 Found 3305 hits with Last Name = 'kang' and Initial = 'j'
Found 3305 hits with Last Name = 'kang' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266
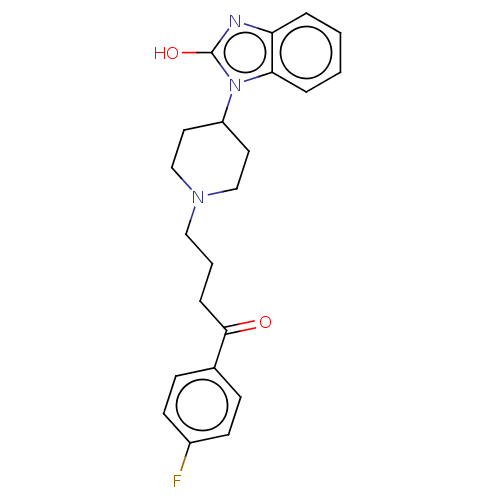
(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D4 receptor by radioligand displacement assay |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50057512
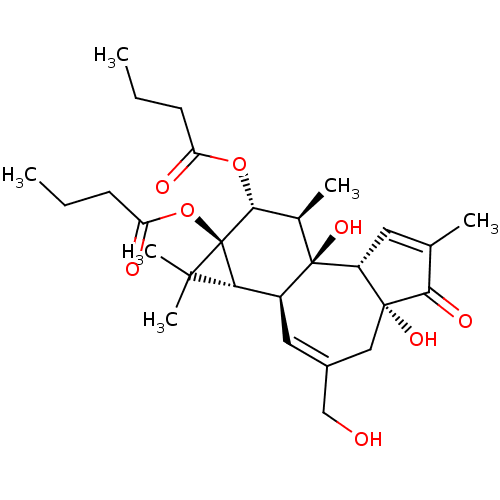
((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Binding Affinity against protein kinase C alpha |
J Med Chem 43: 921-44 (2000)
BindingDB Entry DOI: 10.7270/Q2RN372X |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IABN from human recombinant dopamine D3 receptor expressed in HEK293 cell membrane incubated for 60 mins filtration binding as... |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50232114
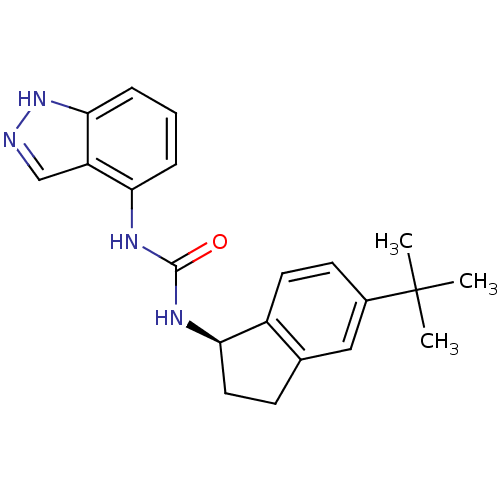
((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 Show InChI InChI=1S/C21H24N4O/c1-21(2,3)14-8-9-15-13(11-14)7-10-18(15)24-20(26)23-17-5-4-6-19-16(17)12-22-25-19/h4-6,8-9,11-12,18H,7,10H2,1-3H3,(H,22,25)(H2,23,24,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176555
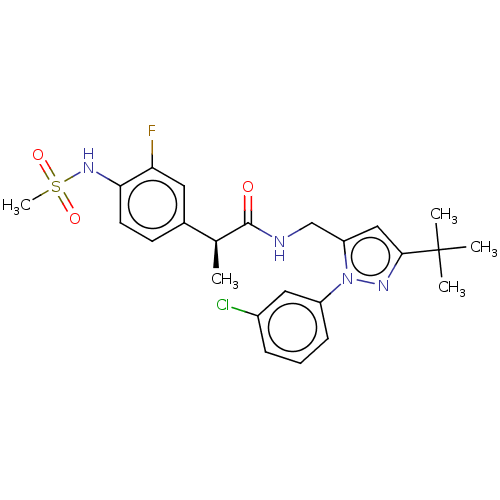
(US9120756, 17)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(C)(C)C)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C24H28ClFN4O3S/c1-15(16-9-10-21(20(26)11-16)29-34(5,32)33)23(31)27-14-19-13-22(24(2,3)4)28-30(19)18-8-6-7-17(25)12-18/h6-13,15,29H,14H2,1-5H3,(H,27,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM176564
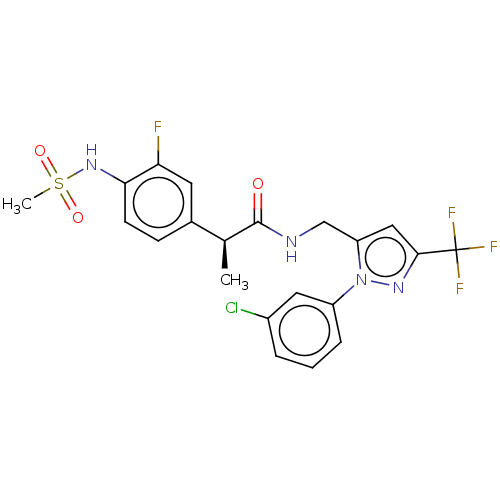
(US9120756, 26)Show SMILES C[C@H](C(=O)NCc1cc(nn1-c1cccc(Cl)c1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C21H19ClF4N4O3S/c1-12(13-6-7-18(17(23)8-13)29-34(2,32)33)20(31)27-11-16-10-19(21(24,25)26)28-30(16)15-5-3-4-14(22)9-15/h3-10,12,29H,11H2,1-2H3,(H,27,31)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50398494

(CHEMBL2177429)Show SMILES C[C@H](C(=O)NCc1ccc(nc1N1CCC(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r| Show InChI InChI=1S/C23H28F4N4O3S/c1-14-8-10-31(11-9-14)21-17(5-7-20(29-21)23(25,26)27)13-28-22(32)15(2)16-4-6-19(18(24)12-16)30-35(3,33)34/h4-7,12,14-15,30H,8-11,13H2,1-3H3,(H,28,32)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128266
BindingDB Entry DOI: 10.7270/Q2CV4NG9 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553855
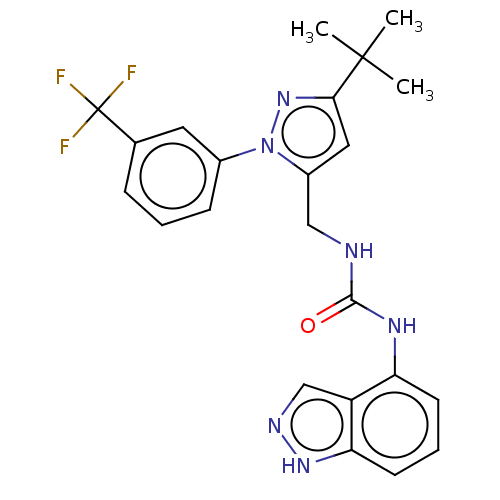
(CHEMBL4782906)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(c1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor (unknown origin) |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50057512

((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:23,t:12| Show InChI InChI=1S/C28H40O8/c1-7-9-20(30)35-24-16(4)27(34)18(22-25(5,6)28(22,24)36-21(31)10-8-2)12-17(14-29)13-26(33)19(27)11-15(3)23(26)32/h11-12,16,18-19,22,24,29,33-34H,7-10,13-14H2,1-6H3/t16-,18+,19-,22?,24-,26-,27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5-HT2B receptor expressed in cell membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 24: 3464-71 (2016)
Article DOI: 10.1016/j.bmc.2016.05.053
BindingDB Entry DOI: 10.7270/Q2X63PVP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553837

(CHEMBL4752231)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(Cl)c3)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553860

(CHEMBL4745407)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1ccc(F)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553861
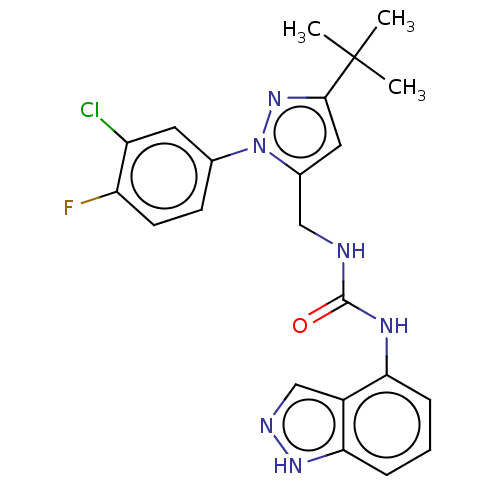
(CHEMBL4792344)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1ccc(F)c(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786
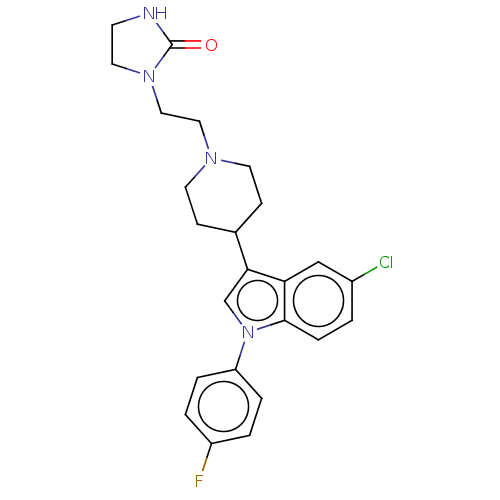
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553836
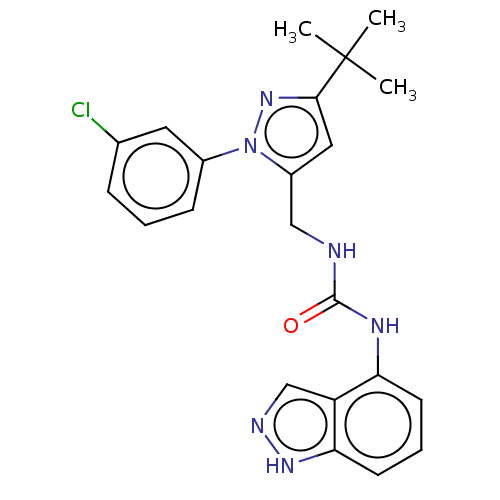
(CHEMBL4753881)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553862
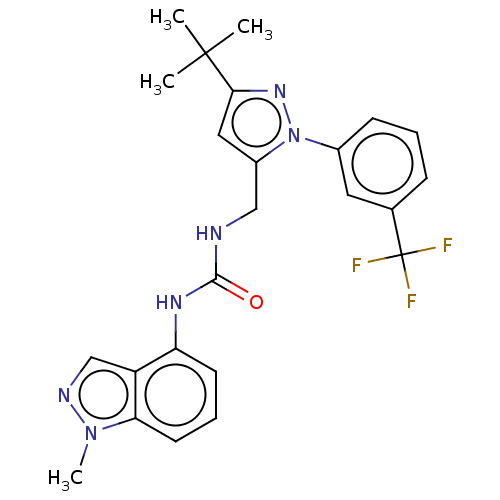
(CHEMBL4756342)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(c3)C(F)(F)F)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553868

(CHEMBL4763969)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3ccc(F)c(Cl)c3)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553843
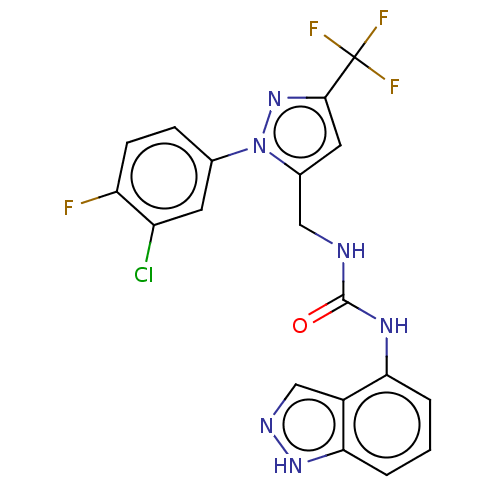
(CHEMBL4763915)Show SMILES Fc1ccc(cc1Cl)-n1nc(cc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507
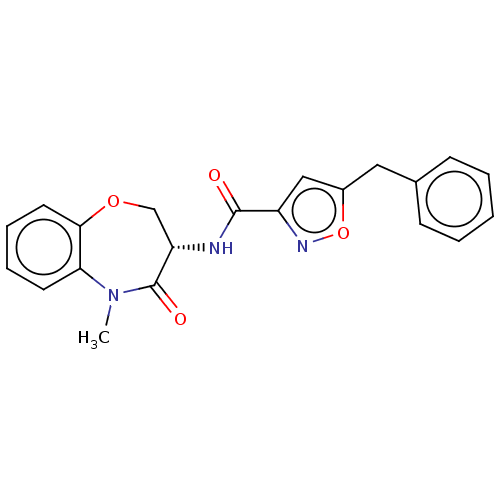
(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C delta type
(Mus musculus) | BDBM50216859

(CHEMBL442232 | {2-(hydroxymethyl)-5-[5-methyl-3-(2...)Show SMILES CC(C)CC(C\C=C1\OC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C |w:9.11| Show InChI InChI=1S/C21H36O6/c1-14(2)10-16(11-15(3)4)8-9-17-18(23)27-21(12-22,26-17)13-25-19(24)20(5,6)7/h9,14-16,22H,8,10-13H2,1-7H3/b17-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553833
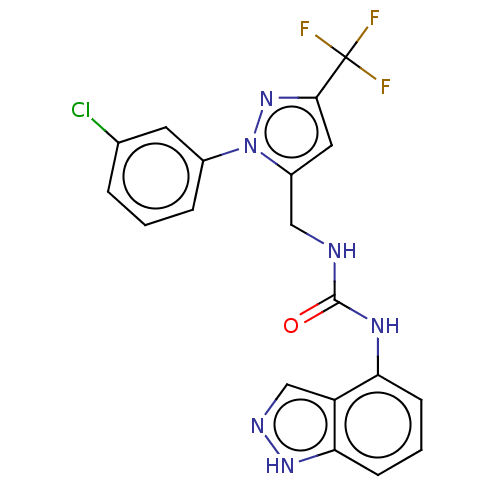
(CHEMBL4752959)Show SMILES FC(F)(F)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50107120
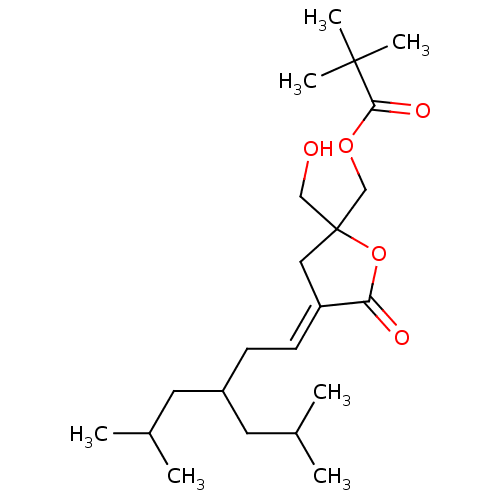
((E) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...)Show SMILES CC(C)CC(C\C=C1/CC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of [3H]PDBu binding to C1b domain of protein kinase C delta with phosphatidylserine |
J Med Chem 48: 5738-48 (2005)
Article DOI: 10.1021/jm050352m
BindingDB Entry DOI: 10.7270/Q27M07G5 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216857
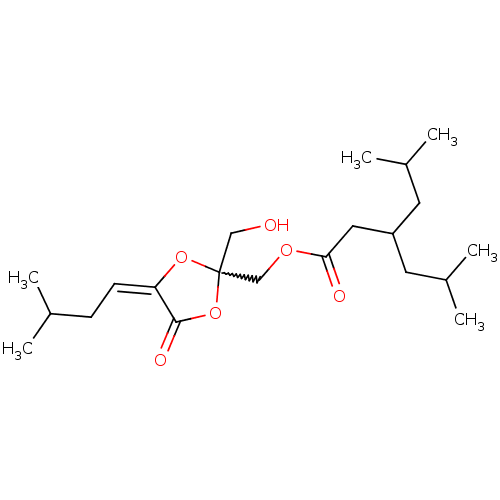
((2-(hydroxymethyl)-4-(3-methylbutylidene)-5-oxo-te...)Show SMILES CC(C)C\C=C1\OC(CO)(COC(=O)CC(CC(C)C)CC(C)C)OC1=O |w:7.9| Show InChI InChI=1S/C21H36O6/c1-14(2)7-8-18-20(24)27-21(12-22,26-18)13-25-19(23)11-17(9-15(3)4)10-16(5)6/h8,14-17,22H,7,9-13H2,1-6H3/b18-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216856
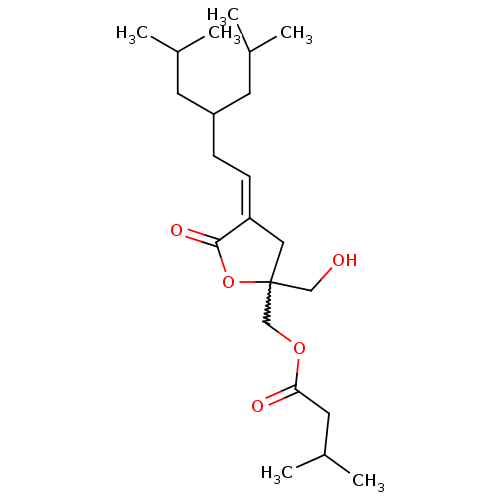
(CHEMBL231617 | Z-{2-(Hydroxymethyl)-4-[5-methyl-3-...)Show SMILES CC(C)CC(C\C=C1\CC(CO)(COC(=O)CC(C)C)OC1=O)CC(C)C |w:9.11| Show InChI InChI=1S/C22H38O5/c1-15(2)9-18(10-16(3)4)7-8-19-12-22(13-23,27-21(19)25)14-26-20(24)11-17(5)6/h8,15-18,23H,7,9-14H2,1-6H3/b19-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553867
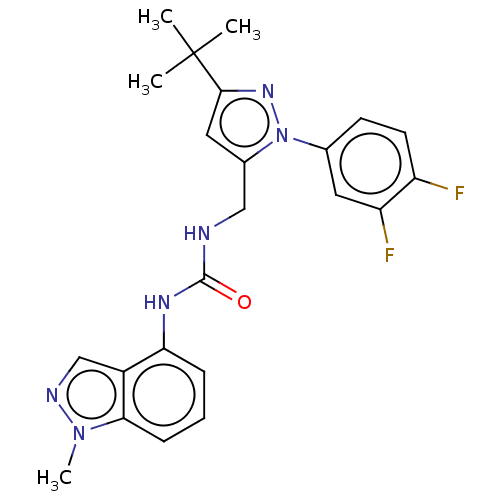
(CHEMBL4790987)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3ccc(F)c(F)c3)C(C)(C)C)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50107112
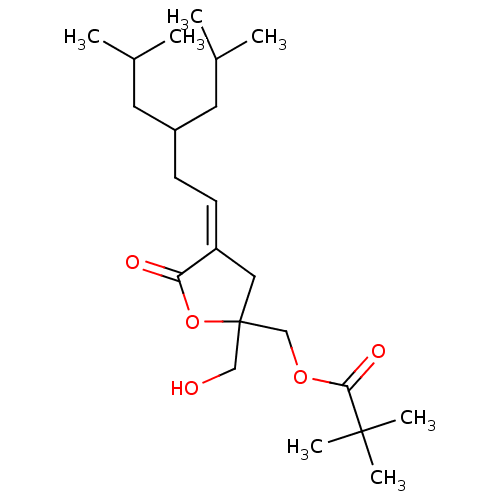
((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...)Show SMILES CC(C)CC(C\C=C1\CC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50107112

((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...)Show SMILES CC(C)CC(C\C=C1\CC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PDBu binding to C1b domain of protein kinase C delta |
J Med Chem 48: 5738-48 (2005)
Article DOI: 10.1021/jm050352m
BindingDB Entry DOI: 10.7270/Q27M07G5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50183266

(Anquil | Benperidol | Benquil | MCN-JR-4584 | R-45...)Show SMILES Oc1nc2ccccc2n1C1CCN(CCCC(=O)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C22H24FN3O2/c23-17-9-7-16(8-10-17)21(27)6-3-13-25-14-11-18(12-15-25)26-20-5-2-1-4-19(20)24-22(26)28/h1-2,4-5,7-10,18H,3,6,11-15H2,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50107112

((Z) 2,2-Dimethyl-propionic acid 2-hydroxymethyl-4-...)Show SMILES CC(C)CC(C\C=C1\CC(CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Binding affinity to mouse wildtype PKCdelta C1a expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
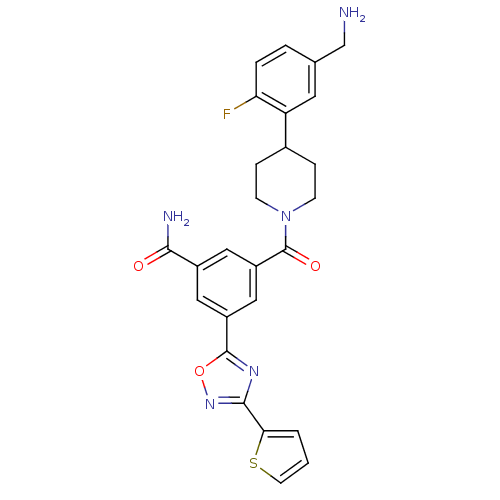
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553854

(CHEMBL4798584)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553839
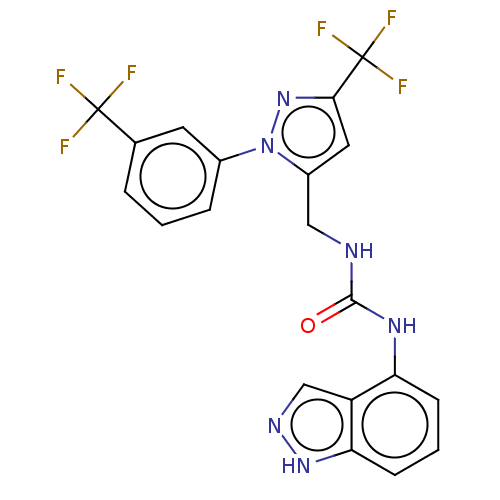
(CHEMBL4778103)Show SMILES FC(F)(F)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1cccc(c1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553846

(CHEMBL4740674)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(c3)C(F)(F)F)C(F)(F)F)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D4 receptor (unknown origin) |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50187521

((E)-{2-(hydroxymethyl)-4-[(4-hydroxyphenyl)methyle...)Show SMILES CC(C)C(CC(=O)OCC1(CO)C\C(=C/c2ccc(O)cc2)C(=O)O1)C(C)C Show InChI InChI=1S/C22H30O6/c1-14(2)19(15(3)4)10-20(25)27-13-22(12-23)11-17(21(26)28-22)9-16-5-7-18(24)8-6-16/h5-9,14-15,19,23-24H,10-13H2,1-4H3/b17-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Binding affinity to PKCdeltaC1b in presence of phospholipid |
J Med Chem 49: 3185-203 (2006)
Article DOI: 10.1021/jm060011o
BindingDB Entry DOI: 10.7270/Q2Z89C1G |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50183070

(CHEMBL3818478)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CCCCc2c[nH]c3ccccc23)CC1 Show InChI InChI=1S/C25H30N4O/c30-24-25(29(19-27-24)21-9-2-1-3-10-21)13-16-28(17-14-25)15-7-6-8-20-18-26-23-12-5-4-11-22(20)23/h1-5,9-12,18,26H,6-8,13-17,19H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human recombinant dopamine D4 receptor incubated for 1.5 hrs by microbeta scintillation counting method |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50184391
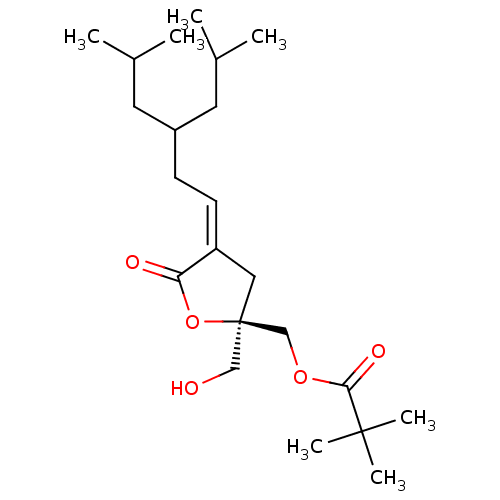
((R,Z)-2-(2-(hydroxymethyl)-4-(3-isobutyl-5-methylh...)Show SMILES CC(C)CC(C\C=C1\C[C@@](CO)(COC(=O)C(C)(C)C)OC1=O)CC(C)C Show InChI InChI=1S/C22H38O5/c1-15(2)10-17(11-16(3)4)8-9-18-12-22(13-23,27-19(18)24)14-26-20(25)21(5,6)7/h9,15-17,23H,8,10-14H2,1-7H3/b18-9-/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Institute of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [20-3H]PDBu from recombinant PKC alpha in presence of phosphatidylserine |
J Med Chem 49: 2028-36 (2006)
Article DOI: 10.1021/jm0509391
BindingDB Entry DOI: 10.7270/Q27D2TQ1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
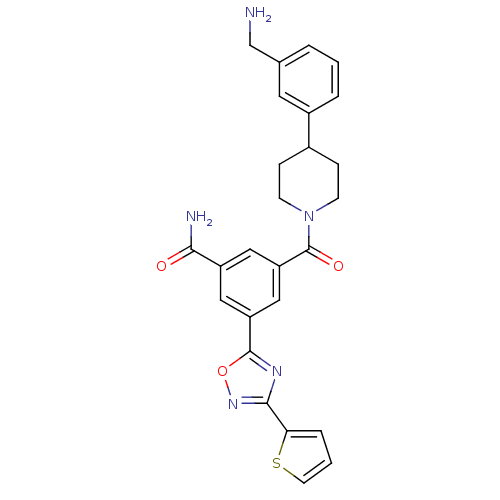
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50183070

(CHEMBL3818478)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CCCCc2c[nH]c3ccccc23)CC1 Show InChI InChI=1S/C25H30N4O/c30-24-25(29(19-27-24)21-9-2-1-3-10-21)13-16-28(17-14-25)15-7-6-8-20-18-26-23-12-5-4-11-22(20)23/h1-5,9-12,18,26H,6-8,13-17,19H2,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from human recombinant dopamine D4 receptor incubated for 1.5 hrs by microbeta scintillation counting method |
Bioorg Med Chem 24: 3671-9 (2016)
Article DOI: 10.1016/j.bmc.2016.06.011
BindingDB Entry DOI: 10.7270/Q2W66NPV |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553852

(CHEMBL4764786)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3ccc(F)c(Cl)c3)C(F)(F)F)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50216860
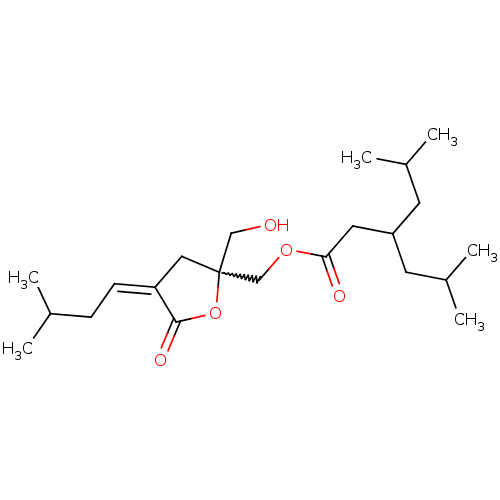
(CHEMBL230048 | Z-[2-(Hydroxymethyl)-4-(3-methylbut...)Show SMILES CC(C)C\C=C1\CC(CO)(COC(=O)CC(CC(C)C)CC(C)C)OC1=O |w:7.9| Show InChI InChI=1S/C22H38O5/c1-15(2)7-8-19-12-22(13-23,27-21(19)25)14-26-20(24)11-18(9-16(3)4)10-17(5)6/h8,15-18,23H,7,9-14H2,1-6H3/b19-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-Frederick
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from mouse wild type C1b domain of PKCdelta expressed in Escherichia coli |
J Med Chem 50: 3465-81 (2007)
Article DOI: 10.1021/jm0702579
BindingDB Entry DOI: 10.7270/Q2RF5TQ5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553856

(CHEMBL4762740)Show SMILES CC(C)(C)c1cc(CNC(=O)Nc2cccc3[nH]ncc23)n(n1)-c1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50182747
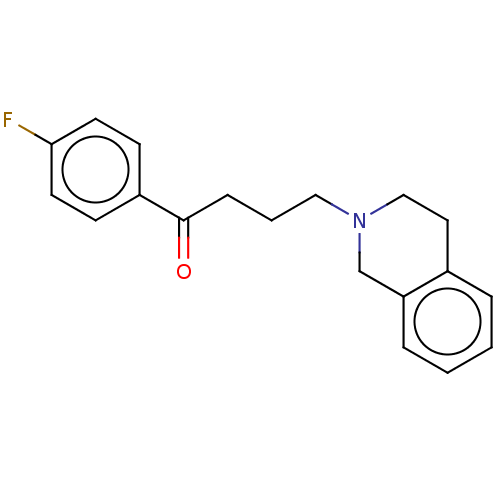
(CHEMBL3818994)Show InChI InChI=1S/C19H20FNO/c20-18-9-7-16(8-10-18)19(22)6-3-12-21-13-11-15-4-1-2-5-17(15)14-21/h1-2,4-5,7-10H,3,6,11-14H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida A&M University
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from rat D4 receptor expressed in cell membranes after 1 hr by liquid scintillation counting |
Bioorg Med Chem 24: 3464-71 (2016)
Article DOI: 10.1016/j.bmc.2016.05.053
BindingDB Entry DOI: 10.7270/Q2X63PVP |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50553834

(CHEMBL4752981)Show SMILES Cn1ncc2c(NC(=O)NCc3cc(nn3-c3cccc(Cl)c3)C(F)(F)F)cccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127548
BindingDB Entry DOI: 10.7270/Q2VD733R |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50085889
(CHEMBL111625 | Decanoic acid 2-hydroxymethyl-4-(3-...)Show SMILES CCCCCCCCCC(=O)OCC1(CO)C\C(=C\CC(C(C)C)C(C)C)C(=O)O1 Show InChI InChI=1S/C25H44O5/c1-6-7-8-9-10-11-12-13-23(27)29-18-25(17-26)16-21(24(28)30-25)14-15-22(19(2)3)20(4)5/h14,19-20,22,26H,6-13,15-18H2,1-5H3/b21-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Binding Affinity against protein kinase C alpha |
J Med Chem 43: 921-44 (2000)
BindingDB Entry DOI: 10.7270/Q2RN372X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data