 Found 3578 hits with Last Name = 'lamb' and Initial = 'j'
Found 3578 hits with Last Name = 'lamb' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142111
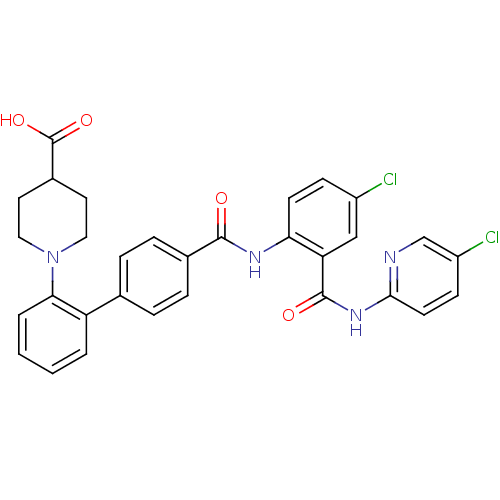
(1-{4'-[4-Chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H26Cl2N4O4/c32-22-9-11-26(25(17-22)30(39)36-28-12-10-23(33)18-34-28)35-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)37-15-13-21(14-16-37)31(40)41/h1-12,17-18,21H,13-16H2,(H,35,38)(H,40,41)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
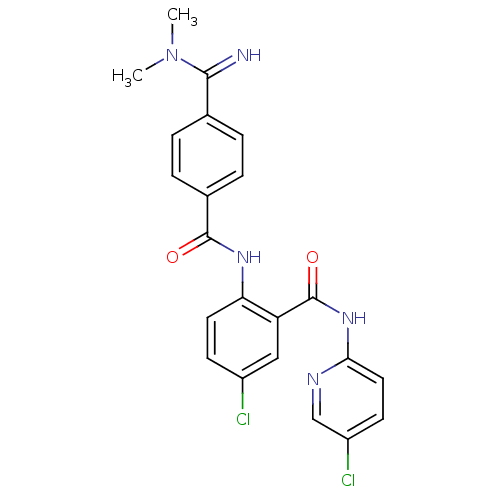
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
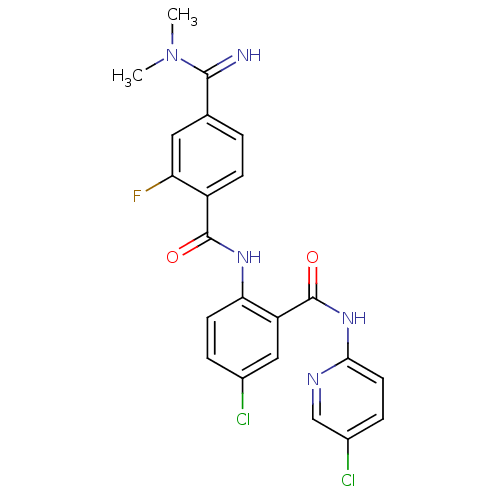
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D3 receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142112
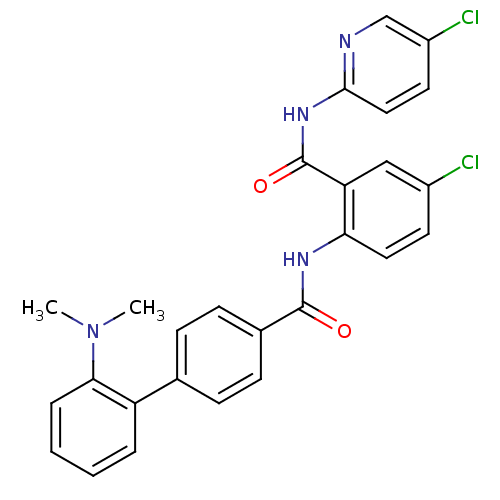
(2'-Dimethylamino-biphenyl-4-carboxylic acid [4-chl...)Show SMILES CN(C)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N4O2/c1-33(2)24-6-4-3-5-21(24)17-7-9-18(10-8-17)26(34)31-23-13-11-19(28)15-22(23)27(35)32-25-14-12-20(29)16-30-25/h3-16H,1-2H3,(H,31,34)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142125
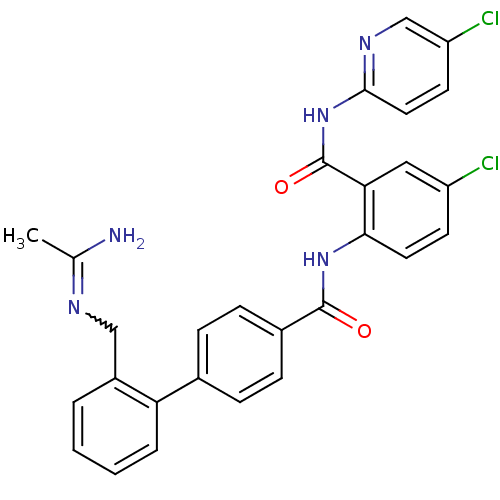
(2'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(N)=NCc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |w:3.3| Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-20-4-2-3-5-23(20)18-6-8-19(9-7-18)27(36)34-25-12-10-21(29)14-24(25)28(37)35-26-13-11-22(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142139
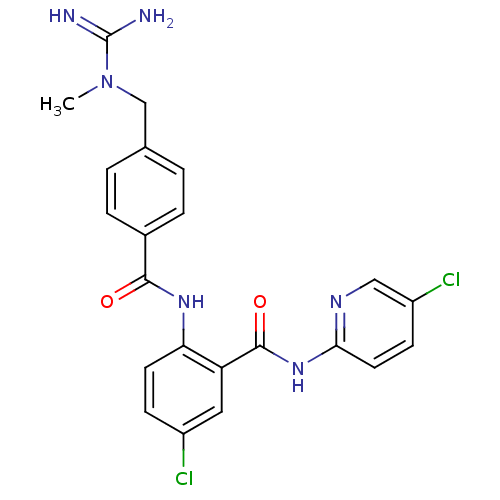
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-{4-[(N-methyl...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(N)=N Show InChI InChI=1S/C22H20Cl2N6O2/c1-30(22(25)26)12-13-2-4-14(5-3-13)20(31)28-18-8-6-15(23)10-17(18)21(32)29-19-9-7-16(24)11-27-19/h2-11H,12H2,1H3,(H3,25,26)(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142090
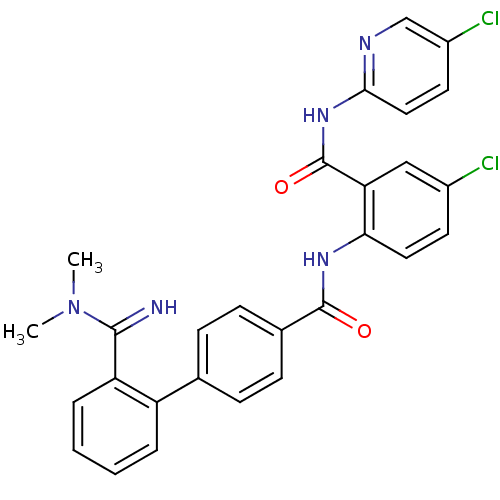
(2'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)22-6-4-3-5-21(22)17-7-9-18(10-8-17)27(36)33-24-13-11-19(29)15-23(24)28(37)34-25-14-12-20(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
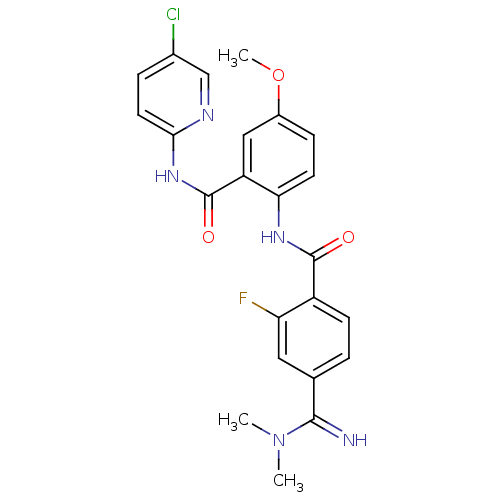
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
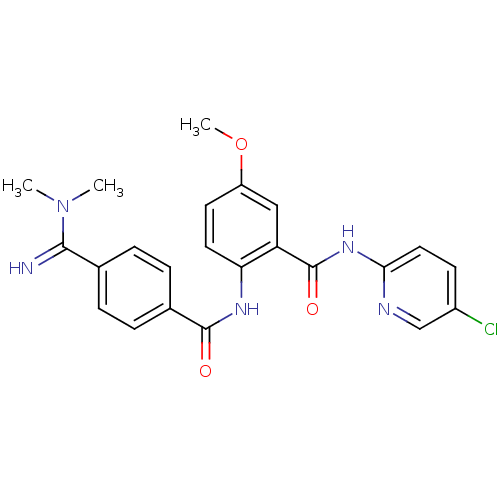
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142118
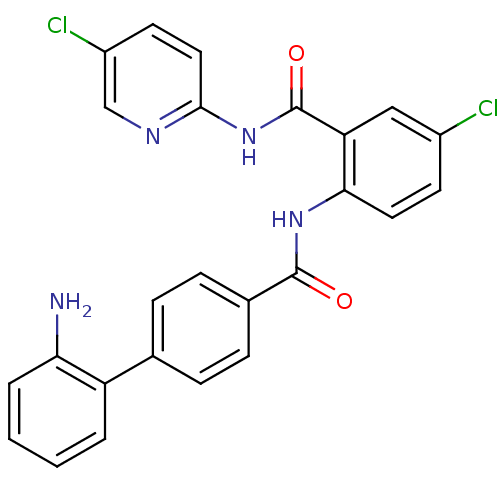
(2'-Amino-biphenyl-4-carboxylic acid [4-chloro-2-(5...)Show SMILES Nc1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C25H18Cl2N4O2/c26-17-9-11-22(20(13-17)25(33)31-23-12-10-18(27)14-29-23)30-24(32)16-7-5-15(6-8-16)19-3-1-2-4-21(19)28/h1-14H,28H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142169
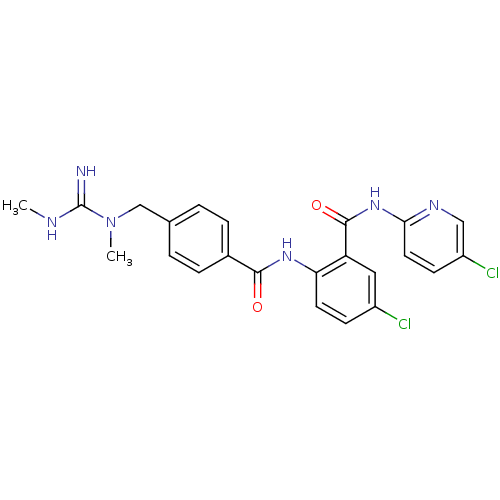
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N'-dime...)Show SMILES CNC(=N)N(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N6O2/c1-27-23(26)31(2)13-14-3-5-15(6-4-14)21(32)29-19-9-7-16(24)11-18(19)22(33)30-20-10-8-17(25)12-28-20/h3-12H,13H2,1-2H3,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142129
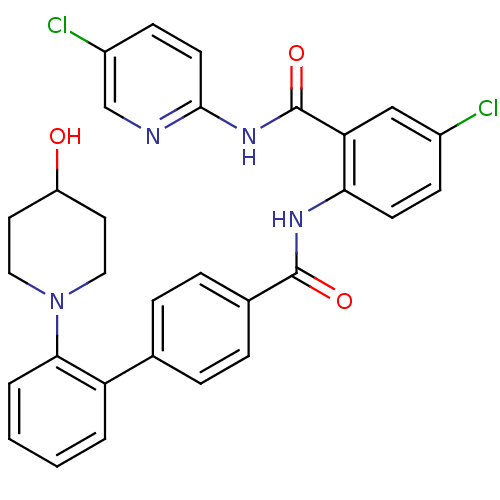
(2'-(4-Hydroxy-piperidin-1-yl)-biphenyl-4-carboxyli...)Show SMILES OC1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H26Cl2N4O3/c31-21-9-11-26(25(17-21)30(39)35-28-12-10-22(32)18-33-28)34-29(38)20-7-5-19(6-8-20)24-3-1-2-4-27(24)36-15-13-23(37)14-16-36/h1-12,17-18,23,37H,13-16H2,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142161
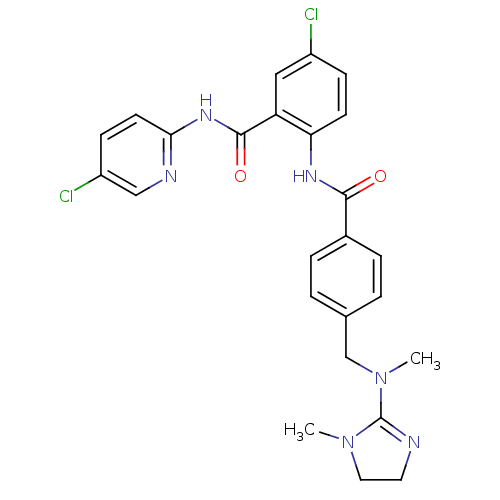
(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-{[methyl-(...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C1=NCCN1C |t:32| Show InChI InChI=1S/C25H24Cl2N6O2/c1-32-12-11-28-25(32)33(2)15-16-3-5-17(6-4-16)23(34)30-21-9-7-18(26)13-20(21)24(35)31-22-10-8-19(27)14-29-22/h3-10,13-14H,11-12,15H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142126
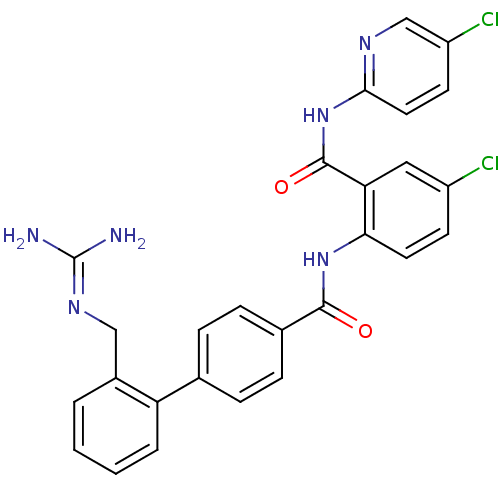
(2'-Guanidinomethyl-biphenyl-4-carboxylic acid [4-c...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1ccccc1-c1ccc(cc1)-[#6](=O)-[#7]-c1ccc(Cl)cc1-[#6](=O)-[#7]-c1ccc(Cl)cn1 Show InChI InChI=1S/C27H22Cl2N6O2/c28-19-9-11-23(22(13-19)26(37)35-24-12-10-20(29)15-32-24)34-25(36)17-7-5-16(6-8-17)21-4-2-1-3-18(21)14-33-27(30)31/h1-13,15H,14H2,(H,34,36)(H4,30,31,33)(H,32,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142142

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N',N'-dim...)Show SMILES CN(C)NCc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H21Cl2N5O2/c1-29(2)26-12-14-3-5-15(6-4-14)21(30)27-19-9-7-16(23)11-18(19)22(31)28-20-10-8-17(24)13-25-20/h3-11,13,26H,12H2,1-2H3,(H,27,30)(H,25,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
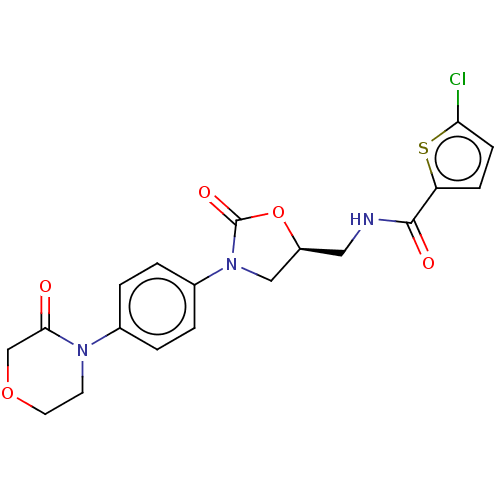
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382312
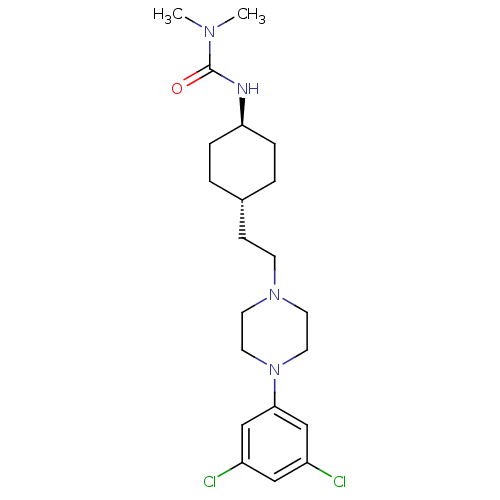
(CHEMBL2024677)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)cc(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(6.35,-23.87,;7.68,-24.65,;7.68,-26.19,;9.02,-23.88,;9.02,-22.34,;10.35,-24.65,;11.68,-23.88,;11.68,-22.34,;13.01,-21.56,;14.34,-22.34,;15.68,-21.57,;17.01,-22.35,;18.34,-21.58,;19.67,-22.36,;21,-21.6,;21.01,-20.06,;19.68,-19.28,;18.34,-20.04,;22.35,-19.3,;23.68,-20.08,;25.02,-19.32,;26.35,-20.1,;25.03,-17.78,;23.69,-17,;23.7,-15.46,;22.36,-17.76,;14.34,-23.88,;13.01,-24.64,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-19-5-3-16(4-6-19)7-8-26-9-11-27(12-10-26)20-14-17(22)13-18(23)15-20/h13-16,19H,3-12H2,1-2H3,(H,24,28)/t16-,19- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142158

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-i...)Show SMILES NC1=NCCN1Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 |t:1| Show InChI InChI=1S/C23H20Cl2N6O2/c24-16-5-7-19(18(11-16)22(33)30-20-8-6-17(25)12-28-20)29-21(32)15-3-1-14(2-4-15)13-31-10-9-27-23(31)26/h1-8,11-12H,9-10,13H2,(H2,26,27)(H,29,32)(H,28,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142177
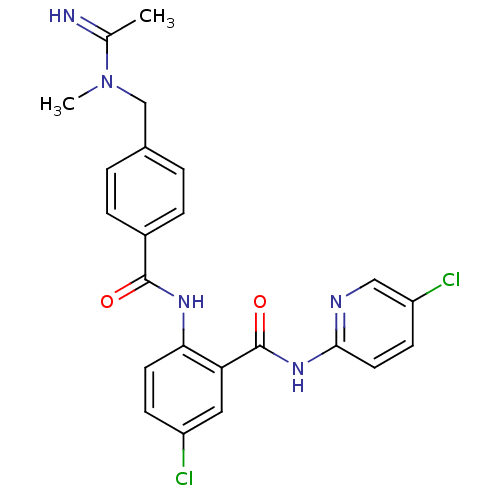
(2-{4-[(Acetimidoyl-methyl-amino)-methyl]-benzoylam...)Show SMILES CN(Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1)C(C)=N Show InChI InChI=1S/C23H21Cl2N5O2/c1-14(26)30(2)13-15-3-5-16(6-4-15)22(31)28-20-9-7-17(24)11-19(20)23(32)29-21-10-8-18(25)12-27-21/h3-12,26H,13H2,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Aurora kinase A-interacting protein
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382310
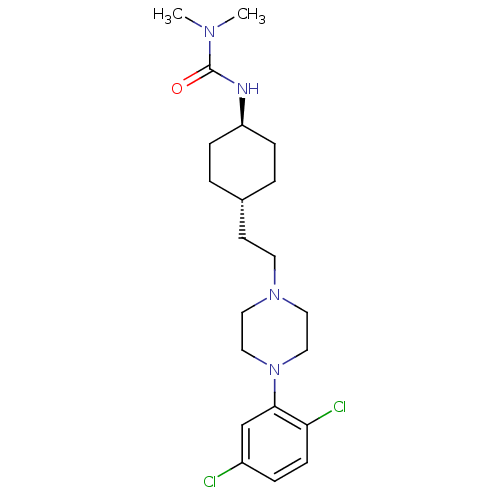
(CHEMBL2024675)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)ccc2Cl)CC1 |r,wU:6.5,wD:9.9,(23.12,-11.63,;24.45,-12.4,;24.45,-13.94,;25.79,-11.64,;25.79,-10.1,;27.12,-12.41,;28.45,-11.64,;28.45,-10.1,;29.78,-9.32,;31.11,-10.1,;32.45,-9.33,;33.78,-10.1,;35.11,-9.34,;36.44,-10.12,;37.77,-9.36,;37.78,-7.82,;36.45,-7.04,;35.11,-7.8,;39.12,-7.06,;39.13,-5.52,;40.46,-4.76,;40.46,-3.22,;41.8,-5.54,;41.79,-7.08,;40.45,-7.84,;40.44,-9.38,;31.11,-11.64,;29.78,-12.4,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-15-17(22)5-8-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140443
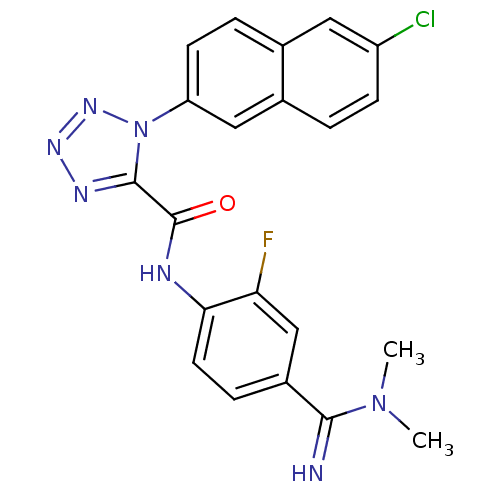
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C21H17ClFN7O/c1-29(2)19(24)14-5-8-18(17(23)11-14)25-21(31)20-26-27-28-30(20)16-7-4-12-9-15(22)6-3-13(12)10-16/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140413
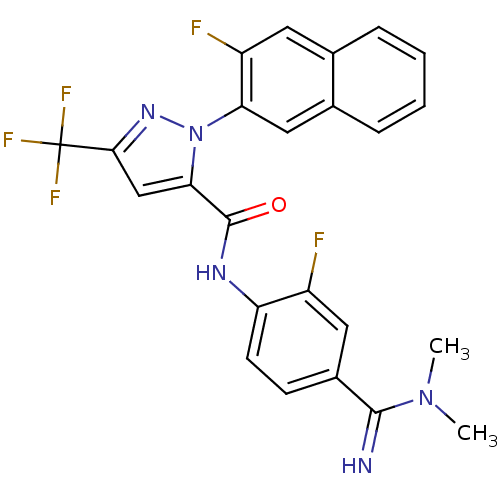
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142089
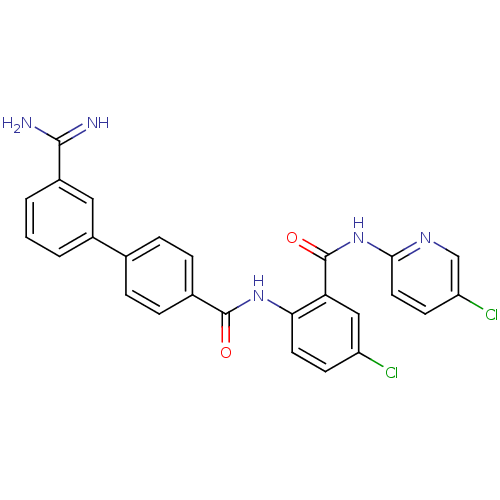
(3'-Carbamimidoyl-biphenyl-4-carboxylic acid [4-chl...)Show SMILES NC(=N)c1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H19Cl2N5O2/c27-19-8-10-22(21(13-19)26(35)33-23-11-9-20(28)14-31-23)32-25(34)16-6-4-15(5-7-16)17-2-1-3-18(12-17)24(29)30/h1-14H,(H3,29,30)(H,32,34)(H,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140424
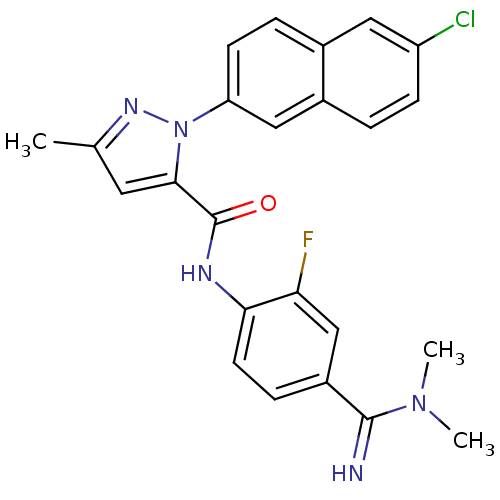
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142100
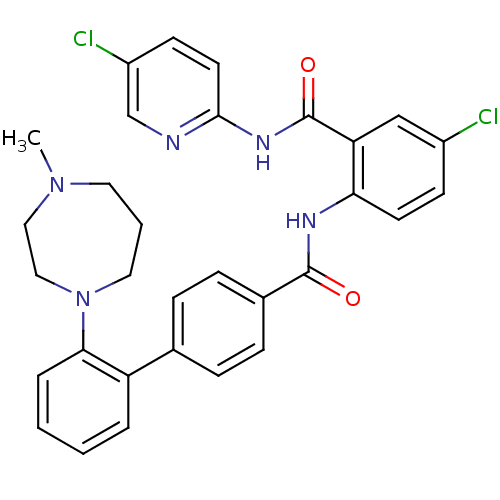
(2'-(4-Methyl-[1,4]diazepan-1-yl)-biphenyl-4-carbox...)Show SMILES CN1CCCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C31H29Cl2N5O2/c1-37-15-4-16-38(18-17-37)28-6-3-2-5-25(28)21-7-9-22(10-8-21)30(39)35-27-13-11-23(32)19-26(27)31(40)36-29-14-12-24(33)20-34-29/h2-3,5-14,19-20H,4,15-18H2,1H3,(H,35,39)(H,34,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2S receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142103
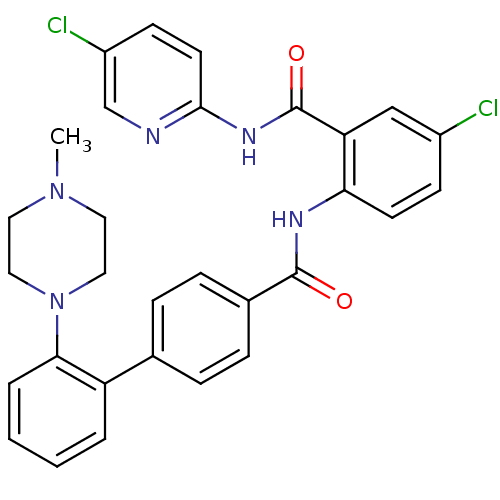
(2'-(4-Methyl-piperazin-1-yl)-biphenyl-4-carboxylic...)Show SMILES CN1CCN(CC1)c1ccccc1-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C30H27Cl2N5O2/c1-36-14-16-37(17-15-36)27-5-3-2-4-24(27)20-6-8-21(9-7-20)29(38)34-26-12-10-22(31)18-25(26)30(39)35-28-13-11-23(32)19-33-28/h2-13,18-19H,14-17H2,1H3,(H,34,38)(H,33,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140410
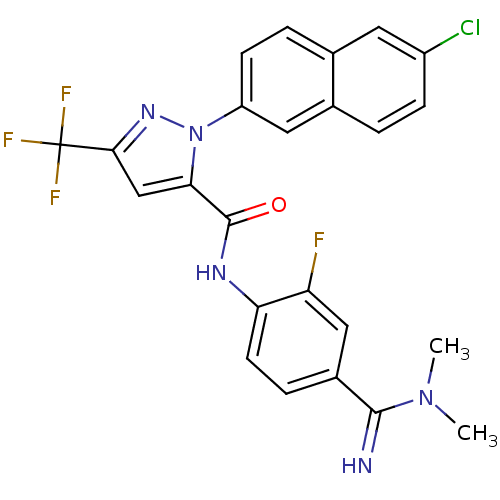
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18ClF4N5O/c1-33(2)22(30)15-5-8-19(18(26)11-15)31-23(35)20-12-21(24(27,28)29)32-34(20)17-7-4-13-9-16(25)6-3-14(13)10-17/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142106
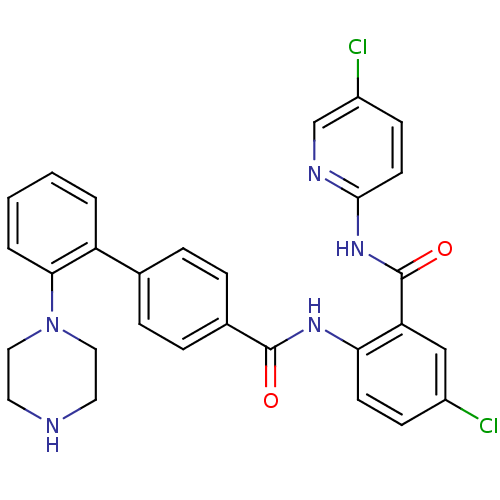
(2'-Piperazin-1-yl-biphenyl-4-carboxylic acid [4-ch...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-c2ccccc2N2CCNCC2)nc1 Show InChI InChI=1S/C29H25Cl2N5O2/c30-21-9-11-25(24(17-21)29(38)35-27-12-10-22(31)18-33-27)34-28(37)20-7-5-19(6-8-20)23-3-1-2-4-26(23)36-15-13-32-14-16-36/h1-12,17-18,32H,13-16H2,(H,34,37)(H,33,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140422
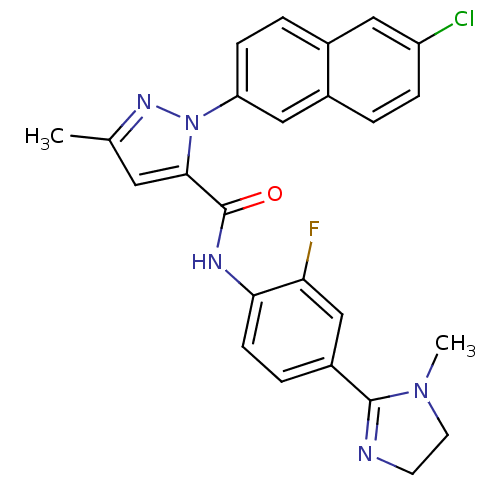
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C25H21ClFN5O/c1-15-11-23(32(30-15)20-7-4-16-12-19(26)6-3-17(16)13-20)25(33)29-22-8-5-18(14-21(22)27)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142154
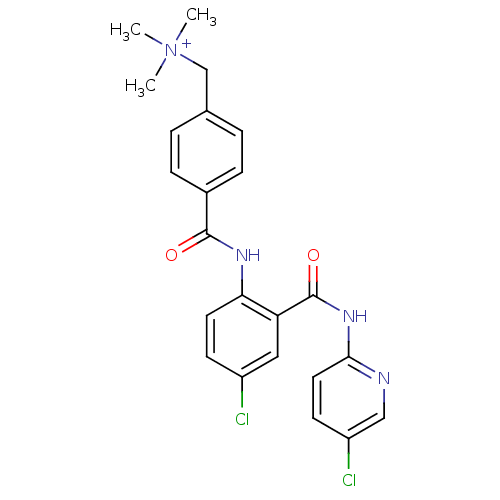
(CHEMBL174499 | {4-[4-Chloro-2-(5-chloro-pyridin-2-...)Show SMILES C[N+](C)(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22Cl2N4O2/c1-29(2,3)14-15-4-6-16(7-5-15)22(30)27-20-10-8-17(24)12-19(20)23(31)28-21-11-9-18(25)13-26-21/h4-13H,14H2,1-3H3,(H-,26,27,28,30,31)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142096
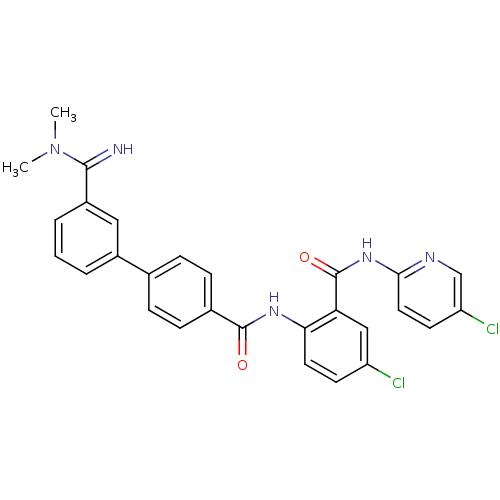
(3'-(N,N-Dimethyl-carbamimidoyl)-biphenyl-4-carboxy...)Show SMILES CN(C)C(=N)c1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-35(2)26(31)20-5-3-4-19(14-20)17-6-8-18(9-7-17)27(36)33-24-12-10-21(29)15-23(24)28(37)34-25-13-11-22(30)16-32-25/h3-16,31H,1-2H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against coagulation factor Xa. |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140415
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18ClF4N5O/c1-34-9-8-31-23(34)16-4-7-20(19(27)12-16)32-24(36)21-13-22(25(28,29)30)33-35(21)18-6-3-14-10-17(26)5-2-15(14)11-18/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142146

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-(4-dimethylam...)Show SMILES CN(C)Cc1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20Cl2N4O2/c1-28(2)13-14-3-5-15(6-4-14)21(29)26-19-9-7-16(23)11-18(19)22(30)27-20-10-8-17(24)12-25-20/h3-12H,13H2,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
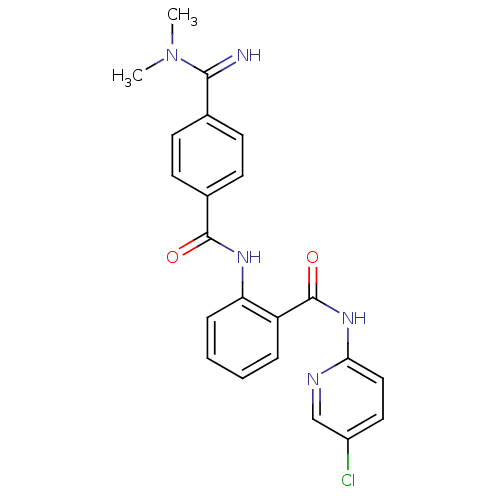
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142163

(5-Chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(2-imino-o...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(CN3CCOC3=N)cc2)nc1 Show InChI InChI=1S/C23H19Cl2N5O3/c24-16-5-7-19(18(11-16)22(32)29-20-8-6-17(25)12-27-20)28-21(31)15-3-1-14(2-4-15)13-30-9-10-33-23(30)26/h1-8,11-12,26H,9-10,13H2,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against coagulation factor X. |
Bioorg Med Chem Lett 14: 989-93 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.080
BindingDB Entry DOI: 10.7270/Q2X34WXD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382306

(CHEMBL2024522)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccccc2Cl)CC1 |r,wU:6.5,wD:9.9,(5.86,-.97,;7.19,-1.74,;7.19,-3.28,;8.53,-.97,;8.53,.57,;9.86,-1.74,;11.19,-.97,;11.19,.57,;12.52,1.35,;13.85,.57,;15.19,1.33,;16.52,.56,;17.85,1.33,;19.18,.55,;20.51,1.31,;20.52,2.85,;19.19,3.62,;17.85,2.86,;21.86,3.61,;21.87,5.14,;23.2,5.91,;24.54,5.13,;24.53,3.58,;23.19,2.83,;23.18,1.29,;13.85,-.97,;12.52,-1.73,)| Show InChI InChI=1S/C21H33ClN4O/c1-24(2)21(27)23-18-9-7-17(8-10-18)11-12-25-13-15-26(16-14-25)20-6-4-3-5-19(20)22/h3-6,17-18H,7-16H2,1-2H3,(H,23,27)/t17-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142091
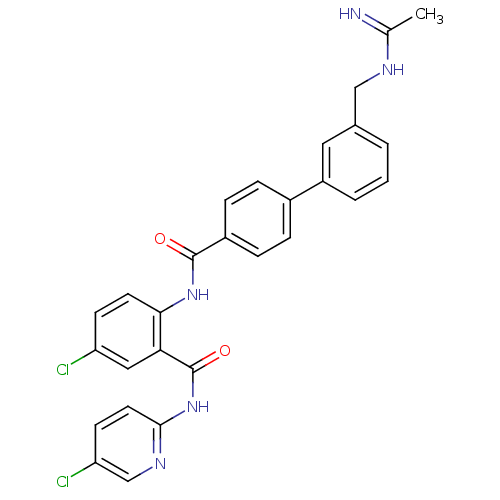
(3'-(Acetimidoylamino-methyl)-biphenyl-4-carboxylic...)Show SMILES CC(=N)NCc1cccc(c1)-c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H23Cl2N5O2/c1-17(31)32-15-18-3-2-4-21(13-18)19-5-7-20(8-6-19)27(36)34-25-11-9-22(29)14-24(25)28(37)35-26-12-10-23(30)16-33-26/h2-14,16H,15H2,1H3,(H2,31,32)(H,34,36)(H,33,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50142094
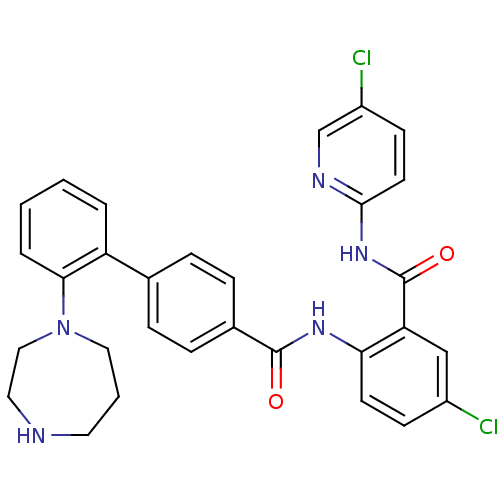
(2'-[1,4]Diazepan-1-yl-biphenyl-4-carboxylic acid [...)Show SMILES Clc1ccc(NC(=O)c2cc(Cl)ccc2NC(=O)c2ccc(cc2)-c2ccccc2N2CCCNCC2)nc1 Show InChI InChI=1S/C30H27Cl2N5O2/c31-22-10-12-26(25(18-22)30(39)36-28-13-11-23(32)19-34-28)35-29(38)21-8-6-20(7-9-21)24-4-1-2-5-27(24)37-16-3-14-33-15-17-37/h1-2,4-13,18-19,33H,3,14-17H2,(H,35,38)(H,34,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 14: 983-7 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.079
BindingDB Entry DOI: 10.7270/Q21V5DF7 |
More data for this
Ligand-Target Pair | |
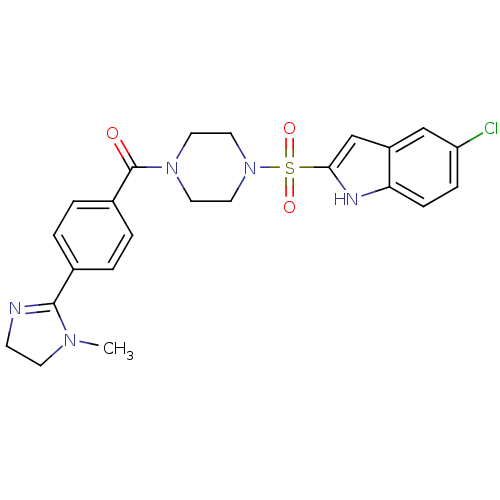
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092
(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
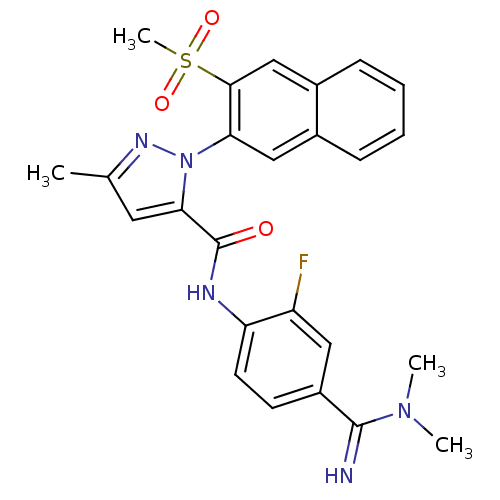
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140418
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C25H24FN5O3S/c1-15-11-22(25(32)28-20-10-9-18(12-19(20)26)24(27)30(2)3)31(29-15)21-13-16-7-5-6-8-17(16)14-23(21)35(4,33)34/h5-14,27H,1-4H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
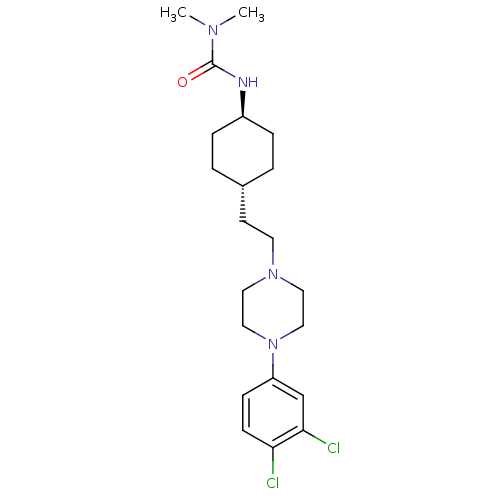
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382311
(CHEMBL2024676)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2ccc(Cl)c(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(-8.97,-23.7,;-7.64,-24.48,;-7.64,-26.02,;-6.31,-23.71,;-6.3,-22.17,;-4.97,-24.48,;-3.64,-23.71,;-3.64,-22.17,;-2.31,-21.39,;-.98,-22.17,;.35,-21.4,;1.69,-22.17,;3.02,-21.41,;4.35,-22.19,;5.68,-21.43,;5.69,-19.89,;4.36,-19.11,;3.02,-19.87,;7.03,-19.13,;7.04,-17.59,;8.37,-16.83,;9.71,-17.61,;11.04,-16.85,;9.7,-19.15,;11.02,-19.93,;8.36,-19.91,;-.98,-23.71,;-2.31,-24.47,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-5-3-16(4-6-17)9-10-26-11-13-27(14-12-26)18-7-8-19(22)20(23)15-18/h7-8,15-17H,3-6,9-14H2,1-2H3,(H,24,28)/t16-,17- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50323373
(1,2-dicyclohexyl-3-(pyridin-3-yl)propan-1-one O-3-...)Show SMILES Clc1cccc(NC(=O)O\N=C(\C(Cc2cccnc2)C2CCCCC2)C2CCCCC2)c1 Show InChI InChI=1S/C27H34ClN3O2/c28-23-14-7-15-24(18-23)30-27(32)33-31-26(22-12-5-2-6-13-22)25(21-10-3-1-4-11-21)17-20-9-8-16-29-19-20/h7-9,14-16,18-19,21-22,25H,1-6,10-13,17H2,(H,30,32)/b31-26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-M-MPEP from rat mGLUR5 |
Bioorg Med Chem Lett 20: 4371-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.075
BindingDB Entry DOI: 10.7270/Q2G1611F |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140409
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 |c:4| Show InChI InChI=1S/C26H24FN5O3S/c1-16-12-23(26(33)29-21-9-8-19(13-20(21)27)25-28-10-11-31(25)2)32(30-16)22-14-17-6-4-5-7-18(17)15-24(22)36(3,34)35/h4-9,12-15H,10-11H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140407
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140417
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C26H23F2N5O/c1-16-12-24(33(31-16)23-15-18-7-3-2-6-17(18)13-21(23)28)26(34)30-22-9-8-19(14-20(22)27)25(29)32-10-4-5-11-32/h2-3,6-9,12-15,29H,4-5,10-11H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data