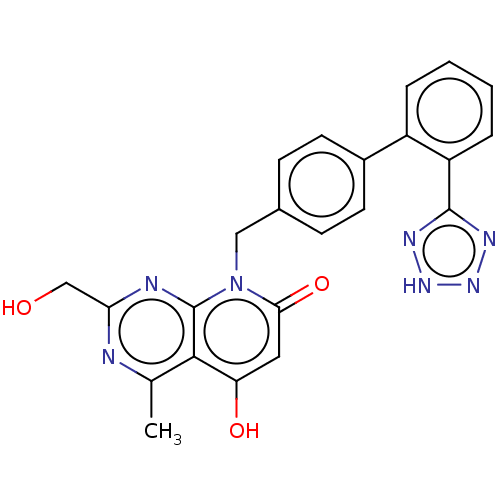 (CHEMBL135574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50040439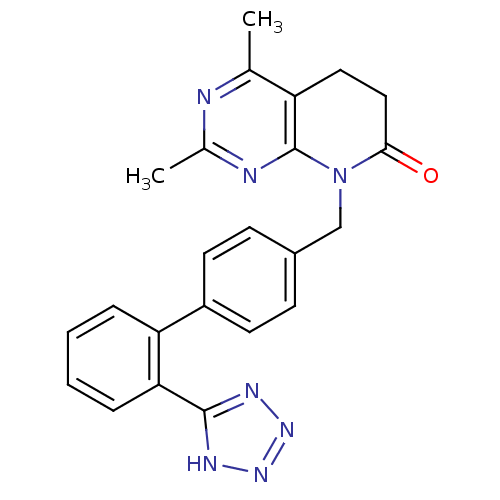 (2,4-Dimethyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in mouse LV2 cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087670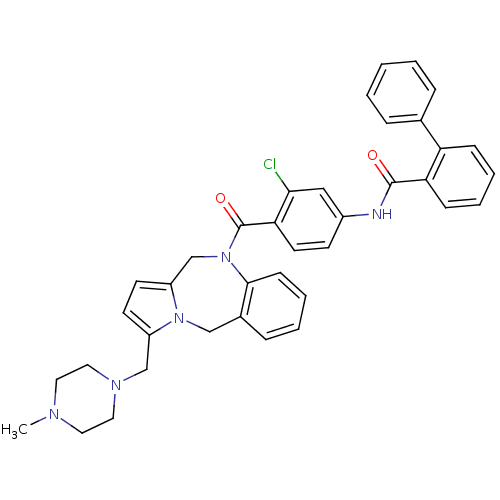 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087664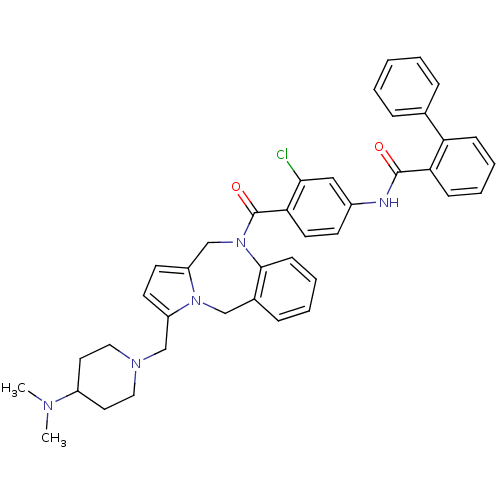 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087675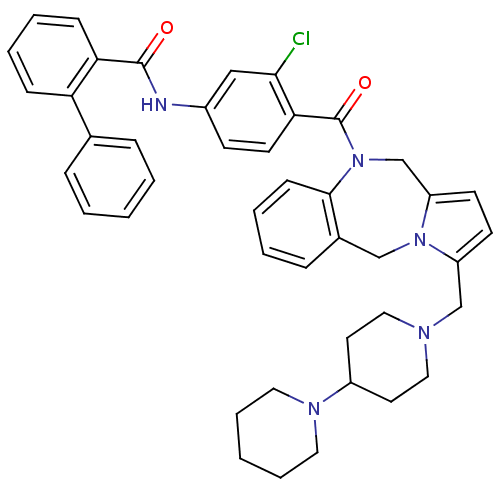 (Biphenyl-2-carboxylic acid [4-(3-[1,4']bipiperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471878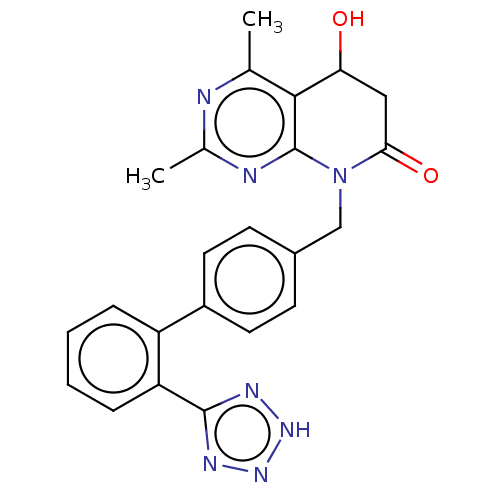 (CHEMBL337067) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087674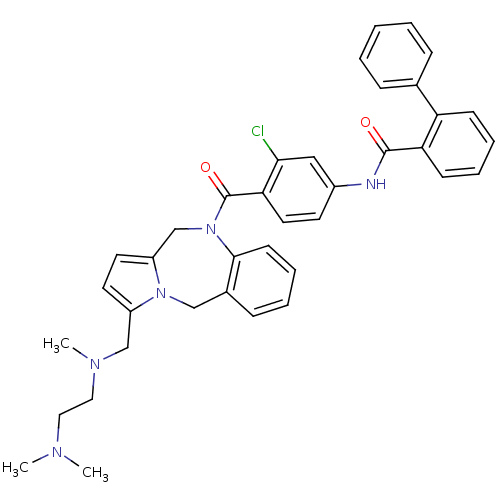 (Biphenyl-2-carboxylic acid [3-chloro-4-(3-{[(2-dim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087667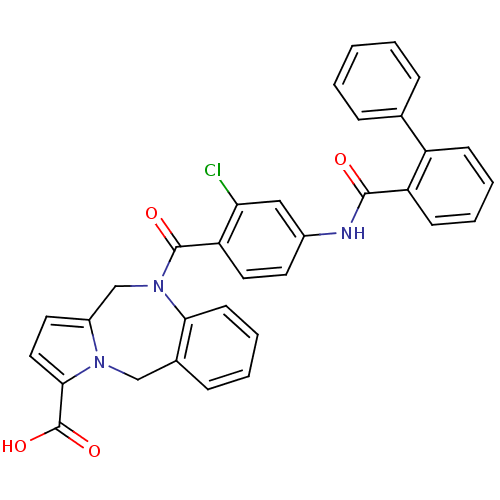 (10-{4-[(Biphenyl-2-carbonyl)-amino]-2-chloro-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087672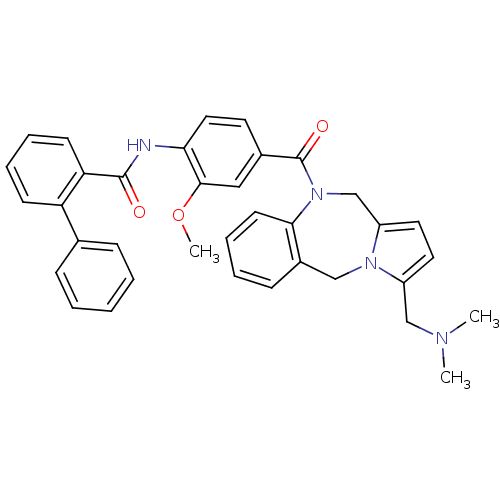 (Biphenyl-2-carboxylic acid [4-(3-dimethylaminometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471879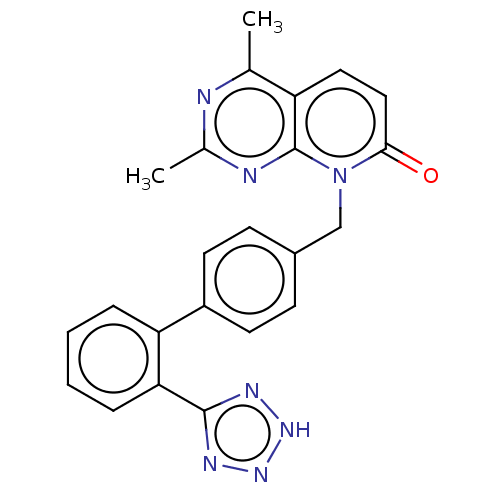 (CHEMBL135309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087673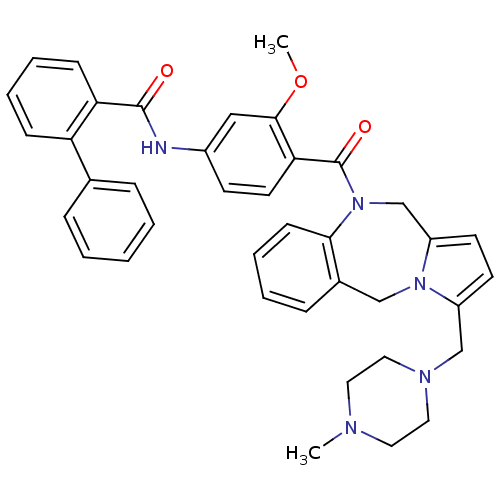 (Biphenyl-2-carboxylic acid {3-methoxy-4-[3-(4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087666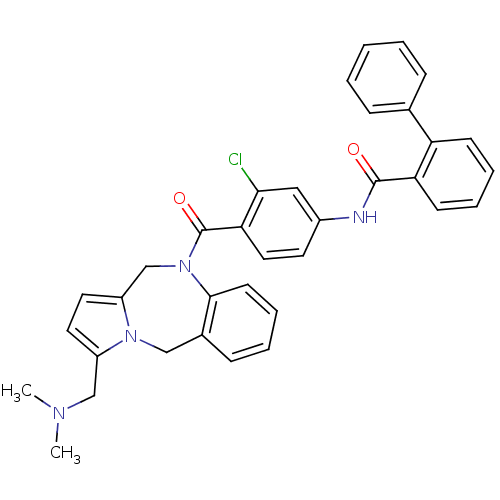 (Biphenyl-2-carboxylic acid [3-chloro-4-(3-dimethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087665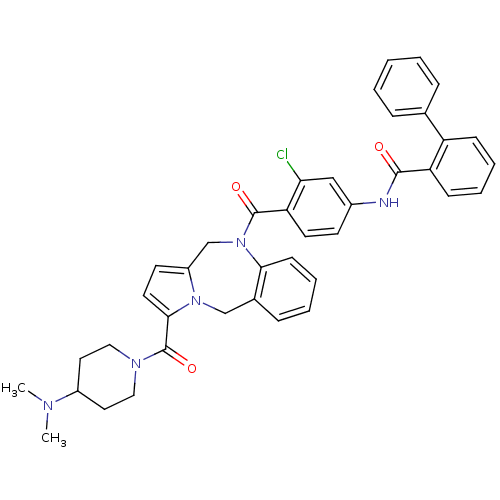 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50222059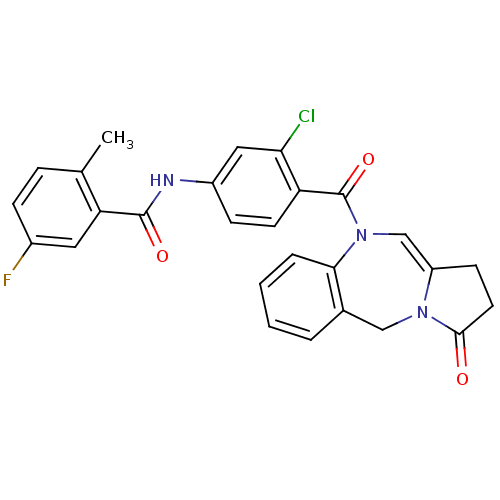 (CHEMBL392363 | N-[3-chloro-4-(3-oxo-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087669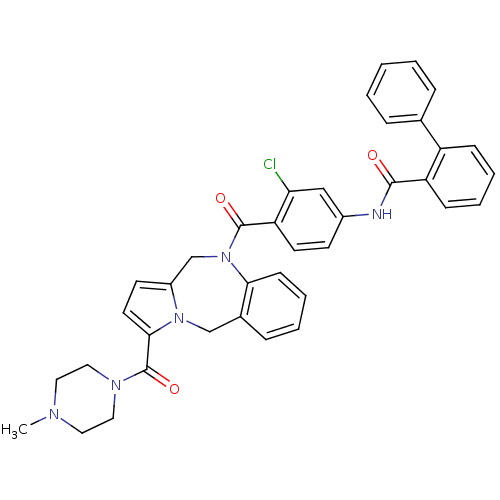 (Biphenyl-2-carboxylic acid {3-chloro-4-[3-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087668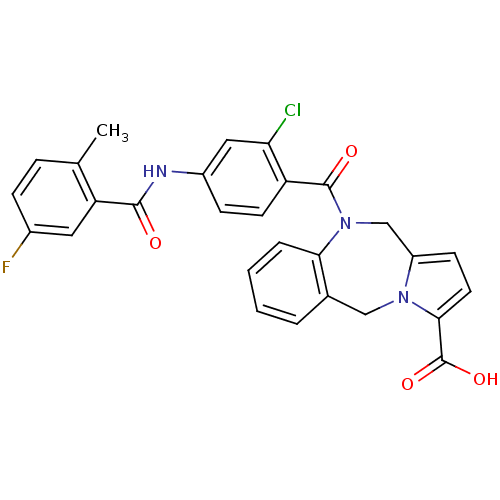 (10-[2-Chloro-4-(5-fluoro-2-methyl-benzoylamino)-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087671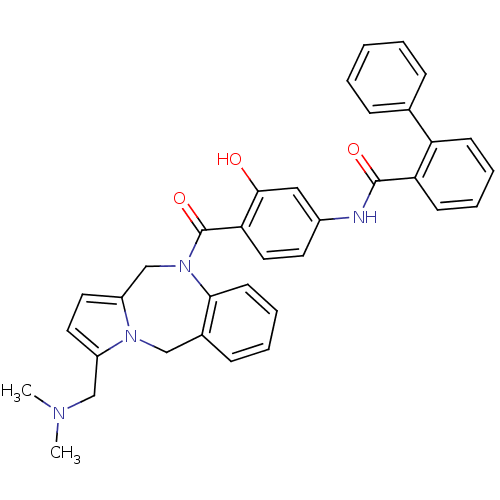 (Biphenyl-2-carboxylic acid [4-(3-dimethylaminometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human vasopressin V2-receptor expressed in murine fibroblast cell (LV2) membranes | Bioorg Med Chem Lett 10: 783-6 (2000) BindingDB Entry DOI: 10.7270/Q24M93RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50222058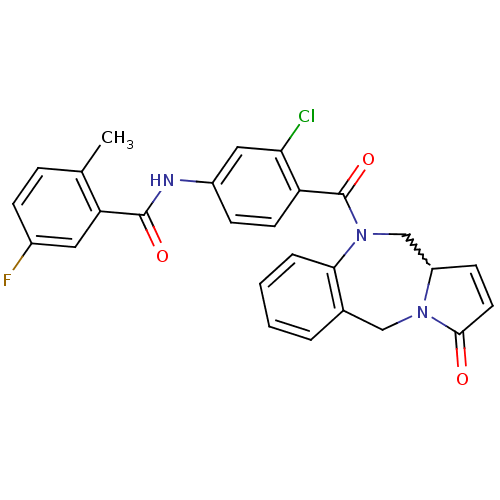 (CHEMBL237772 | N-[3-chloro-4-(3-oxo-11,11a-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16314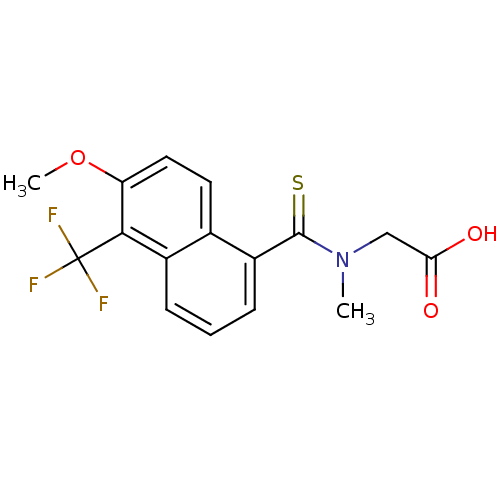 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of aldose reductase from the partially purified bovine lens | J Med Chem 33: 2892-9 (1990) BindingDB Entry DOI: 10.7270/Q27H1HK5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50177593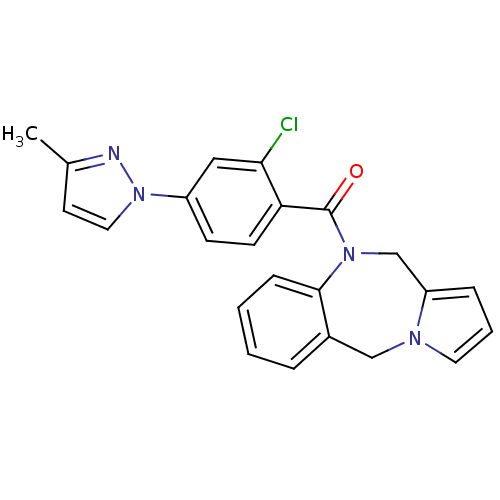 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 80.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in mouse LV2 cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50016901 ((2-Methyl-3-oxo-3H-phenalen-1-yl)-acetic acid | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50177593 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 519 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of aldose reductase (or polyol accumulation) in isolated rat sciatic nerve by compound at 10e-5 M concentration | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50016902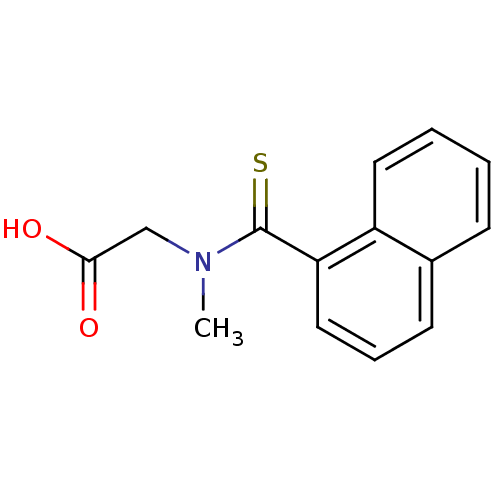 (CHEMBL168106 | [Methyl-(naphthalene-1-carbothioyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of bovine lens aldose reductase | J Med Chem 32: 757-65 (1989) BindingDB Entry DOI: 10.7270/Q22B8X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50026173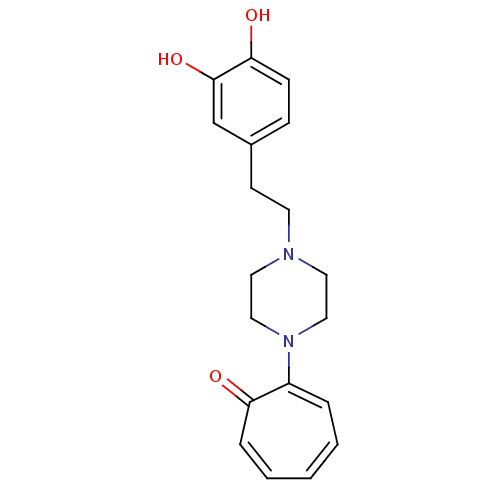 (2-{4-[2-(3,4-Dimethoxy-phenyl)-2-hydroxy-ethyl]-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50177593 ((5H,11H-benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 778 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50026171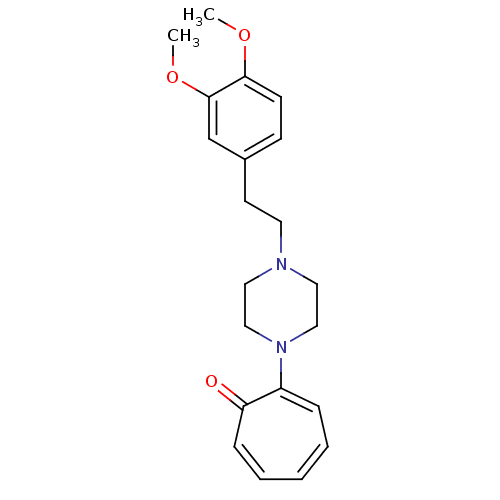 (2-[4-(2-Hydroxy-2-phenyl-ethyl)-piperazin-1-yl]-cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50222060 (1-(2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benza...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50222059 (CHEMBL392363 | N-[3-chloro-4-(3-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50222060 (1-(2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50222059 (CHEMBL392363 | N-[3-chloro-4-(3-oxo-2,3-dihydro-1H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50222057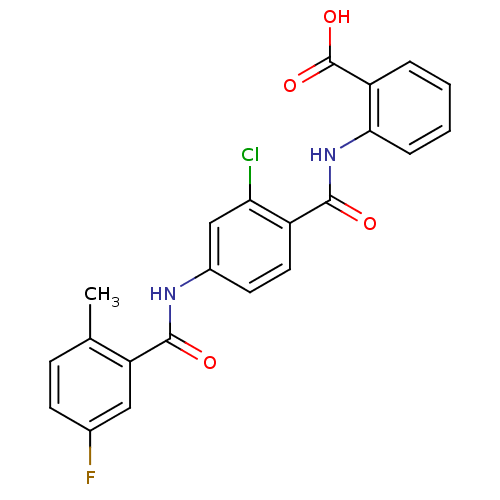 (2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benzamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50222057 (2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benzamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50222058 (CHEMBL237772 | N-[3-chloro-4-(3-oxo-11,11a-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50222057 (2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benzamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]Manning ligand from human vasopressin V1a receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50222058 (CHEMBL237772 | N-[3-chloro-4-(3-oxo-11,11a-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]oxytocin from human oxytocin receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50222060 (1-(2-(2-chloro-4-(5-fluoro-2-methylbenzamido)benza...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50026172 (2-{4-[2-(3,4-Dimethoxy-phenyl)-2-hydroxy-1-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50452265 (CHEMBL2115477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50452267 (Ciladopa) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]WB-4101 from rat brain alpha-1 adrenergic receptor | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50222061 (10-[2-chloro-4-(3-methyl-pyrazol-1-yl)-benzoyl]-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V2 receptor expressed in mouse LV2 cells | Bioorg Med Chem Lett 17: 5796-800 (2007) Article DOI: 10.1016/j.bmcl.2007.08.053 BindingDB Entry DOI: 10.7270/Q2NV9J05 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50452266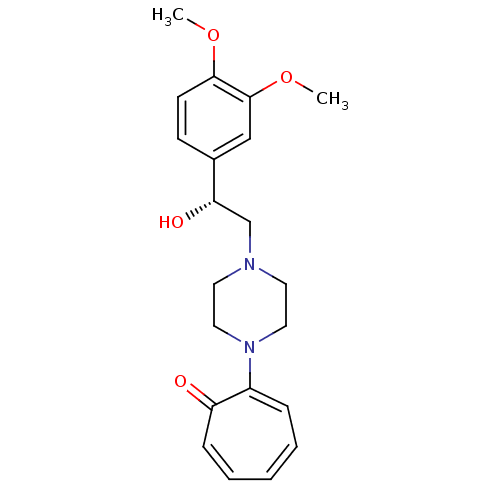 (CHEMBL2115071) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace [3H]WB-4101 from rat brain alpha-1 adrenergic receptor | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50452265 (CHEMBL2115477) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was for its ability to displace [3H]-haloperidol binding to rat striatal Dopamine receptor D2 | J Med Chem 29: 186-93 (1986) BindingDB Entry DOI: 10.7270/Q2N29VZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |