 Found 3426 hits with Last Name = 'duffy' and Initial = 'j'
Found 3426 hits with Last Name = 'duffy' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513934
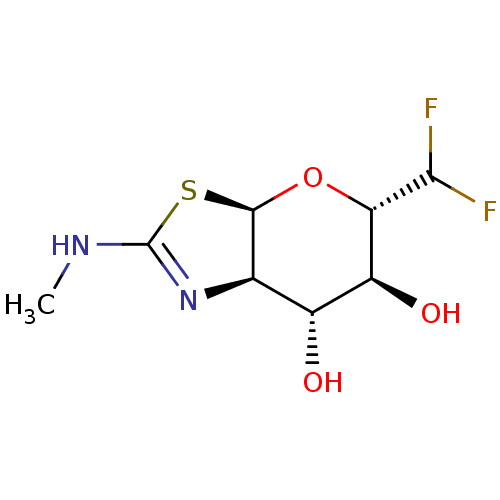
(CHEMBL4443587)Show SMILES [H][C@]12O[C@H](C(F)F)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NC)S2 |r,t:14| Show InChI InChI=1S/C8H12F2N2O3S/c1-11-8-12-2-3(13)4(14)5(6(9)10)15-7(2)16-8/h2-7,13-14H,1H3,(H,11,12)/t2-,3-,4+,5+,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50323697

((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...)Show SMILES CCNC1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C9H16N2O4S/c1-2-10-9-11-5-7(14)6(13)4(3-12)15-8(5)16-9/h4-8,12-14H,2-3H2,1H3,(H,10,11)/t4-,5-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305149
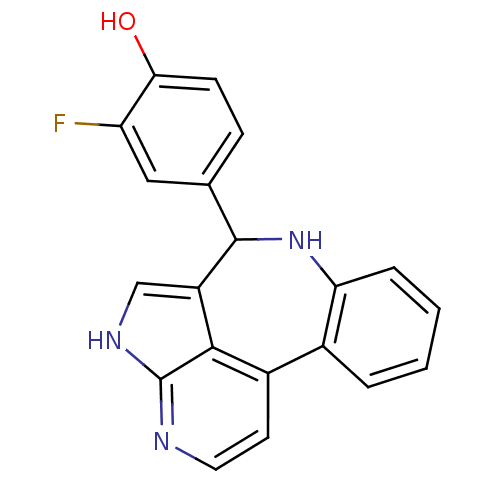
(2-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show InChI InChI=1S/C20H14FN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513931
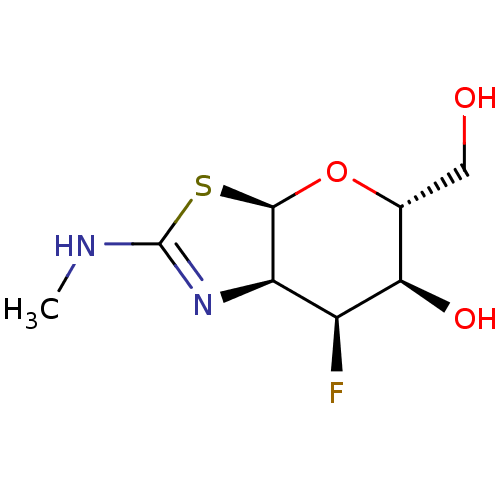
(CHEMBL4444446)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@@H](F)[C@@]1([H])N=C(NC)S2 |r,t:13| Show InChI InChI=1S/C8H13FN2O3S/c1-10-8-11-5-4(9)6(13)3(2-12)14-7(5)15-8/h3-7,12-13H,2H2,1H3,(H,10,11)/t3-,4+,5-,6-,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM205423
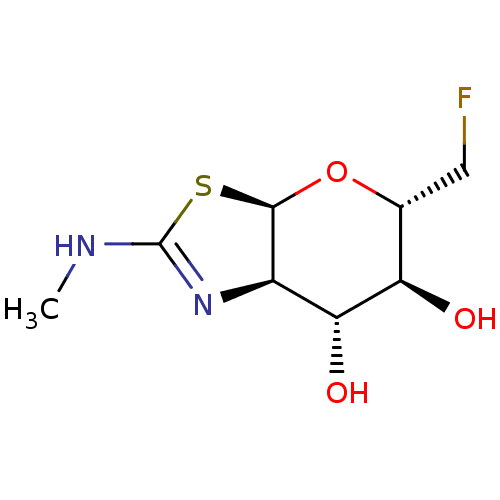
(US9243020, 17 | US9815861, Example 17)Show SMILES CNC1=N[C@H]2[C@H](O[C@H](CF)[C@@H](O)[C@@H]2O)S1 |r,t:2| Show InChI InChI=1S/C8H13FN2O3S/c1-10-8-11-4-6(13)5(12)3(2-9)14-7(4)15-8/h3-7,12-13H,2H2,1H3,(H,10,11)/t3-,4-,5-,6-,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289804

(Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)NC(CC1(CC1)OC)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H30ClFN4O7/c1-39-25(37)31-17-6-4-16(5-7-17)23(35)32-20(14-27(40-2)11-12-27)24(36)34-13-3-10-28(15-34)21-19(33-26(38)41-28)9-8-18(29)22(21)30/h4-9,20H,3,10-15H2,1-2H3,(H,31,37)(H,32,35)(H,33,38)/t20?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305150
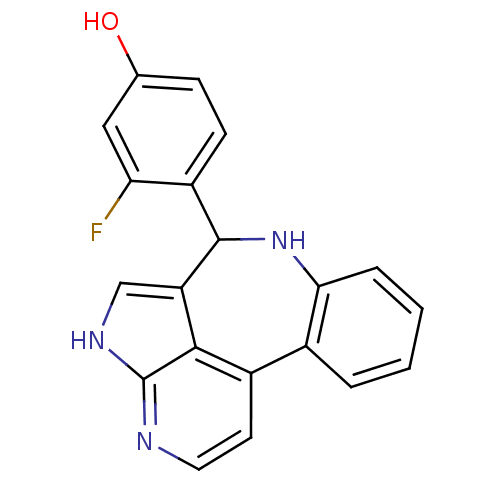
(3-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C20H14FN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289851
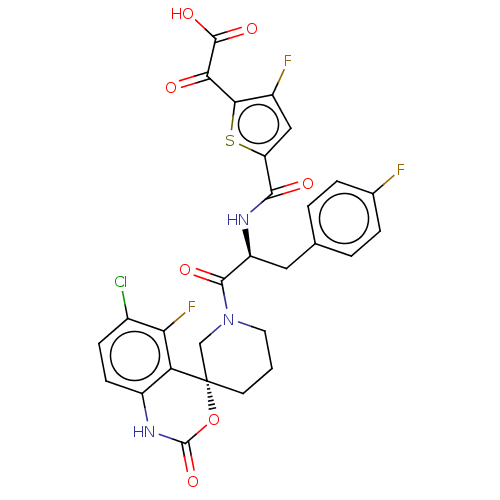
(2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...)Show SMILES OC(=O)C(=O)c1sc(cc1F)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H21ClF3N3O7S/c29-15-6-7-17-20(21(15)32)28(42-27(41)34-17)8-1-9-35(12-28)25(38)18(10-13-2-4-14(30)5-3-13)33-24(37)19-11-16(31)23(43-19)22(36)26(39)40/h2-7,11,18H,1,8-10,12H2,(H,33,37)(H,34,41)(H,39,40)/t18-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289807
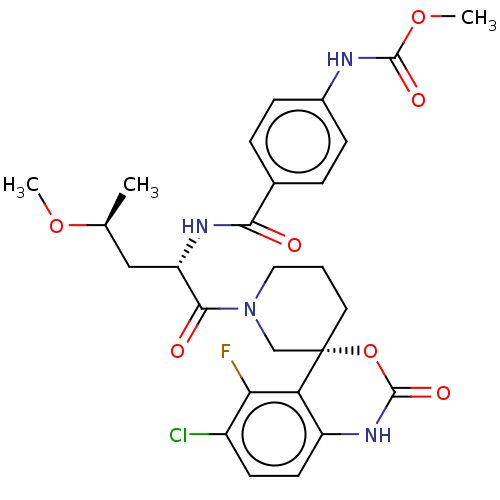
(US10093683, Example 118A | US10093683, Example 118...)Show SMILES CO[C@@H](C)C[C@H](NC(=O)c1ccc(NC(=O)OC)cc1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C27H30ClFN4O7/c1-15(38-2)13-20(31-23(34)16-5-7-17(8-6-16)30-25(36)39-3)24(35)33-12-4-11-27(14-33)21-19(32-26(37)40-27)10-9-18(28)22(21)29/h5-10,15,20H,4,11-14H2,1-3H3,(H,30,36)(H,31,34)(H,32,37)/t15-,20-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305157
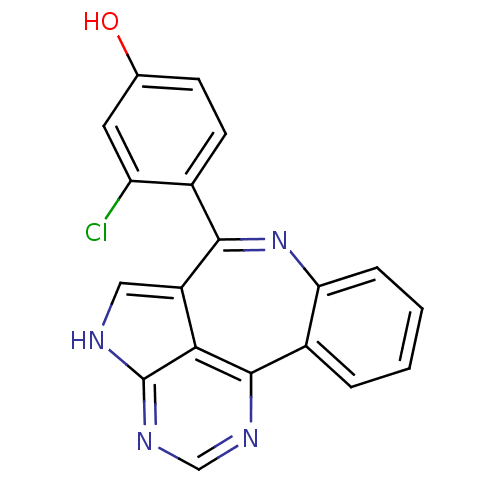
(3-chloro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(c(Cl)c1)-c1nc2ccccc2c2ncnc3[nH]cc1c23 Show InChI InChI=1S/C19H11ClN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305155
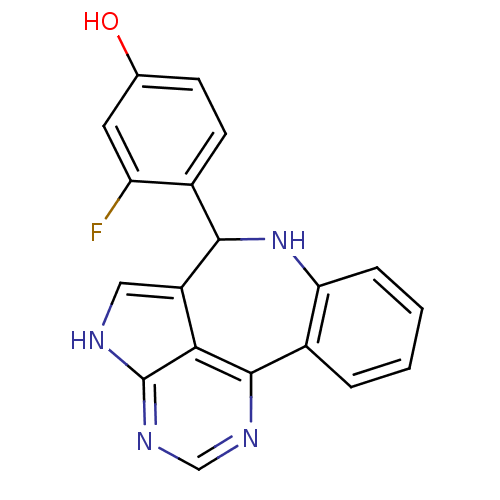
(3-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ncnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C19H13FN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305156
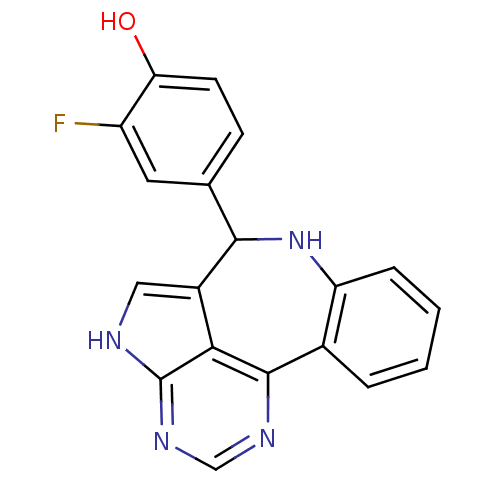
(2-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show InChI InChI=1S/C19H13FN4O/c20-13-7-10(5-6-15(13)25)17-12-8-21-19-16(12)18(22-9-23-19)11-3-1-2-4-14(11)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305148
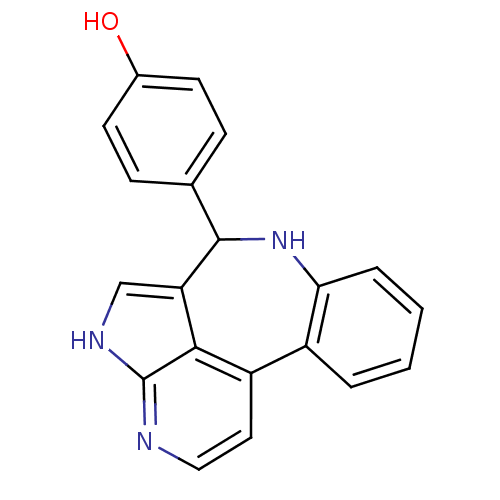
(4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,17...)Show InChI InChI=1S/C20H15N3O/c24-13-7-5-12(6-8-13)19-16-11-22-20-18(16)15(9-10-21-20)14-3-1-2-4-17(14)23-19/h1-11,19,23-24H,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305153
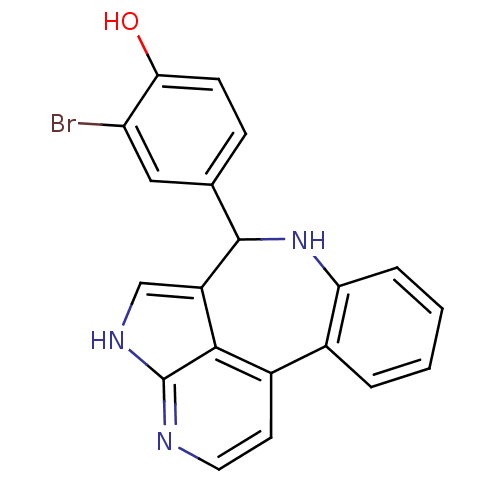
(2-bromo-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}....)Show InChI InChI=1S/C20H14BrN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50323697

((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...)Show SMILES CCNC1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C9H16N2O4S/c1-2-10-9-11-5-7(14)6(13)4(3-12)15-8(5)16-9/h4-8,12-14H,2-3H2,1H3,(H,10,11)/t4-,5-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA expressed in Escherichia coli assessed as inhibitory constant using 4-MUGlcNAc as substrate incubated for 20 mins... |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289844
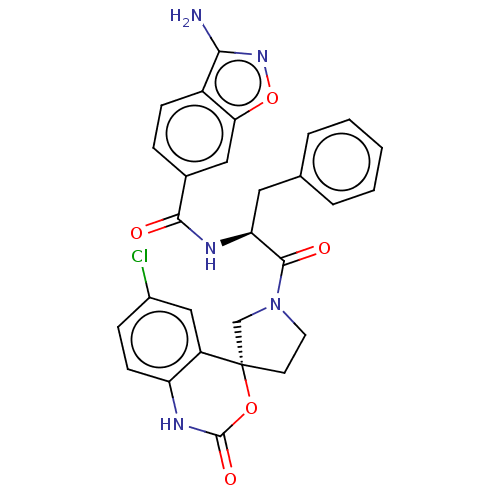
(3-amino-N—((S)-1-(R)-6-chloro-2-oxo-1,2-dihyd...)Show SMILES Nc1noc2cc(ccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC[C@@]2(C1)OC(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C28H24ClN5O5/c29-18-7-9-21-20(14-18)28(38-27(37)32-21)10-11-34(15-28)26(36)22(12-16-4-2-1-3-5-16)31-25(35)17-6-8-19-23(13-17)39-33-24(19)30/h1-9,13-14,22H,10-12,15H2,(H2,30,33)(H,31,35)(H,32,37)/t22-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM205425

(US9243020, 20 | US9815861, Example 20)Show SMILES CN(C)C1=N[C@H]2[C@H](O[C@H](C(F)F)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C9H14F2N2O3S/c1-13(2)9-12-3-4(14)5(15)6(7(10)11)16-8(3)17-9/h3-8,14-15H,1-2H3/t3-,4-,5+,6+,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289807

(US10093683, Example 118A | US10093683, Example 118...)Show SMILES CO[C@@H](C)C[C@H](NC(=O)c1ccc(NC(=O)OC)cc1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C27H30ClFN4O7/c1-15(38-2)13-20(31-23(34)16-5-7-17(8-6-16)30-25(36)39-3)24(35)33-12-4-11-27(14-33)21-19(32-26(37)40-27)10-9-18(28)22(21)29/h5-10,15,20H,4,11-14H2,1-3H3,(H,30,36)(H,31,34)(H,32,37)/t15-,20-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.52 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM141965
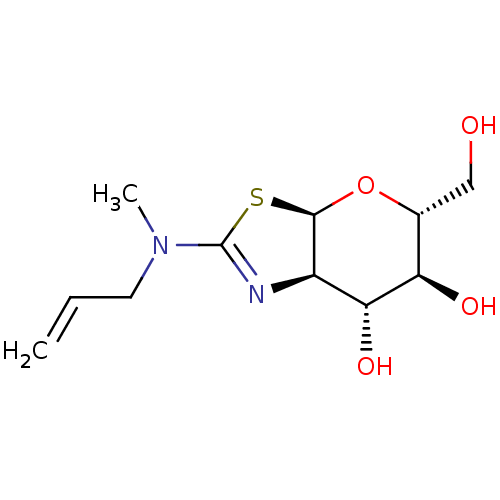
(US8927507, 16)Show SMILES CN(CC=C)C1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)S1 |r,t:5| Show InChI InChI=1S/C11H18N2O4S/c1-3-4-13(2)11-12-7-9(16)8(15)6(5-14)17-10(7)18-11/h3,6-10,14-16H,1,4-5H2,2H3/t6-,7-,8-,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289795

(Methyl 2-amino-3-(5-fluoropyridin-2-yl)propanoate ...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)NC(Cc1ccc(F)cn1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C29H26ClF2N5O6/c1-42-27(40)34-18-6-3-16(4-7-18)25(38)35-22(13-19-8-5-17(31)14-33-19)26(39)37-12-2-11-29(15-37)23-21(36-28(41)43-29)10-9-20(30)24(23)32/h3-10,14,22H,2,11-13,15H2,1H3,(H,34,40)(H,35,38)(H,36,41)/t22?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.82 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM205422
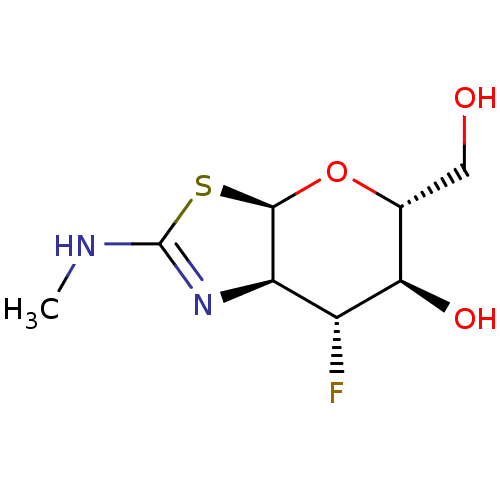
(US9243020, 9 | US9815861, Example 9)Show SMILES CNC1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2F)S1 |r,t:2| Show InChI InChI=1S/C8H13FN2O3S/c1-10-8-11-5-4(9)6(13)3(2-12)14-7(5)15-8/h3-7,12-13H,2H2,1H3,(H,10,11)/t3-,4-,5-,6-,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305154
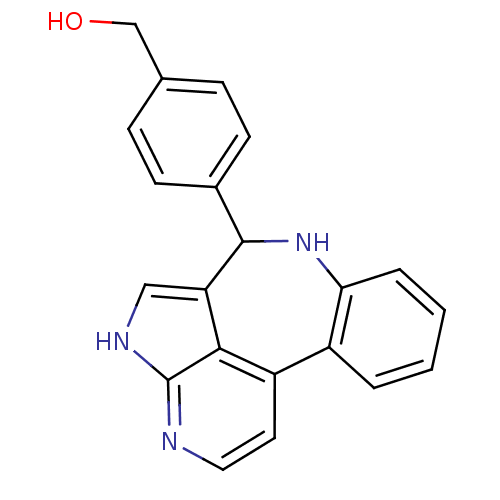
((4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,1...)Show InChI InChI=1S/C21H17N3O/c25-12-13-5-7-14(8-6-13)20-17-11-23-21-19(17)16(9-10-22-21)15-3-1-2-4-18(15)24-20/h1-11,20,24-25H,12H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289853
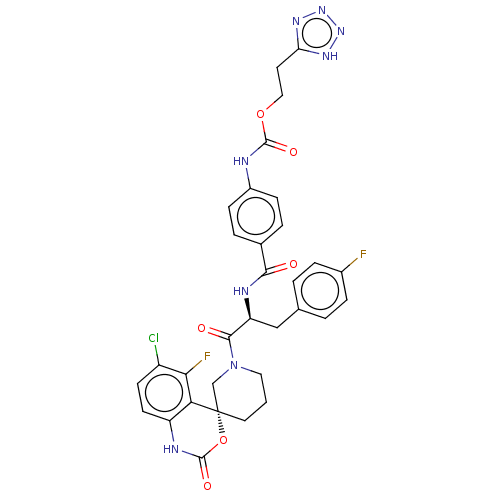
(2-(1H-tetrazol-5-yl)ethyl (4-(((S)-1-((R)-6-chloro...)Show SMILES Fc1ccc(C[C@H](NC(=O)c2ccc(NC(=O)OCCc3nnn[nH]3)cc2)C(=O)N2CCC[C@@]3(C2)OC(=O)Nc2ccc(Cl)c(F)c32)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.22 | -48.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513947
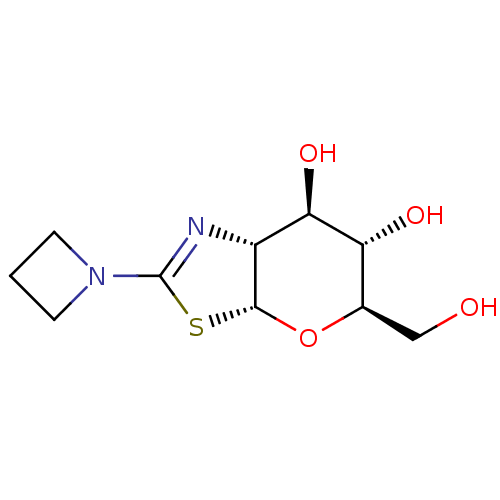
(CHEMBL4545886)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N1CCC1 |r,c:13| Show InChI InChI=1S/C10H16N2O4S/c13-4-5-7(14)8(15)6-9(16-5)17-10(11-6)12-2-1-3-12/h5-9,13-15H,1-4H2/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220180
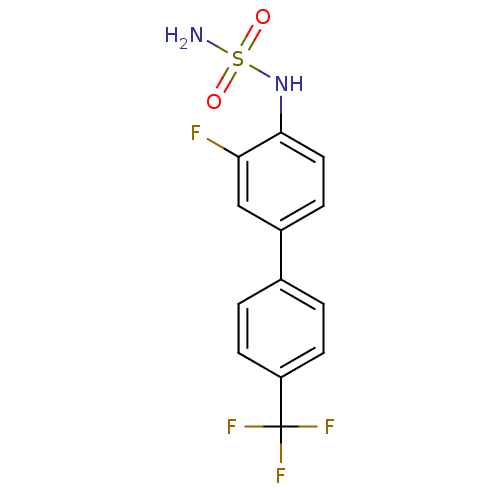
(CHEMBL243669 | N-[3-fluoro-4'-(trifluoromethyl)-4-...)Show InChI InChI=1S/C13H10F4N2O2S/c14-11-7-9(3-6-12(11)19-22(18,20)21)8-1-4-10(5-2-8)13(15,16)17/h1-7,19H,(H2,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305152
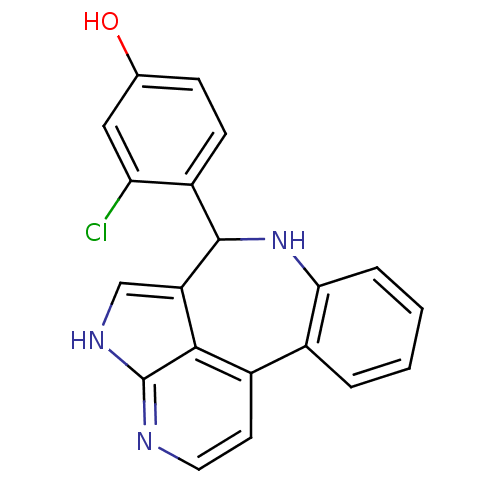
(3-chloro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(Cl)c1 Show InChI InChI=1S/C20H14ClN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289805
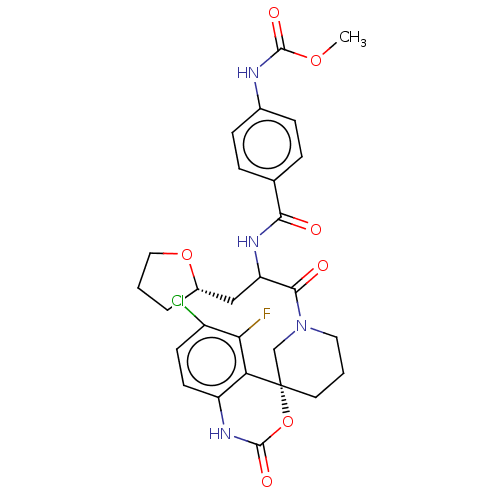
(US10093683, Example 116A | US10093683, Example 116...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)NC(C[C@@H]1CCCO1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H30ClFN4O7/c1-39-26(37)31-17-7-5-16(6-8-17)24(35)32-21(14-18-4-2-13-40-18)25(36)34-12-3-11-28(15-34)22-20(33-27(38)41-28)10-9-19(29)23(22)30/h5-10,18,21H,2-4,11-15H2,1H3,(H,31,37)(H,32,35)(H,33,38)/t18-,21?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.21 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513960
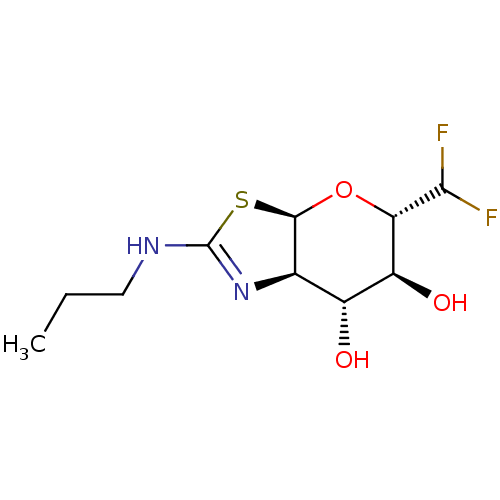
(CHEMBL4561925)Show SMILES [H][C@]12O[C@H](C(F)F)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NCCC)S2 |r,t:14| Show InChI InChI=1S/C10H16F2N2O3S/c1-2-3-13-10-14-4-5(15)6(16)7(8(11)12)17-9(4)18-10/h4-9,15-16H,2-3H2,1H3,(H,13,14)/t4-,5-,6+,7+,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513955
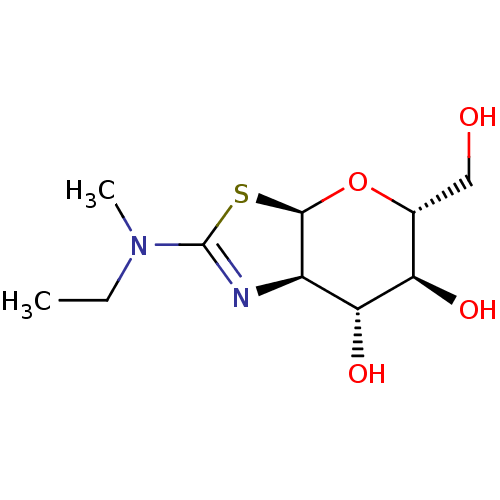
(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513957
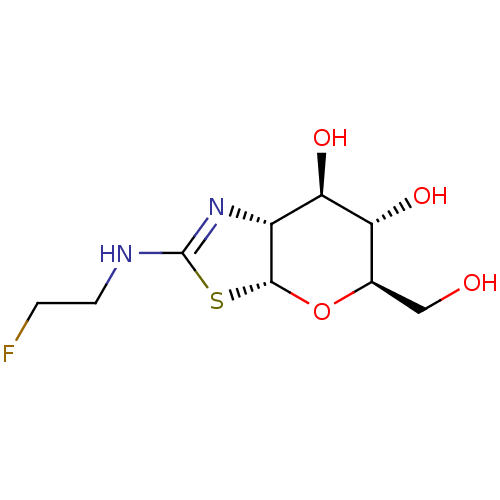
(CHEMBL4456515)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NCCF)S2 |r,t:13| Show InChI InChI=1S/C9H15FN2O4S/c10-1-2-11-9-12-5-7(15)6(14)4(3-13)16-8(5)17-9/h4-8,13-15H,1-3H2,(H,11,12)/t4-,5-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50220182
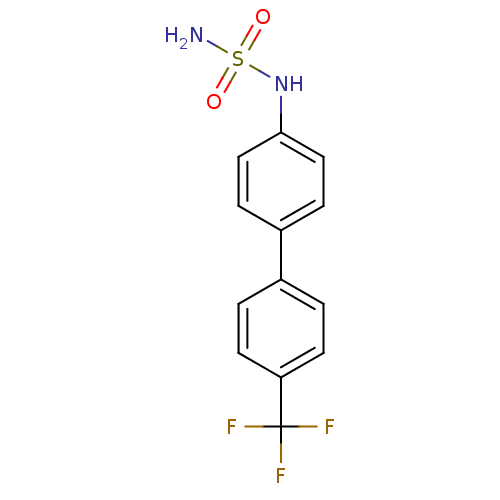
(CHEMBL390629 | N-[4'-(trifluoromethyl)-4-biphenyly...)Show InChI InChI=1S/C13H11F3N2O2S/c14-13(15,16)11-5-1-9(2-6-11)10-3-7-12(8-4-10)18-21(17,19)20/h1-8,18H,(H2,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human KSP motor domain by ATPase assay |
J Med Chem 50: 4939-52 (2007)
Article DOI: 10.1021/jm070435y
BindingDB Entry DOI: 10.7270/Q29023H4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50378535
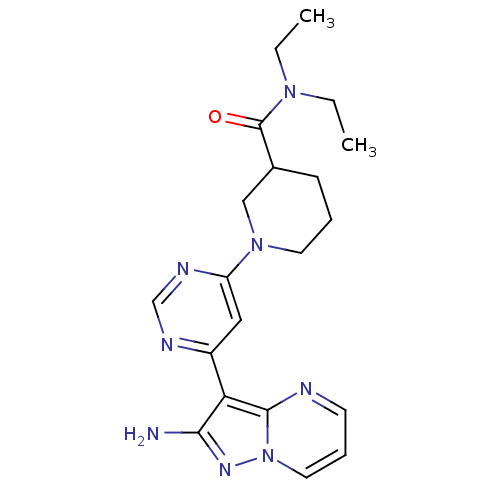
(CHEMBL1204012)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H26N8O/c1-3-26(4-2)20(29)14-7-5-9-27(12-14)16-11-15(23-13-24-16)17-18(21)25-28-10-6-8-22-19(17)28/h6,8,10-11,13-14H,3-5,7,9,12H2,1-2H3,(H2,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289805

(US10093683, Example 116A | US10093683, Example 116...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)NC(C[C@@H]1CCCO1)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H30ClFN4O7/c1-39-26(37)31-17-7-5-16(6-8-17)24(35)32-21(14-18-4-2-13-40-18)25(36)34-12-3-11-28(15-34)22-20(33-27(38)41-28)10-9-19(29)23(22)30/h5-10,18,21H,2-4,11-15H2,1H3,(H,31,37)(H,32,35)(H,33,38)/t18-,21?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 7.51 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM205424
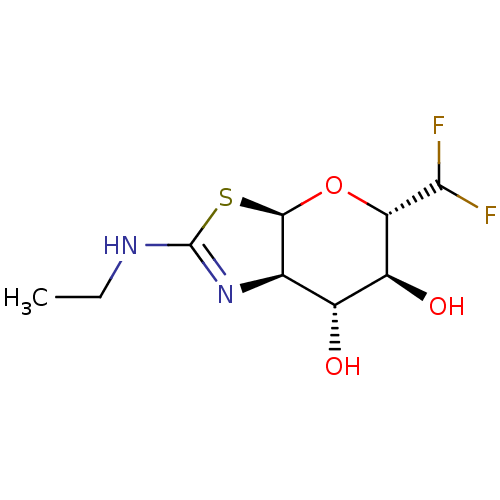
(US9243020, 19 | US9815861, Example 19)Show SMILES CCNC1=N[C@H]2[C@H](O[C@H](C(F)F)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C9H14F2N2O3S/c1-2-12-9-13-3-4(14)5(15)6(7(10)11)16-8(3)17-9/h3-8,14-15H,2H2,1H3,(H,12,13)/t3-,4-,5+,6+,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513958
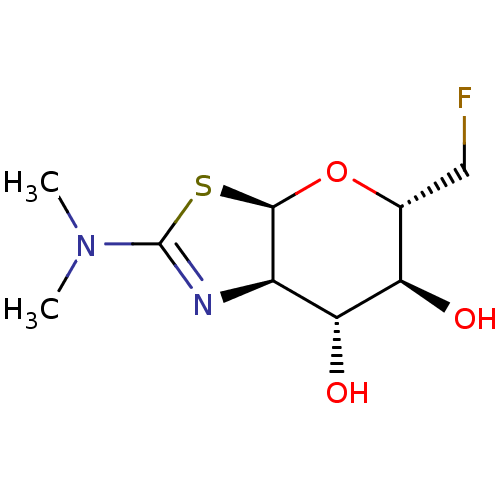
(CHEMBL4473252)Show SMILES [H][C@]12O[C@H](CF)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)C |r,c:13| Show InChI InChI=1S/C9H15FN2O3S/c1-12(2)9-11-5-7(14)6(13)4(3-10)15-8(5)16-9/h4-8,13-14H,3H2,1-2H3/t4-,5-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289849
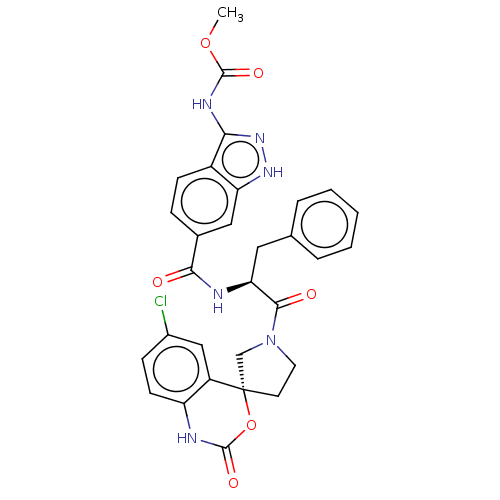
(Methyl 3-amino-6-(((S)-1-((R)-6-chloro-2-oxo-1,2-d...)Show SMILES COC(=O)Nc1n[nH]c2cc(ccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC[C@@]2(C1)OC(=O)Nc1ccc(Cl)cc21 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513939
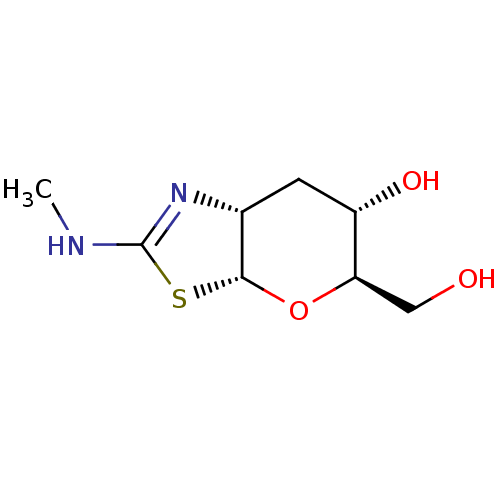
(CHEMBL4516713)Show SMILES [H][C@@]12C[C@H](O)[C@@H](CO)O[C@]1([H])SC(NC)=N2 |r,c:15| Show InChI InChI=1S/C8H14N2O3S/c1-9-8-10-4-2-5(12)6(3-11)13-7(4)14-8/h4-7,11-12H,2-3H2,1H3,(H,9,10)/t4-,5+,6-,7-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289787

(US10093683, Example 14b)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CC[C@@]2(C1)OC(=O)Nc1ccc(C)cc21 |r| Show InChI InChI=1S/C30H30N4O6/c1-19-8-13-24-23(16-19)30(40-29(38)33-24)14-15-34(18-30)27(36)25(17-20-6-4-3-5-7-20)32-26(35)21-9-11-22(12-10-21)31-28(37)39-2/h3-13,16,25H,14-15,17-18H2,1-2H3,(H,31,37)(H,32,35)(H,33,38)/t25-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50079596
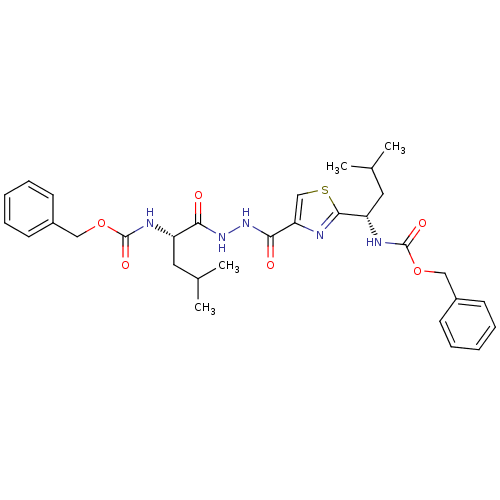
(((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)c1csc(n1)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H39N5O6S/c1-20(2)15-24(33-30(39)41-17-22-11-7-5-8-12-22)27(37)35-36-28(38)26-19-43-29(32-26)25(16-21(3)4)34-31(40)42-18-23-13-9-6-10-14-23/h5-14,19-21,24-25H,15-18H2,1-4H3,(H,33,39)(H,34,40)(H,35,37)(H,36,38)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human osteoclast cathepsin K |
Bioorg Med Chem Lett 9: 1907-10 (1999)
BindingDB Entry DOI: 10.7270/Q2M61JGJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289811
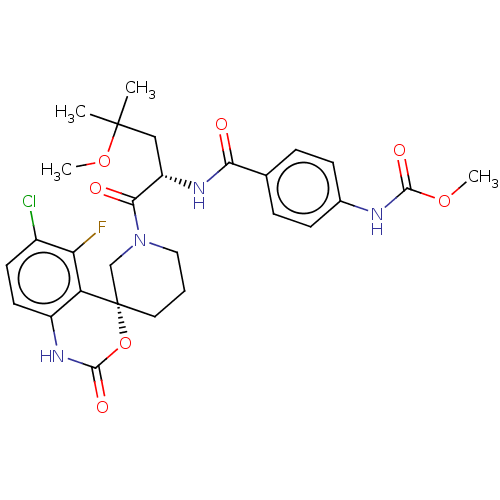
(Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)N[C@@H](CC(C)(C)OC)C(=O)N1CCC[C@@]2(C1)OC(=O)Nc1ccc(Cl)c(F)c21 |r| Show InChI InChI=1S/C28H32ClFN4O7/c1-27(2,40-4)14-20(32-23(35)16-6-8-17(9-7-16)31-25(37)39-3)24(36)34-13-5-12-28(15-34)21-19(33-26(38)41-28)11-10-18(29)22(21)30/h6-11,20H,5,12-15H2,1-4H3,(H,31,37)(H,32,35)(H,33,38)/t20-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 10.2 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM205421
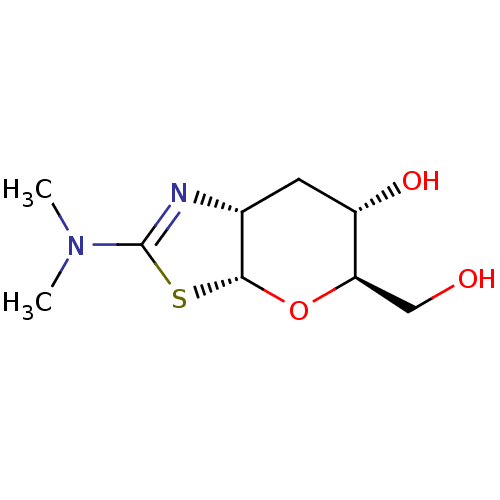
(US9243020, 4 | US9815861, Example 4)Show SMILES CN(C)C1=N[C@@H]2C[C@H](O)[C@@H](CO)O[C@@H]2S1 |r,t:3| Show InChI InChI=1S/C9H16N2O3S/c1-11(2)9-10-5-3-6(13)7(4-12)14-8(5)15-9/h5-8,12-13H,3-4H2,1-2H3/t5-,6+,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289801

(Methyl (4-(((S)-1-((R)-6-chloro-2-oxo-1,2-dihydros...)Show SMILES COC(=O)Nc1ccc(cc1)C(=O)N[C@@H](CC(C)(C)O)C(=O)N1CC[C@@]2(C1)OC(=O)Nc1ccc(Cl)cc21 |r| Show InChI InChI=1S/C26H29ClN4O7/c1-25(2,36)13-20(29-21(32)15-4-7-17(8-5-15)28-23(34)37-3)22(33)31-11-10-26(14-31)18-12-16(27)6-9-19(18)30-24(35)38-26/h4-9,12,20,36H,10-11,13-14H2,1-3H3,(H,28,34)(H,29,32)(H,30,35)/t20-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM142083

(US8933040, 7)Show SMILES OC[C@H]1O[C@@H]2SC(=N[C@@H]2[C@@H](O)[C@@H]1O)N1CCCC1 |r,c:6| Show InChI InChI=1S/C11H18N2O4S/c14-5-6-8(15)9(16)7-10(17-6)18-11(12-7)13-3-1-2-4-13/h6-10,14-16H,1-5H2/t6-,7-,8-,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305151
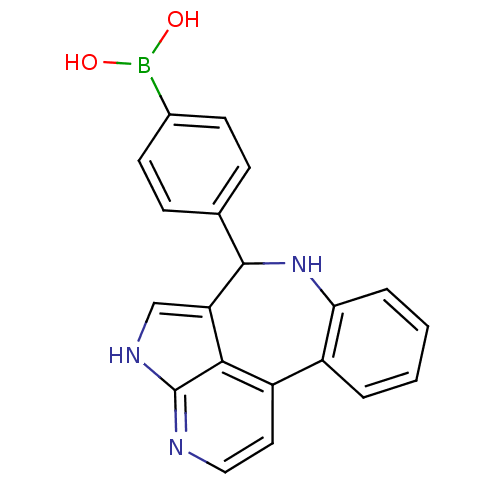
((4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,1...)Show SMILES OB(O)c1ccc(cc1)C1Nc2ccccc2-c2ccnc3[nH]cc1c23 Show InChI InChI=1S/C20H16BN3O2/c25-21(26)13-7-5-12(6-8-13)19-16-11-23-20-18(16)15(9-10-22-20)14-3-1-2-4-17(14)24-19/h1-11,19,24-26H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513940
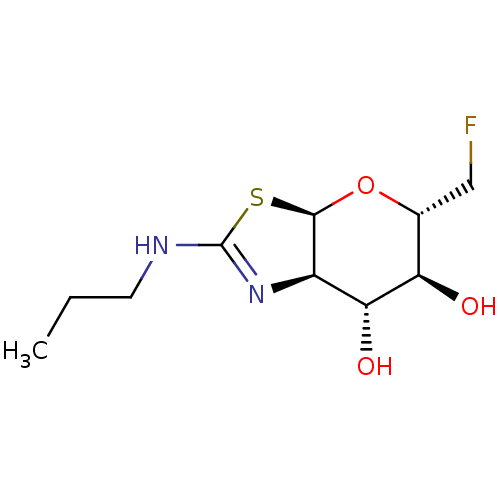
(CHEMBL4586062)Show SMILES [H][C@]12O[C@H](CF)[C@@H](O)[C@H](O)[C@@]1([H])N=C(NCCC)S2 |r,t:13| Show InChI InChI=1S/C10H17FN2O3S/c1-2-3-12-10-13-6-8(15)7(14)5(4-11)16-9(6)17-10/h5-9,14-15H,2-4H2,1H3,(H,12,13)/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50513951
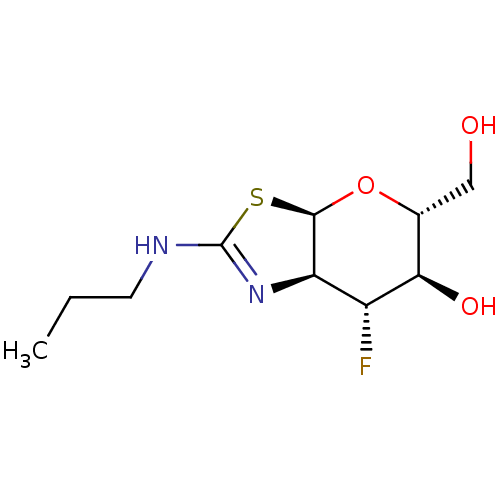
(CHEMBL4583010)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](F)[C@@]1([H])N=C(NCCC)S2 |r,t:13| Show InChI InChI=1S/C10H17FN2O3S/c1-2-3-12-10-13-7-6(11)8(15)5(4-14)16-9(7)17-10/h5-9,14-15H,2-4H2,1H3,(H,12,13)/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090
BindingDB Entry DOI: 10.7270/Q21G0QMH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM289803

(N—((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dih...)Show SMILES Fc1c(Cl)ccc2NC(=O)O[C@]3(CCCN(C3)C(=O)C(Cc3cncnc3)NC(=O)c3ccc4[nH]c(=O)ccc4c3)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 15.6 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... |
US Patent US10093683 (2018)
BindingDB Entry DOI: 10.7270/Q2RR2197 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data