 Found 2501 hits with Last Name = 'lou' and Initial = 'j'
Found 2501 hits with Last Name = 'lou' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50605126

(CHEMBL5187340)Show SMILES COc1cc(ccc1NC(=O)[C@@H]1N[C@@H](CC(C)(C)C)[C@@](CNCC2CCNCC2)([C@H]1c1cccc(Cl)c1F)c1ccc(Cl)cc1F)C(O)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00095
BindingDB Entry DOI: 10.7270/Q2V69PP3 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498230

(CHEMBL3577576)Show SMILES [H][C@]12OC[C@H](OC)[C@@]1([H])[C@H](CO2)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C29H40N2O9S/c1-19(2)15-31(41(34,35)22-12-10-21(36-3)11-13-22)16-24(32)23(14-20-8-6-5-7-9-20)30-29(33)40-26-18-39-28-27(26)25(37-4)17-38-28/h5-13,19,23-28,32H,14-18H2,1-4H3,(H,30,33)/t23-,24+,25-,26-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50498229

(CHEMBL3577575)Show SMILES [H][C@@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@]1([H])C(F)(F)CO2 |r| Show InChI InChI=1S/C27H35F2N3O7S/c1-17(2)13-32(40(35,36)20-10-8-19(30)9-11-20)14-22(33)21(12-18-6-4-3-5-7-18)31-26(34)39-23-15-37-25-24(23)27(28,29)16-38-25/h3-11,17,21-25,33H,12-16,30H2,1-2H3,(H,31,34)/t21-,22+,23-,24-,25-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 protease |
J Med Chem 58: 5088-95 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00474
BindingDB Entry DOI: 10.7270/Q27D2Z42 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536
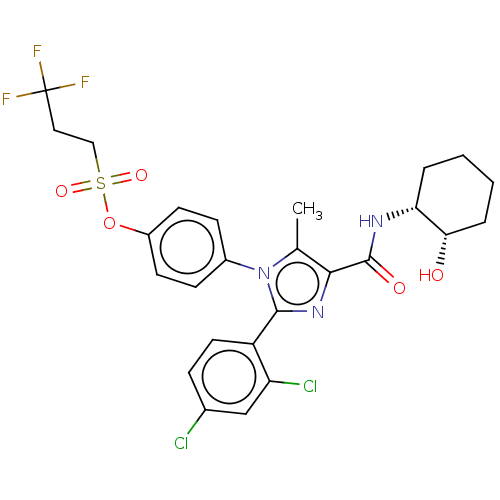
(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114327
BindingDB Entry DOI: 10.7270/Q2NP28GR |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243536

(CHEMBL4062749)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@@H]1CCCC[C@@H]1O |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-21-4-2-3-5-22(21)35)33-24(19-11-6-16(27)14-20(19)28)34(15)17-7-9-18(10-8-17)39-40(37,38)13-12-26(29,30)31/h6-11,14,21-22,35H,2-5,12-13H2,1H3,(H,32,36)/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254
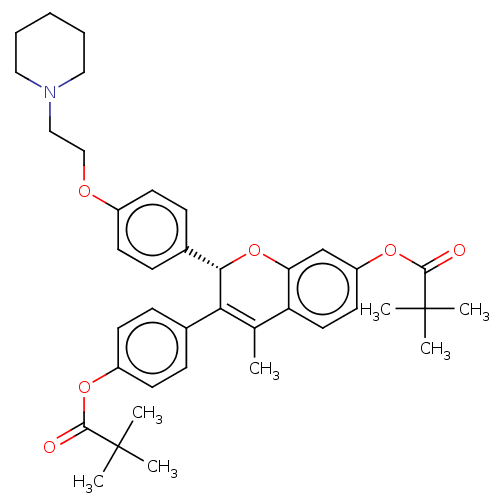
(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471254

(CHEMBL308234)Show SMILES CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1 |r,t:1| Show InChI InChI=1S/C39H47NO6/c1-26-32-20-19-31(45-37(42)39(5,6)7)25-33(32)46-35(34(26)27-11-17-30(18-12-27)44-36(41)38(2,3)4)28-13-15-29(16-14-28)43-24-23-40-21-9-8-10-22-40/h11-20,25,35H,8-10,21-24H2,1-7H3/t35-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471256
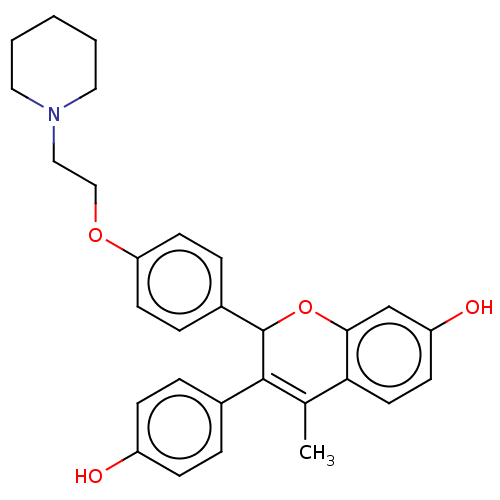
(CHEMBL291808)Show SMILES CC1=C(C(Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated T-47D cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311170
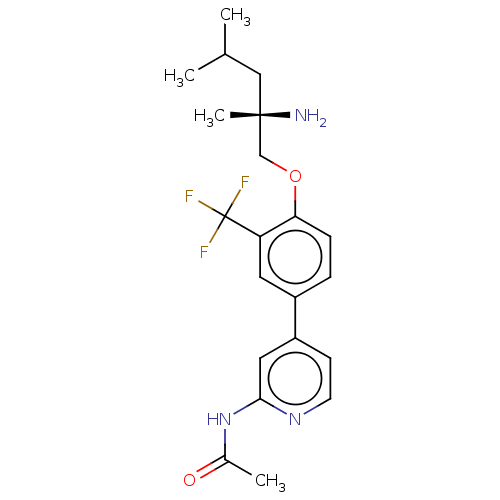
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O2/c1-13(2)11-20(4,25)12-29-18-6-5-15(9-17(18)21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311182

((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C21H26F3N3O4/c1-13(2)11-20(3,25)12-30-16-6-5-14(9-17(16)31-21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311157
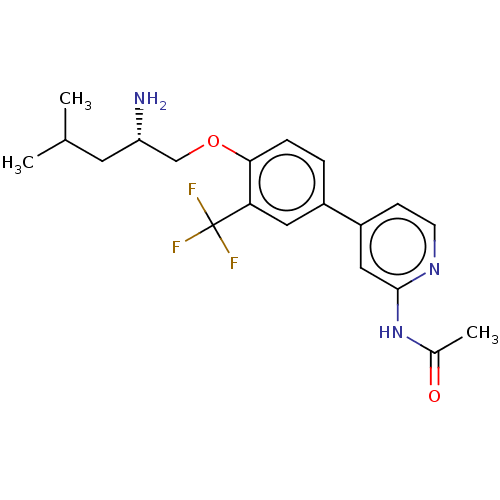
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-12(2)8-16(24)11-28-18-5-4-14(9-17(18)20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311155
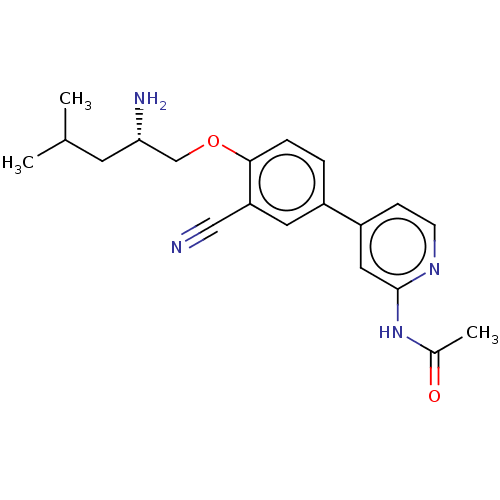
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-cyanop...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24N4O2/c1-13(2)8-18(22)12-26-19-5-4-15(9-17(19)11-21)16-6-7-23-20(10-16)24-14(3)25/h4-7,9-10,13,18H,8,12,22H2,1-3H3,(H,23,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311180
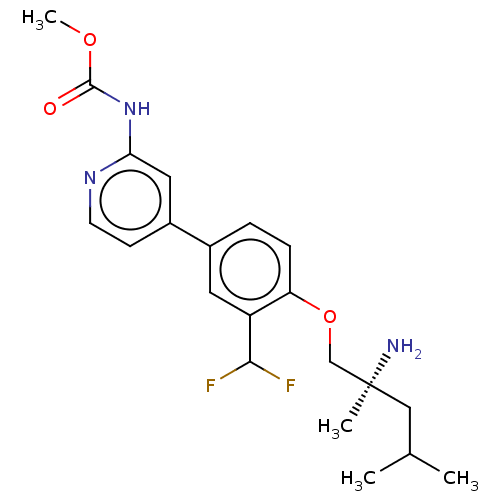
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)F |r| Show InChI InChI=1S/C21H27F2N3O3/c1-13(2)11-21(3,24)12-29-17-6-5-14(9-16(17)19(22)23)15-7-8-25-18(10-15)26-20(27)28-4/h5-10,13,19H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311158
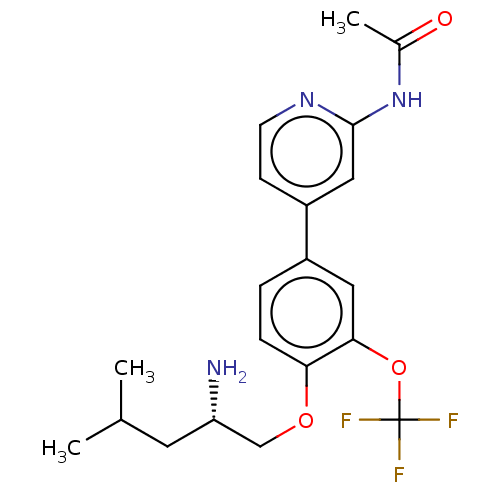
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O3/c1-12(2)8-16(24)11-28-17-5-4-14(9-18(17)29-20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311268
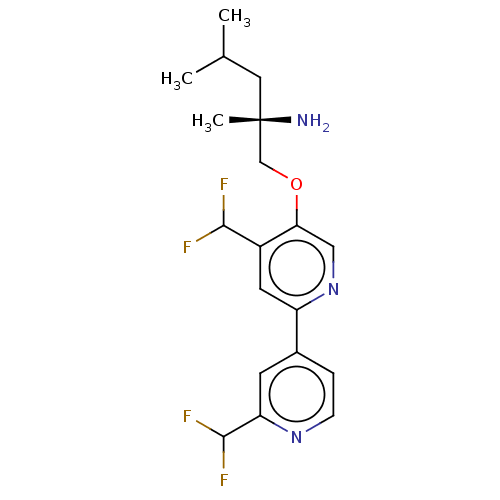
((S)-1-((2′,4-bis(difluoromethyl)-[2,4′...)Show SMILES CC(C)C[C@](C)(N)COc1cnc(cc1C(F)F)-c1ccnc(c1)C(F)F |r| Show InChI InChI=1S/C19H23F4N3O/c1-11(2)8-19(3,24)10-27-16-9-26-14(7-13(16)17(20)21)12-4-5-25-15(6-12)18(22)23/h4-7,9,11,17-18H,8,10,24H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311176
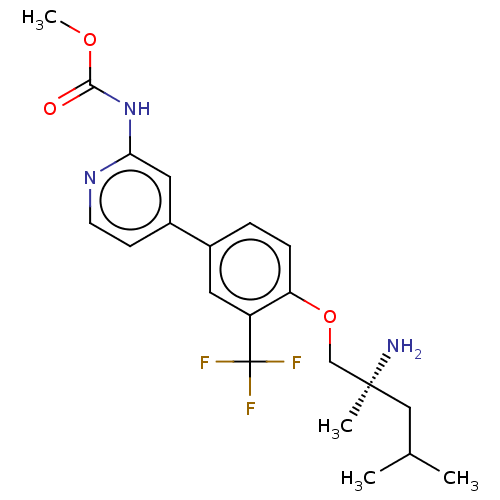
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(3,25)12-30-17-6-5-14(9-16(17)21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311179
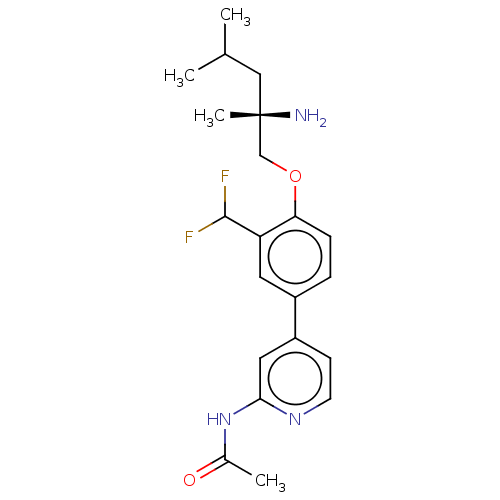
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(d...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H27F2N3O2/c1-13(2)11-21(4,24)12-28-18-6-5-15(9-17(18)20(22)23)16-7-8-25-19(10-16)26-14(3)27/h5-10,13,20H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311189
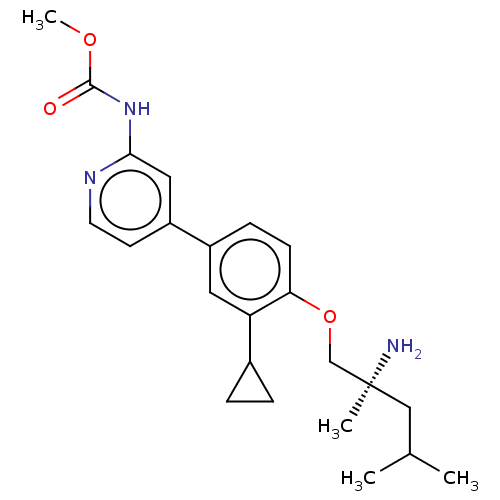
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C1CC1 |r| Show InChI InChI=1S/C23H31N3O3/c1-15(2)13-23(3,24)14-29-20-8-7-17(11-19(20)16-5-6-16)18-9-10-25-21(12-18)26-22(27)28-4/h7-12,15-16H,5-6,13-14,24H2,1-4H3,(H,25,26,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311160
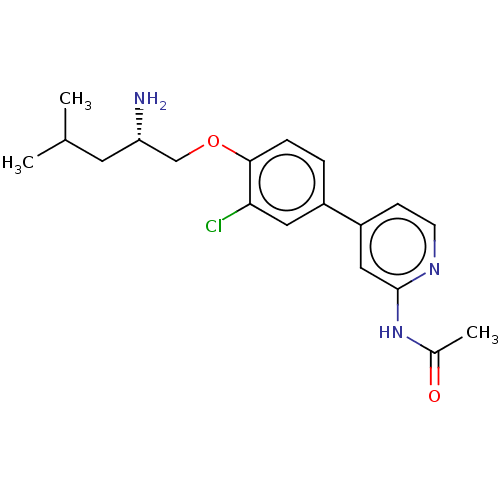
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-chloro...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1Cl)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-12(2)8-16(21)11-25-18-5-4-14(9-17(18)20)15-6-7-22-19(10-15)23-13(3)24/h4-7,9-10,12,16H,8,11,21H2,1-3H3,(H,22,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311181
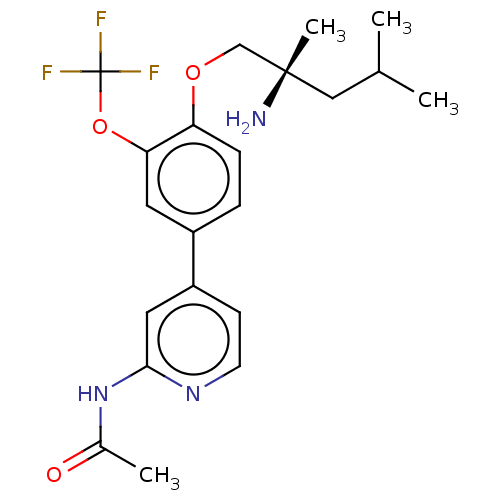
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(4,25)12-29-17-6-5-15(9-18(17)30-21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311264
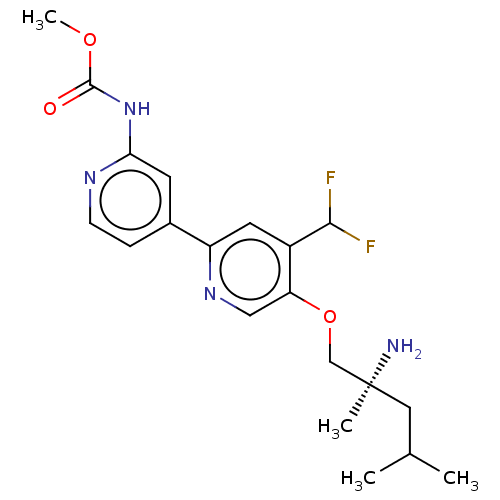
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(C(F)F)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)9-20(3,23)11-29-16-10-25-15(8-14(16)18(21)22)13-5-6-24-17(7-13)26-19(27)28-4/h5-8,10,12,18H,9,11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311261
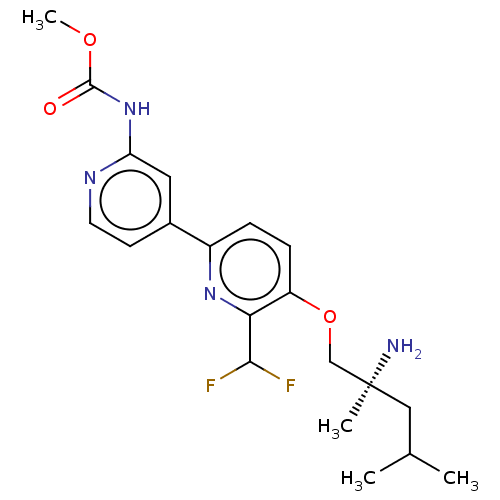
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-6-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(n1)C(F)F |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)10-20(3,23)11-29-15-6-5-14(25-17(15)18(21)22)13-7-8-24-16(9-13)26-19(27)28-4/h5-9,12,18H,10-11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311178
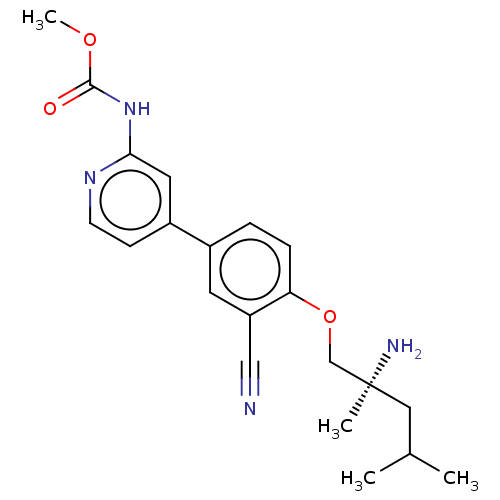
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C#N |r| Show InChI InChI=1S/C21H26N4O3/c1-14(2)11-21(3,23)13-28-18-6-5-15(9-17(18)12-22)16-7-8-24-19(10-16)25-20(26)27-4/h5-10,14H,11,13,23H2,1-4H3,(H,24,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311165
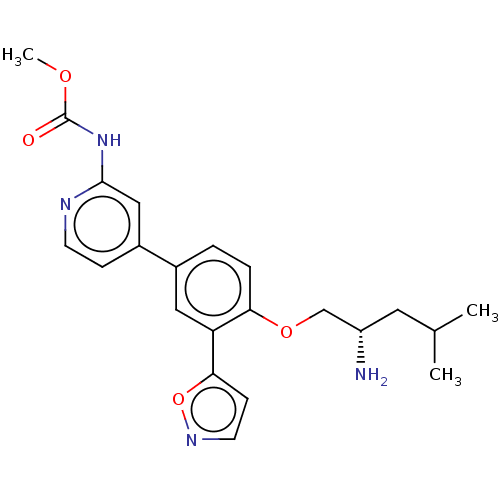
((S)-methyl (4-(4-((2-amino-4-methylpentyl)oxy)-3-(...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@H](N)CC(C)C)c(c1)-c1ccno1 |r| Show InChI InChI=1S/C22H26N4O4/c1-14(2)10-17(23)13-29-19-5-4-15(11-18(19)20-7-9-25-30-20)16-6-8-24-21(12-16)26-22(27)28-3/h4-9,11-12,14,17H,10,13,23H2,1-3H3,(H,24,26,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604135
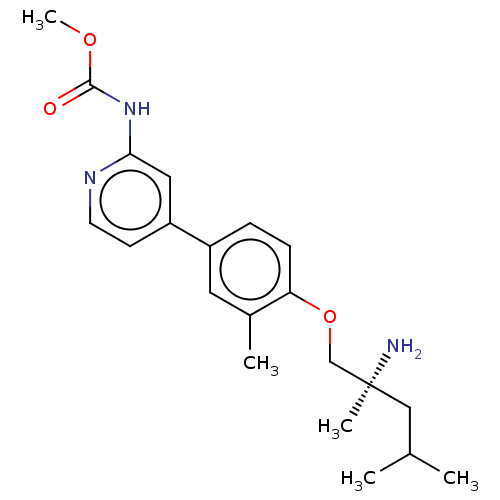
(CHEMBL5198877)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(C)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311159
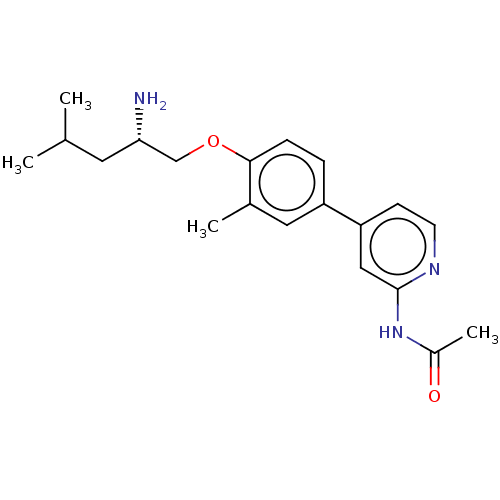
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-methyl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H27N3O2/c1-13(2)9-18(21)12-25-19-6-5-16(10-14(19)3)17-7-8-22-20(11-17)23-15(4)24/h5-8,10-11,13,18H,9,12,21H2,1-4H3,(H,22,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311177
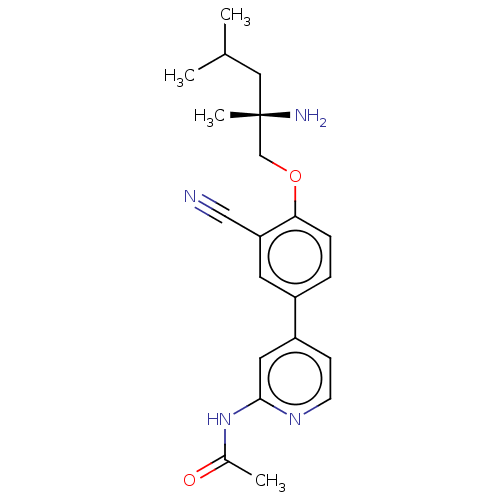
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-cy...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26N4O2/c1-14(2)11-21(4,23)13-27-19-6-5-16(9-18(19)12-22)17-7-8-24-20(10-17)25-15(3)26/h5-10,14H,11,13,23H2,1-4H3,(H,24,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604134
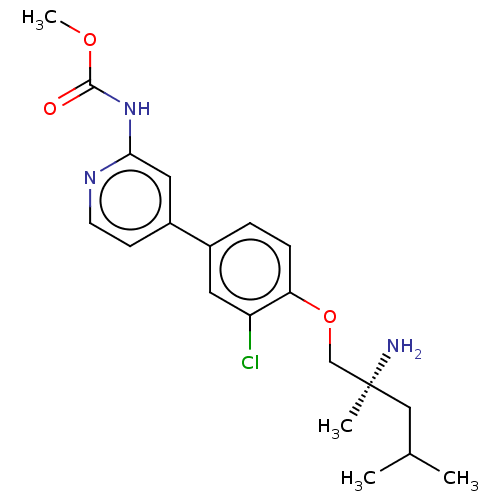
(CHEMBL5187778)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471255

(Acolbifene | EM-652 | SCH-57068)Show SMILES CC1=C([C@@H](Oc2cc(O)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C29H31NO4/c1-20-26-14-11-24(32)19-27(26)34-29(28(20)21-5-9-23(31)10-6-21)22-7-12-25(13-8-22)33-18-17-30-15-3-2-4-16-30/h5-14,19,29,31-32H,2-4,15-18H2,1H3/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent binding affinity against estradiol-stimulated ZR-75-1-cell proliferation |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589
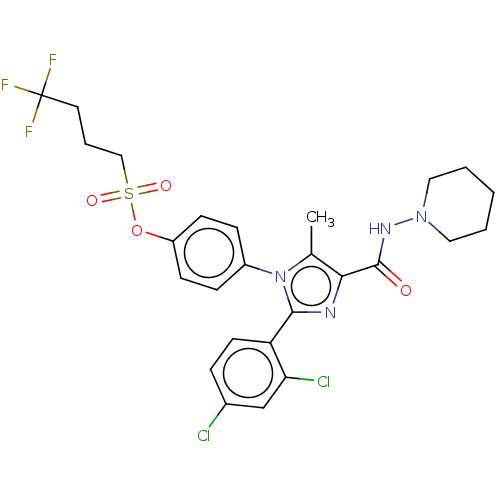
(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625
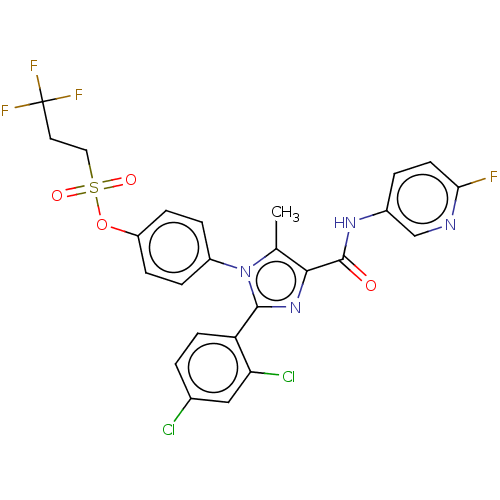
(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243589

(CHEMBL4095223)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCCC(F)(F)F)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H27Cl2F3N4O4S/c1-17-23(25(36)33-34-13-3-2-4-14-34)32-24(21-11-6-18(27)16-22(21)28)35(17)19-7-9-20(10-8-19)39-40(37,38)15-5-12-26(29,30)31/h6-11,16H,2-5,12-15H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545
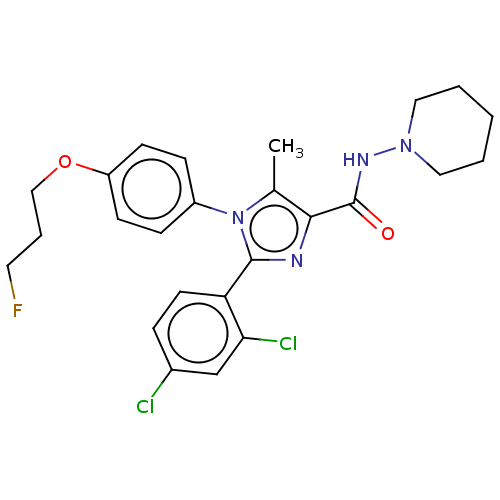
(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608
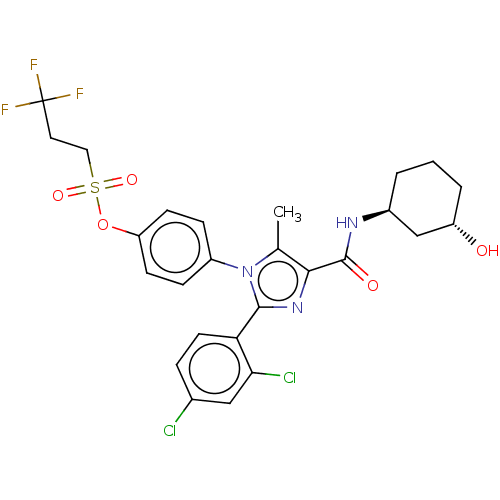
(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243625

(CHEMBL4094098)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(F)nc1 Show InChI InChI=1S/C25H18Cl2F4N4O4S/c1-14-22(24(36)33-16-3-9-21(28)32-13-16)34-23(19-8-2-15(26)12-20(19)27)35(14)17-4-6-18(7-5-17)39-40(37,38)11-10-25(29,30)31/h2-9,12-13H,10-11H2,1H3,(H,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243608

(CHEMBL4100882)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)N[C@H]1CCC[C@H](O)C1 |r| Show InChI InChI=1S/C26H26Cl2F3N3O5S/c1-15-23(25(36)32-17-3-2-4-19(35)14-17)33-24(21-10-5-16(27)13-22(21)28)34(15)18-6-8-20(9-7-18)39-40(37,38)12-11-26(29,30)31/h5-10,13,17,19,35H,2-4,11-12,14H2,1H3,(H,32,36)/t17-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243545

(CHEMBL4078689)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OCCCF)cc1)C(=O)NN1CCCCC1 Show InChI InChI=1S/C25H27Cl2FN4O2/c1-17-23(25(33)30-31-13-3-2-4-14-31)29-24(21-11-6-18(26)16-22(21)27)32(17)19-7-9-20(10-8-19)34-15-5-12-28/h6-11,16H,2-5,12-15H2,1H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM50604141
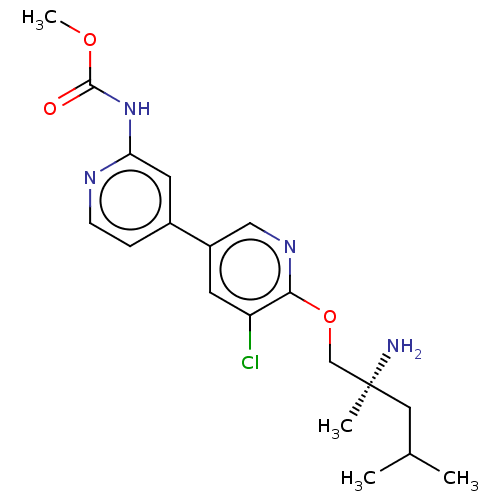
(CHEMBL5188433)Show SMILES COC(=O)Nc1cc(ccn1)-c1cnc(OC[C@@](C)(N)CC(C)C)c(Cl)c1 |r| | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311380
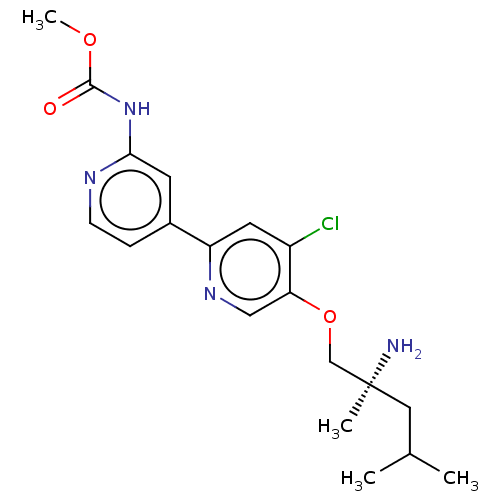
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(Cl)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C19H25ClN4O3/c1-12(2)9-19(3,21)11-27-16-10-23-15(8-14(16)20)13-5-6-22-17(7-13)24-18(25)26-4/h5-8,10,12H,9,11,21H2,1-4H3,(H,22,24,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human Breast cancer cytosol in 2.5%DMF |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec
Curated by ChEMBL
| Assay Description
Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol |
J Med Chem 40: 2117-22 (1997)
Article DOI: 10.1021/jm970095o
BindingDB Entry DOI: 10.7270/Q2474DKC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243588
(CHEMBL4067800)Show SMILES CCCCS(=O)(=O)Oc1ccc(cc1)-n1c(C)c(nc1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C26H30Cl2N4O4S/c1-3-4-16-37(34,35)36-21-11-9-20(10-12-21)32-18(2)24(26(33)30-31-14-6-5-7-15-31)29-25(32)22-13-8-19(27)17-23(22)28/h8-13,17H,3-7,14-16H2,1-2H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50243626
(CHEMBL4089821)Show SMILES Cc1c(nc(-c2ccc(Cl)cc2Cl)n1-c1ccc(OS(=O)(=O)CCC(F)(F)F)cc1)C(=O)Nc1ccc(C)cn1 Show InChI InChI=1S/C26H21Cl2F3N4O4S/c1-15-3-10-22(32-14-15)33-25(36)23-16(2)35(24(34-23)20-9-4-17(27)13-21(20)28)18-5-7-19(8-6-18)39-40(37,38)12-11-26(29,30)31/h3-10,13-14H,11-12H2,1-2H3,(H,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from recombinant human CB1 receptor expressed in beta-galactosidase expressing CHOK1 cell membranes after 60 mins by scin... |
J Med Chem 60: 9545-9564 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00861
BindingDB Entry DOI: 10.7270/Q2X92DQ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data