

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50458516 (CHEMBL4210652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527166 (CHEMBL4589666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527182 (CHEMBL4458115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527163 (CHEMBL4445050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527184 (CHEMBL4454026) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527185 (CHEMBL4580168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527167 (CHEMBL4445668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527162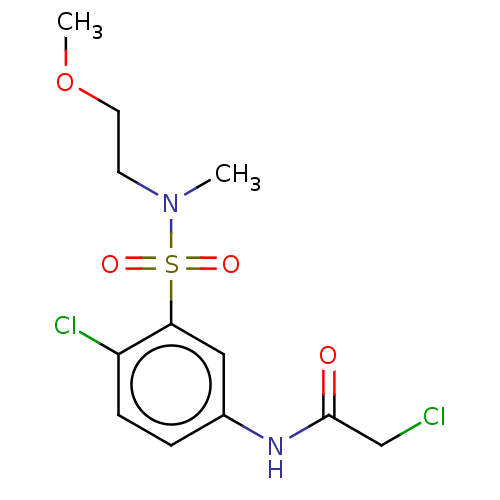 (CHEMBL4458365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527165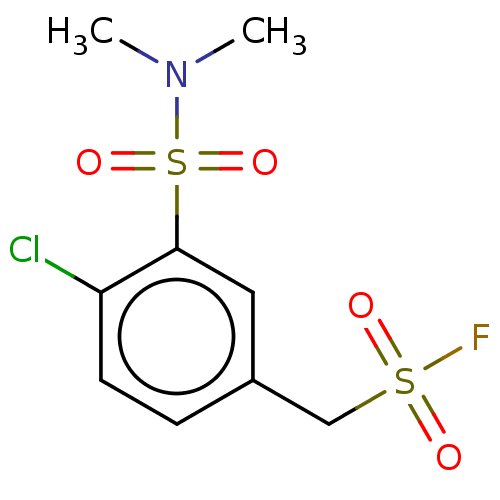 (CHEMBL4554461) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase omega-1 (Homo sapiens (Human)) | BDBM50527169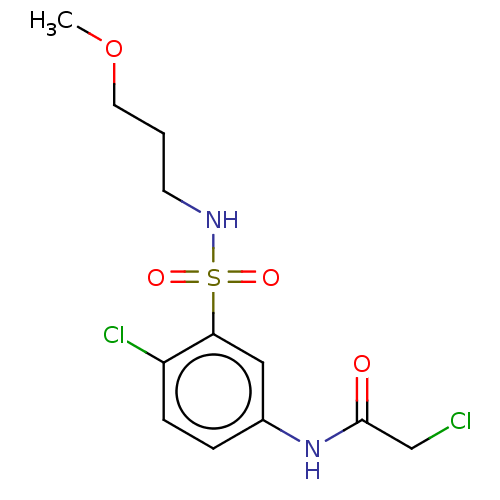 (CHEMBL4437777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ... | J Med Chem 63: 2894-2914 (2020) Article DOI: 10.1021/acs.jmedchem.9b01391 BindingDB Entry DOI: 10.7270/Q24Q7ZDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged Bcl-Xl by luminescence proximity assay | J Med Chem 56: 5514-40 (2014) Article DOI: 10.1021/jm400556w BindingDB Entry DOI: 10.7270/Q2X92CQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50020938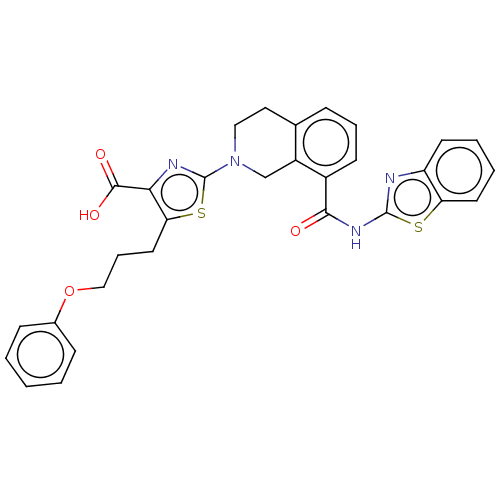 (CHEMBL3287301) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 26-mer BIMBH3 peptide from GST-tagged Bcl-Xl (unknown origin) by AlphaScreen assay | ACS Med Chem Lett 5: 662-7 (2014) Article DOI: 10.1021/ml500030p BindingDB Entry DOI: 10.7270/Q2KP83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM109236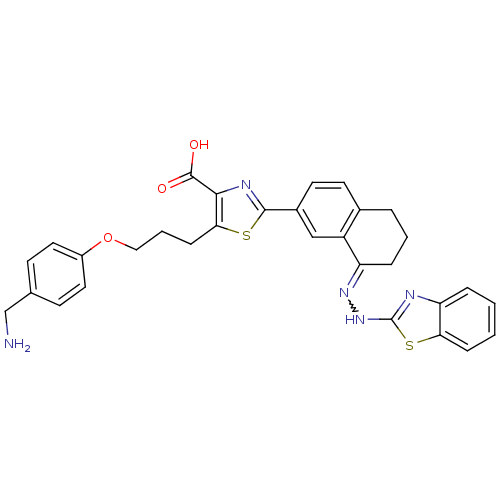 (WEHI-539) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description Each 384-well plate was prepared with 4.75 µL of working assay buffer (50 mM HEPES, 10 mM DTT, 100 mM NaCl, 0.05% Tween029 and 0.1 mg/mL casein,... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM109235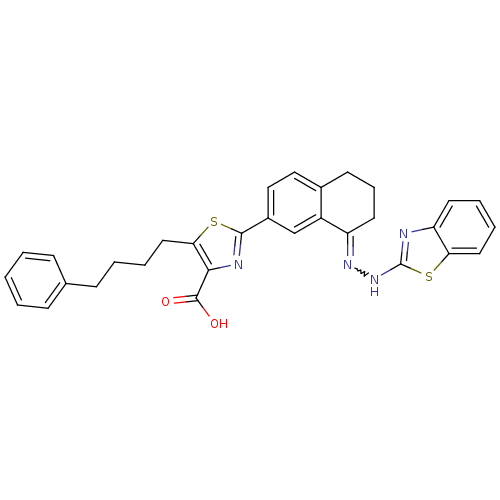 ((E)-2-(8-(2-(Benzo[d]thiazol-2-yl)hydrazono)-5,6,7...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description Each 384-well plate was prepared with 4.75 µL of working assay buffer (50 mM HEPES, 10 mM DTT, 100 mM NaCl, 0.05% Tween029 and 0.1 mg/mL casein,... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518833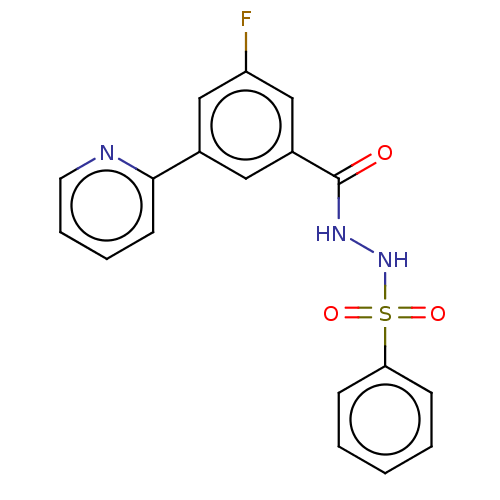 (CHEMBL4528993 | US10829446, Compound 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50339781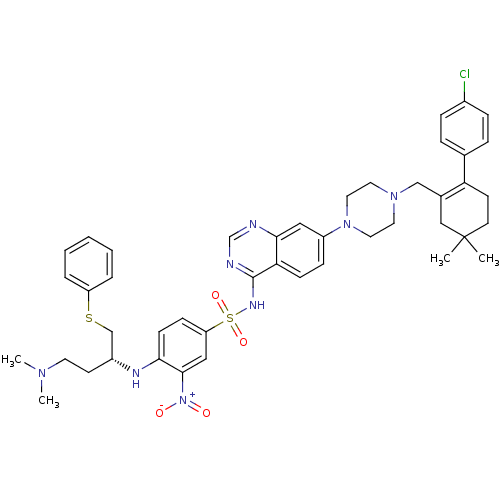 (CHEMBL1689143 | N-(7-{4-[2-(4-Chloro-phenyl)-5,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of biotinylated Bim-BH3 from GST-tagged Bcl-Xl by AlphaSCREEN competitive assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50339783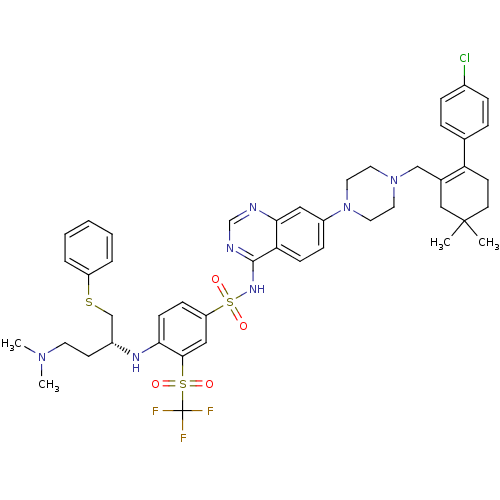 (CHEMBL1689145 | N-(7-{4-[2-(4-Chloro-phenyl)-5,5-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of biotinylated Bim-BH3 from GST-tagged Bcl-Xl by AlphaSCREEN competitive assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of biotinylated Bim-BH3 from GST-tagged Bcl-Xl by AlphaSCREEN competitive assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308131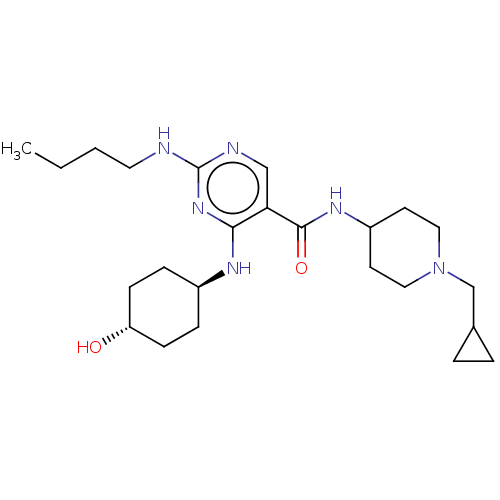 (US9649309, Compound UNC2876A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113822 BindingDB Entry DOI: 10.7270/Q2TX3KFC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50020936 (CHEMBL3287299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 26-mer BIMBH3 peptide from GST-tagged Bcl-Xl (unknown origin) by AlphaScreen assay | ACS Med Chem Lett 5: 662-7 (2014) Article DOI: 10.1021/ml500030p BindingDB Entry DOI: 10.7270/Q2KP83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50602486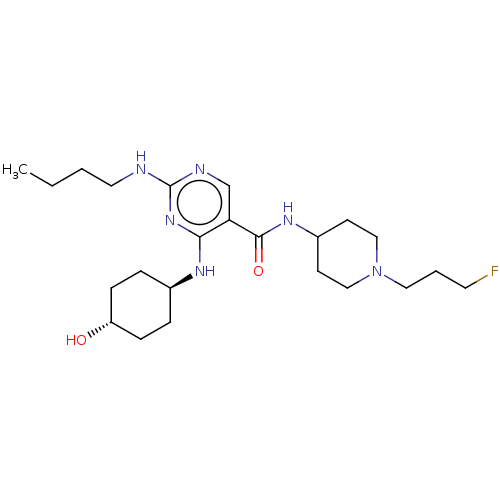 (CHEMBL5196154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113822 BindingDB Entry DOI: 10.7270/Q2TX3KFC | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50602485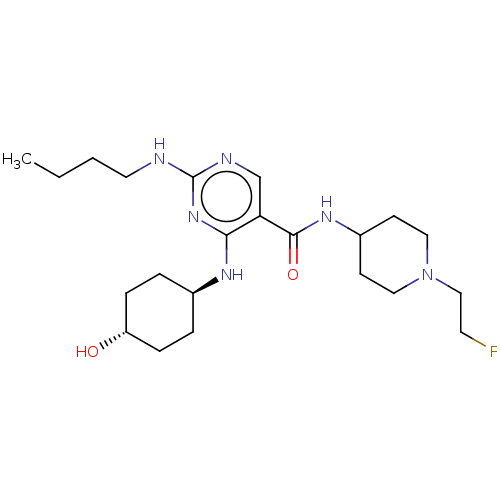 (CHEMBL5171919) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113822 BindingDB Entry DOI: 10.7270/Q2TX3KFC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50339783 (CHEMBL1689145 | N-(7-{4-[2-(4-Chloro-phenyl)-5,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of wild type mBimBH3 from human Bcl-2 by solution competition assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description Each 384-well plate was prepared with 4.75 µL of working assay buffer (50 mM HEPES, 10 mM DTT, 100 mM NaCl, 0.05% Tween029 and 0.1 mg/mL casein,... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527224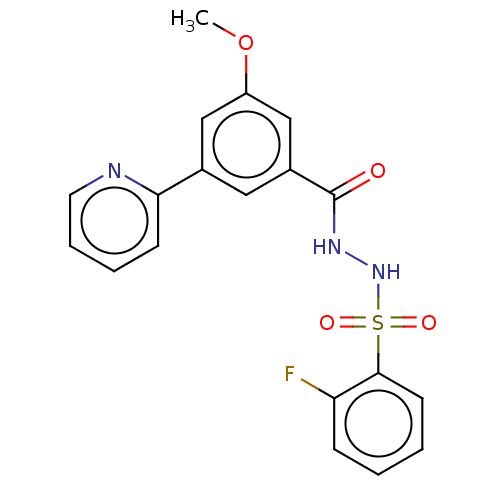 (CHEMBL4468833 | US10829446, Compound 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527244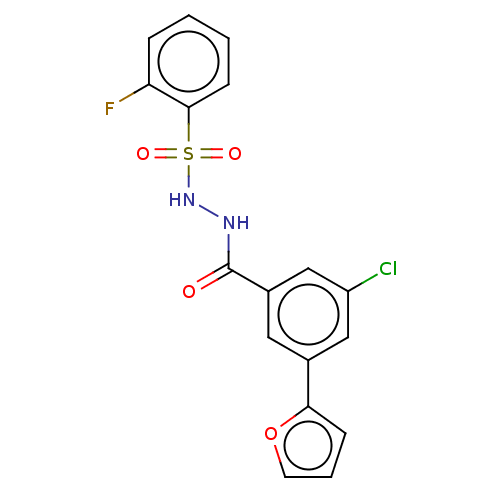 (CHEMBL4437798 | US10829446, Compound 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50339779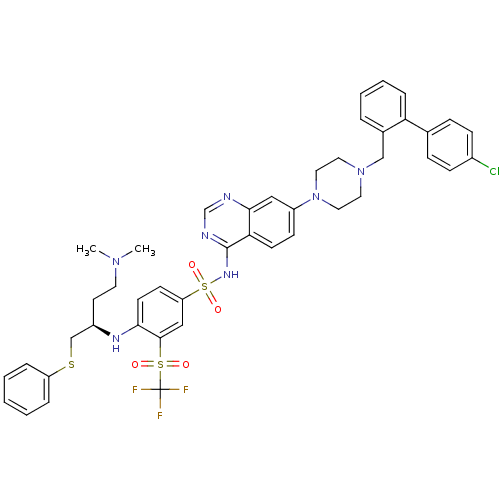 (CHEMBL1689141 | N-{7-[4-(4'-Chloro-biphenyl-2-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of biotinylated Bim-BH3 from GST-tagged Bcl-Xl by AlphaSCREEN competitive assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527381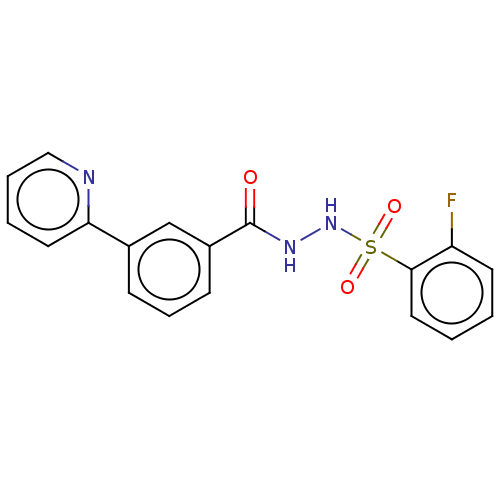 (CHEMBL4466823 | US10829446, Compound 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50436680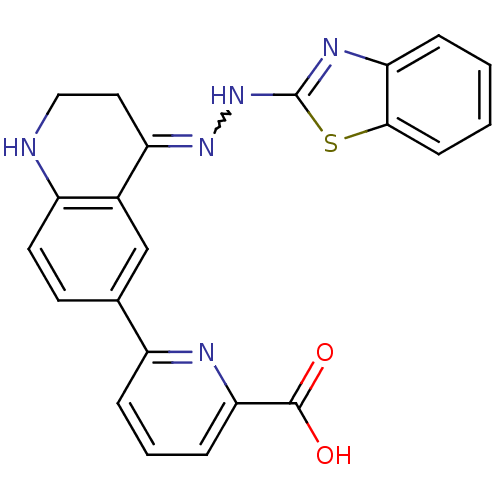 (CHEMBL2398255) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged Bcl-Xl by luminescence proximity assay | J Med Chem 56: 5514-40 (2014) Article DOI: 10.1021/jm400556w BindingDB Entry DOI: 10.7270/Q2X92CQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of wild type mBimBH3 from human Bcl-2 by solution competition assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM109234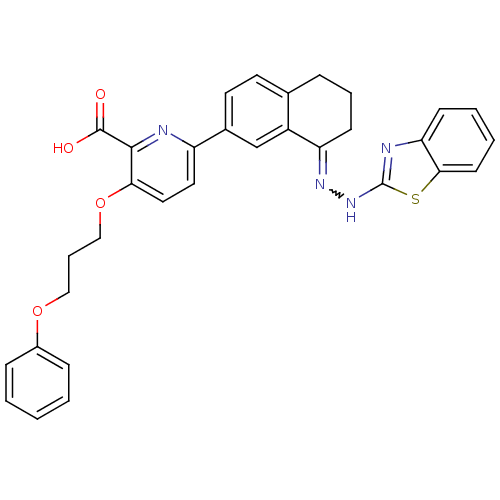 (6-(8-(2-(Benzo[d]thiazol-2-yl)hydrazono)-5,6,7,8-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description Each 384-well plate was prepared with 4.75 µL of working assay buffer (50 mM HEPES, 10 mM DTT, 100 mM NaCl, 0.05% Tween029 and 0.1 mg/mL casein,... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527355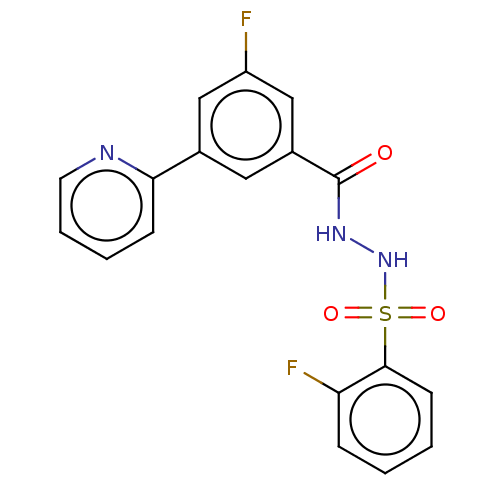 (CHEMBL4456585 | US10829446, Compound 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Binding affinity to Bcl-2 (unknown origin) by surface plasmon resonance assay | J Med Chem 56: 5514-40 (2014) Article DOI: 10.1021/jm400556w BindingDB Entry DOI: 10.7270/Q2X92CQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50020939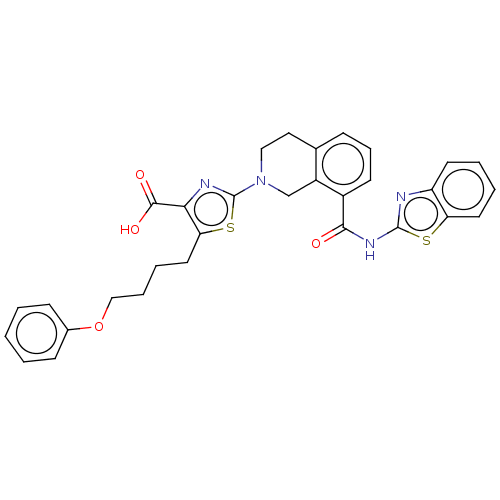 (CHEMBL3287302) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Displacement of 26-mer BIMBH3 peptide from GST-tagged Bcl-Xl (unknown origin) by AlphaScreen assay | ACS Med Chem Lett 5: 662-7 (2014) Article DOI: 10.1021/ml500030p BindingDB Entry DOI: 10.7270/Q2KP83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527250 (CHEMBL4475883 | US10829446, Compound 67) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50339777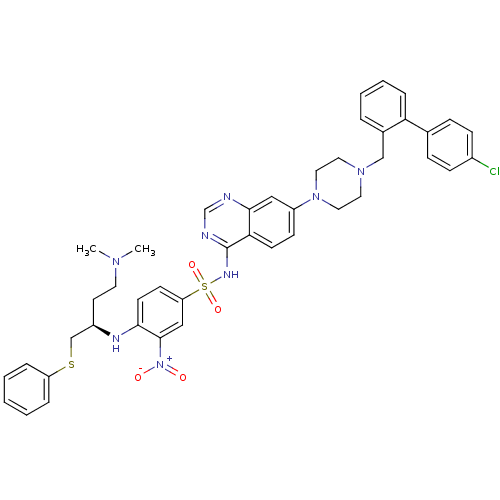 ((R)-N-(7-(4-((4'-chlorobiphenyl-2-yl)methyl)pipera...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of biotinylated Bim-BH3 from GST-tagged Bcl-Xl by AlphaSCREEN competitive assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM109236 (WEHI-539) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.10 | 1.40 | n/a | n/a | n/a | 7.4 | n/a |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description The protocols for tight and weak binders were described in Biacore S51 assay set-up with a flow rate of 30 µL/min. For His-Bcl-2 protein assays... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527238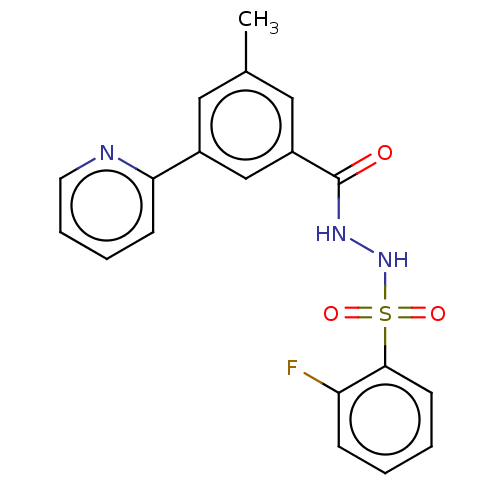 (CHEMBL4539384 | US10829446, Compound 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518832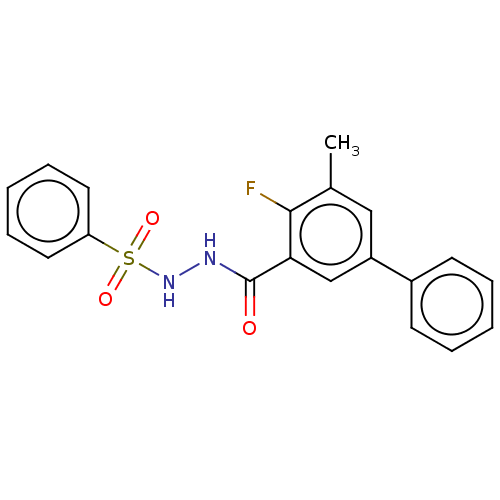 (CHEMBL4455897 | US10829446, Compound 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50339777 ((R)-N-(7-(4-((4'-chlorobiphenyl-2-yl)methyl)pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of wild type mBimBH3 from human Bcl-2 by solution competition assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527245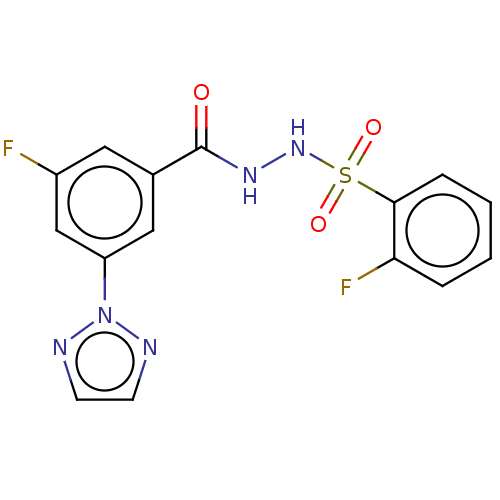 (CHEMBL4521125 | US10829446, Compound 89) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527328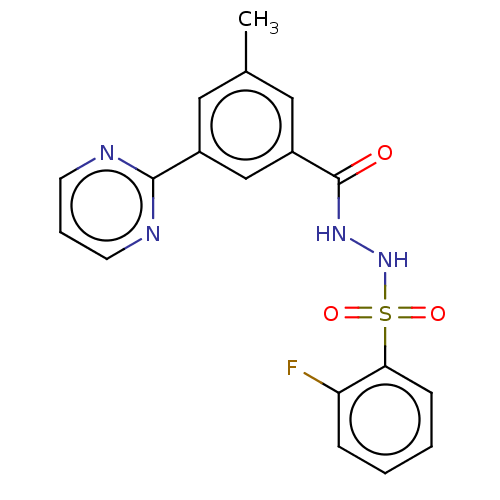 (CHEMBL4441439 | US10829446, Compound 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527225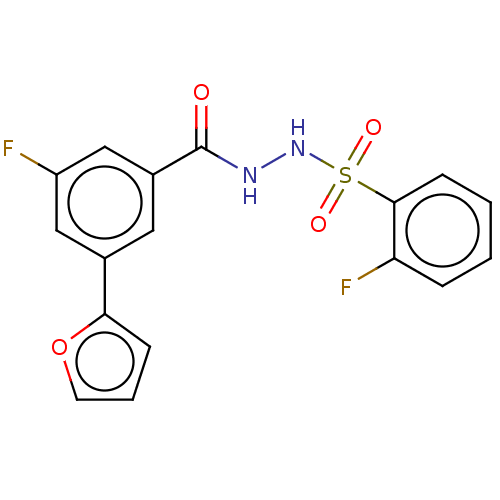 (CHEMBL4565790 | US10829446, Compound 101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50518831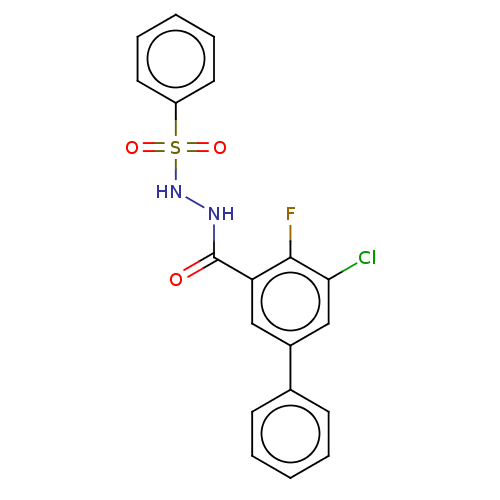 (CHEMBL4464835 | US10829446, Compound 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Cancer Therapeutics CRC Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 62: 7146-7159 (2019) Article DOI: 10.1021/acs.jmedchem.9b00665 BindingDB Entry DOI: 10.7270/Q26D5XCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50436679 (CHEMBL2398250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Binding affinity to human GST-tagged Bcl-Xl by luminescence proximity assay | J Med Chem 56: 5514-40 (2014) Article DOI: 10.1021/jm400556w BindingDB Entry DOI: 10.7270/Q2X92CQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436 (Homo sapiens (Human)) | BDBM50277889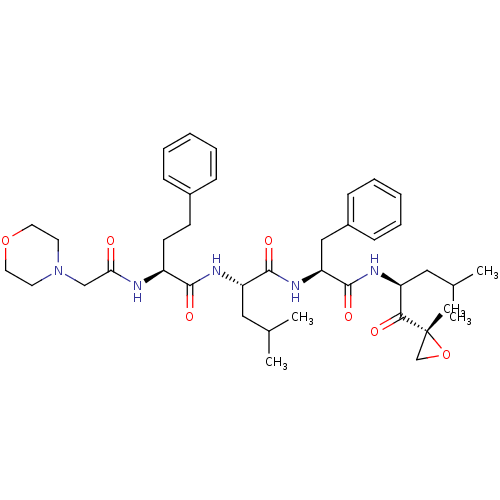 (CARFILZOMIB | CHEMBL451887) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of 20S proteasome activity in human ANBL-6 cells | J Med Chem 61: 7448-7470 (2018) Article DOI: 10.1021/acs.jmedchem.8b00318 BindingDB Entry DOI: 10.7270/Q23T9KVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT6A [507-778] (Homo sapiens) | BDBM50527243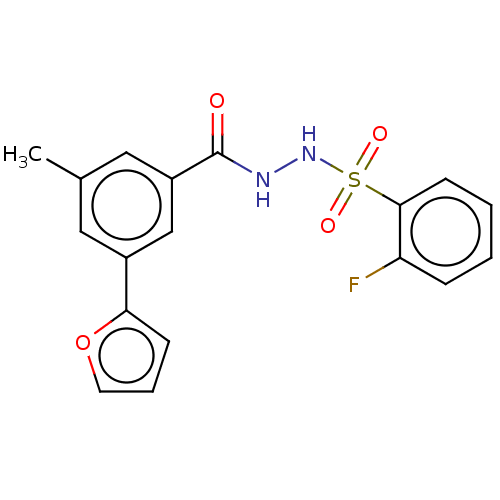 (CHEMBL4458272 | US10829446, Compound 153) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ... | J Med Chem 63: 4655-4684 (2020) Article DOI: 10.1021/acs.jmedchem.9b02071 BindingDB Entry DOI: 10.7270/Q2W66Q7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50339781 (CHEMBL1689143 | N-(7-{4-[2-(4-Chloro-phenyl)-5,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of wild type mBimBH3 from human Bcl-2 by solution competition assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50339779 (CHEMBL1689141 | N-{7-[4-(4'-Chloro-biphenyl-2-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Walter and Eliza Hall Institute of Medical Research Curated by ChEMBL | Assay Description Displacement of wild type mBimBH3 from human Bcl-2 by solution competition assay | J Med Chem 54: 1914-26 (2011) Article DOI: 10.1021/jm101596e BindingDB Entry DOI: 10.7270/Q27P8ZP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform Bcl-X(L) of Bcl-2-like protein 1 (Bcl-xL) (Homo sapiens (Human)) | BDBM109233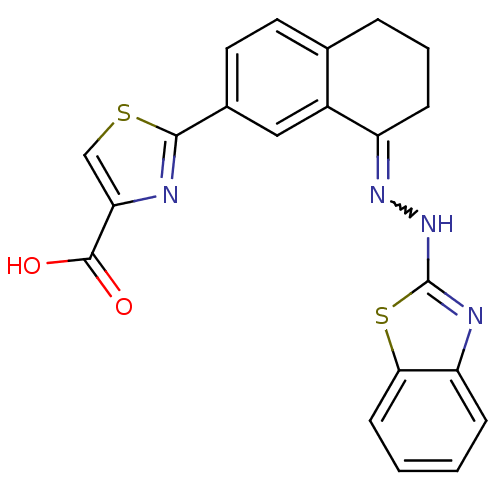 (2-(8-(2-(Benzothiazol-2-yl)hydrazono)-5,6,7,8-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia | Assay Description The protocols for tight and weak binders were described in Biacore S51 assay set-up with a flow rate of 30 µL/min. For His-Bcl-2 protein assays... | Nat Chem Biol 9: 390-7 (2013) Article DOI: 10.1038/nchembio.1246 BindingDB Entry DOI: 10.7270/Q2QJ7FZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1096 total ) | Next | Last >> |