 Found 164 hits with Last Name = 'reymond' and Initial = 'jl'
Found 164 hits with Last Name = 'reymond' and Initial = 'jl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50296742

(1,4-diazabicyclo[3.2.2]nonane-(4-bromo)-phenyl car...)Show SMILES Brc1ccc(OC(=O)N2CCN3CCC2CC3)cc1 |TLB:6:8:13.12:15.16,(1.51,4.96,;.33,3.96,;.6,2.44,;-.59,1.45,;-2.03,1.98,;-3.21,.98,;-4.67,1.51,;-4.94,3.03,;-5.85,.51,;-5.48,-.96,;-6.49,-.24,;-7.87,-.31,;-7.95,1.4,;-7.33,2.56,;-7.26,1.14,;-8.56,.43,;-8.86,-1.01,;-2.31,3.49,;-1.14,4.49,)| Show InChI InChI=1S/C14H17BrN2O2/c15-11-1-3-13(4-2-11)19-14(18)17-10-9-16-7-5-12(17)6-8-16/h1-4,12H,5-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha7 nAChR |
ACS Med Chem Lett 1: 422-426 (2010)
Article DOI: 10.1021/ml100125f
BindingDB Entry DOI: 10.7270/Q29S1RB9 |
More data for this
Ligand-Target Pair | |
Natural resistance-associated macrophage protein 2
(Homo sapiens (Human)) | BDBM50387726

(CHEMBL2058878)Show InChI InChI=1S/C16H16N4OS2/c17-15(18)22-7-9-3-1-5-11-12-6-2-4-10(8-23-16(19)20)14(12)21-13(9)11/h1-6H,7-8H2,(H3,17,18)(H3,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human DMT1 expressed in HEK293T cells by measuring inhibition of radiolabeled-55Fe2+ uptake by Dixon plot analysis |
Citation and Details
Article DOI: 10.1039/d0md00085j
BindingDB Entry DOI: 10.7270/Q2J96B1J |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM50327886
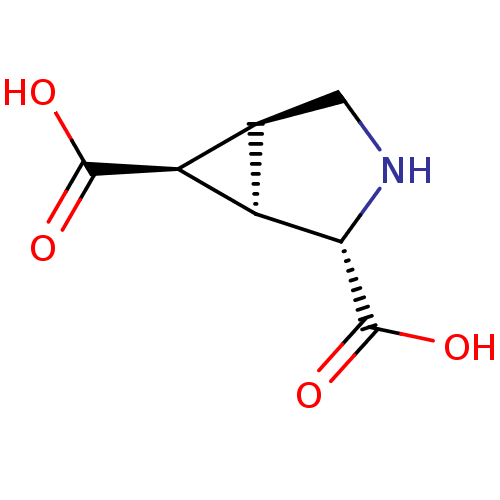
((1R,2S,5S,6S)-3-azabicyclo[3.1.0]hexane-2,6-dicarb...)Show SMILES OC(=O)[C@H]1[C@H]2CN[C@@H]([C@@H]12)C(O)=O |r| Show InChI InChI=1S/C7H9NO4/c9-6(10)4-2-1-8-5(3(2)4)7(11)12/h2-5,8H,1H2,(H,9,10)(H,11,12)/t2-,3+,4-,5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM50197105

((2S,1'S,2'R)-2-(2'-Carboxycyclobutyl)glycine | CHE...)Show InChI InChI=1S/C7H11NO4/c8-5(7(11)12)3-1-2-4(3)6(9)10/h3-5H,1-2,8H2,(H,9,10)(H,11,12)/t3-,4+,5-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM50327885
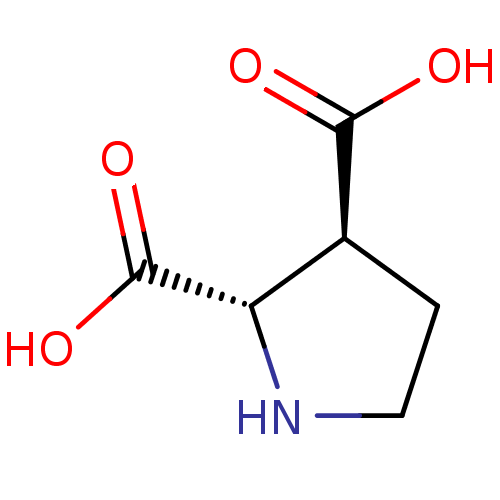
((2S,3S)-pyrrolidine-2,3-dicarboxylic acid | CHEMBL...)Show InChI InChI=1S/C6H9NO4/c8-5(9)3-1-2-7-4(3)6(10)11/h3-4,7H,1-2H2,(H,8,9)(H,10,11)/t3-,4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM50180145
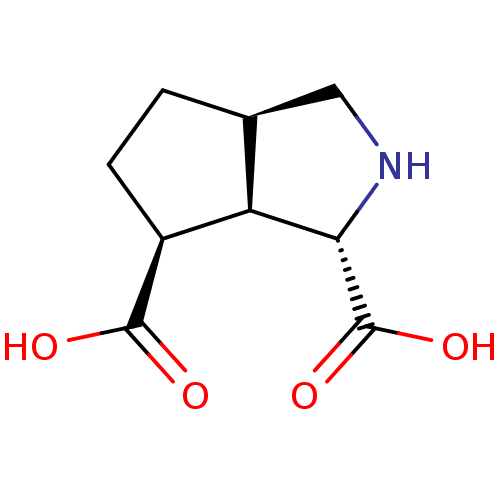
((1R,4S,5R,6S)-3-azabicyclo[3.3.0]octane-4,6-dicarb...)Show SMILES OC(=O)[C@H]1CC[C@H]2CN[C@@H]([C@@H]12)C(O)=O Show InChI InChI=1S/C9H13NO4/c11-8(12)5-2-1-4-3-10-7(6(4)5)9(13)14/h4-7,10H,1-3H2,(H,11,12)(H,13,14)/t4-,5-,6+,7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50175305
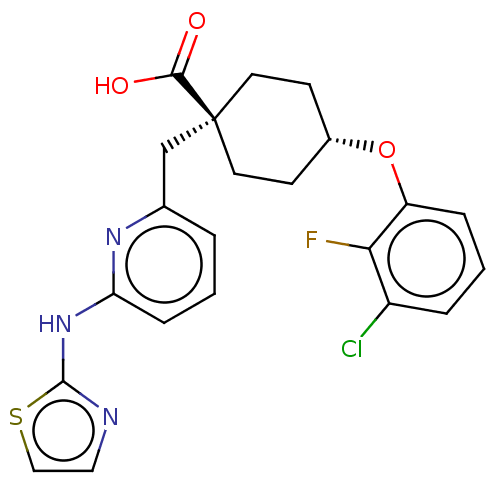
(CHEMBL3600873)Show SMILES OC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:19.24,(2.4,1.65,;1.33,2.27,;1.34,3.5,;,1.54,;-1.33,2.27,;-2.67,1.5,;-2.64,-.04,;-3.96,-.84,;-5.31,-.09,;-5.34,1.45,;-6.69,2.19,;-8,1.39,;-8.11,-.13,;-9.61,-.48,;-10.41,.83,;-9.4,2,;-4.02,2.24,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;0,-3.08,;1.34,-3.85,;2.67,-3.07,;4.01,-3.84,;4.01,-5.38,;2.68,-6.15,;2.68,-7.39,;1.34,-5.39,;.28,-6.01,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B/Inner centromere protein
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assay |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase in human HeLa Kyoto cells assessed as effect on distribution of phspho-histone H3 ser10 level incubated for 20 hrs |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase B/Inner centromere protein
(Homo sapiens (Human)) | BDBM50175305

(CHEMBL3600873)Show SMILES OC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:19.24,(2.4,1.65,;1.33,2.27,;1.34,3.5,;,1.54,;-1.33,2.27,;-2.67,1.5,;-2.64,-.04,;-3.96,-.84,;-5.31,-.09,;-5.34,1.45,;-6.69,2.19,;-8,1.39,;-8.11,-.13,;-9.61,-.48,;-10.41,.83,;-9.4,2,;-4.02,2.24,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;0,-3.08,;1.34,-3.85,;2.67,-3.07,;4.01,-3.84,;4.01,-5.38,;2.68,-6.15,;2.68,-7.39,;1.34,-5.39,;.28,-6.01,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15-,22- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assay |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198027
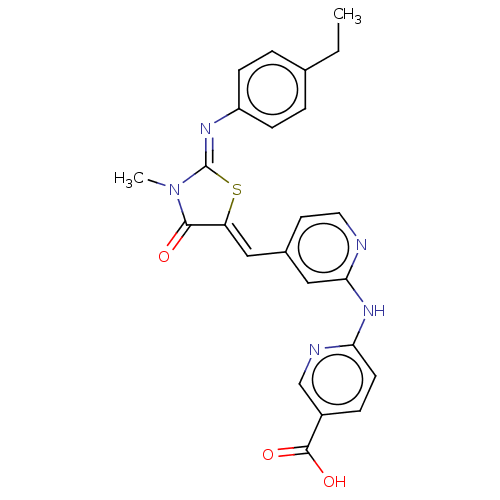
(CHEMBL3921246)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H21N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-14H,3H2,1-2H3,(H,31,32)(H,25,26,28)/b19-12-,27-24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198027

(CHEMBL3921246)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H21N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-14H,3H2,1-2H3,(H,31,32)(H,25,26,28)/b19-12-,27-24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198081
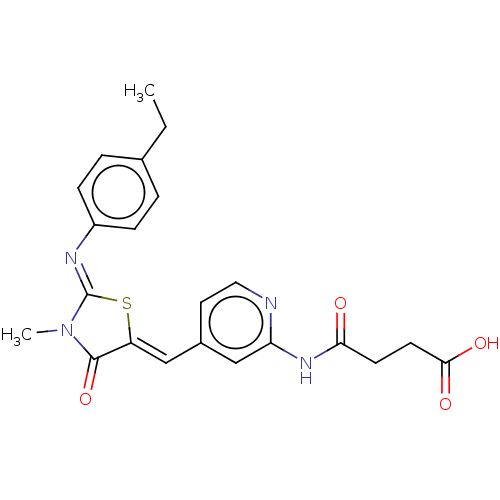
(CHEMBL3915550)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(NC(=O)CCC(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C22H22N4O4S/c1-3-14-4-6-16(7-5-14)24-22-26(2)21(30)17(31-22)12-15-10-11-23-18(13-15)25-19(27)8-9-20(28)29/h4-7,10-13H,3,8-9H2,1-2H3,(H,28,29)(H,23,25,27)/b17-12-,24-22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198080

(CHEMBL3932394)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(NC(=O)CC(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C21H20N4O4S/c1-3-13-4-6-15(7-5-13)23-21-25(2)20(29)16(30-21)10-14-8-9-22-17(11-14)24-18(26)12-19(27)28/h4-11H,3,12H2,1-2H3,(H,27,28)(H,22,24,26)/b16-10-,23-21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198082

(CHEMBL3904239)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cc3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C25H22N4O3S/c1-3-16-4-8-20(9-5-16)28-25-29(2)23(30)21(33-25)14-17-12-13-26-22(15-17)27-19-10-6-18(7-11-19)24(31)32/h4-15H,3H2,1-2H3,(H,26,27)(H,31,32)/b21-14-,28-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198086
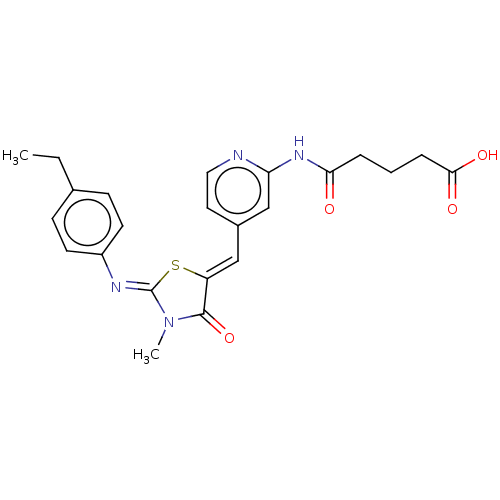
(CHEMBL3985313)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(NC(=O)CCCC(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C23H24N4O4S/c1-3-15-7-9-17(10-8-15)25-23-27(2)22(31)18(32-23)13-16-11-12-24-19(14-16)26-20(28)5-4-6-21(29)30/h7-14H,3-6H2,1-2H3,(H,29,30)(H,24,26,28)/b18-13-,25-23- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50277545

(4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...)Show SMILES COc1cc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2OC)ccc1C(O)=O |c:11| Show InChI InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase autophosphorylation at Thr288 in human HeLa Kyoto cells incubated for 20 hrs |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50175305

(CHEMBL3600873)Show SMILES OC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:19.24,(2.4,1.65,;1.33,2.27,;1.34,3.5,;,1.54,;-1.33,2.27,;-2.67,1.5,;-2.64,-.04,;-3.96,-.84,;-5.31,-.09,;-5.34,1.45,;-6.69,2.19,;-8,1.39,;-8.11,-.13,;-9.61,-.48,;-10.41,.83,;-9.4,2,;-4.02,2.24,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;0,-3.08,;1.34,-3.85,;2.67,-3.07,;4.01,-3.84,;4.01,-5.38,;2.68,-6.15,;2.68,-7.39,;1.34,-5.39,;.28,-6.01,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15-,22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B kinase in human HeLa Kyoto cells assessed as effect on distribution of phspho-histone H3 ser10 level incubated for 20 hrs |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 2
(Homo sapiens (Human)) | BDBM50327884
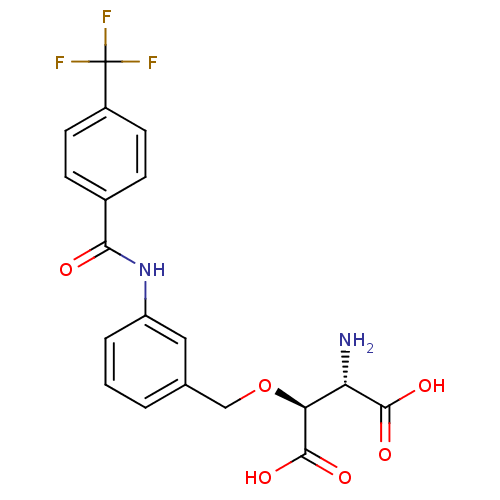
((2S,3S)-2-amino-3-(3-(4-(trifluoromethyl)benzamido...)Show SMILES N[C@@H]([C@H](OCc1cccc(NC(=O)c2ccc(cc2)C(F)(F)F)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H17F3N2O6/c20-19(21,22)12-6-4-11(5-7-12)16(25)24-13-3-1-2-10(8-13)9-30-15(18(28)29)14(23)17(26)27/h1-8,14-15H,9,23H2,(H,24,25)(H,26,27)(H,28,29)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human GLT1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198052

(CHEMBL3896597)Show SMILES CCc1ccc(cc1)\N=C1/SC(Cc2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H23N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-11,13-14,19H,3,12H2,1-2H3,(H,31,32)(H,25,26,28)/b27-24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198052

(CHEMBL3896597)Show SMILES CCc1ccc(cc1)\N=C1/SC(Cc2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H23N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-11,13-14,19H,3,12H2,1-2H3,(H,31,32)(H,25,26,28)/b27-24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase autophosphorylation at Thr288 in human HeLa Kyoto cells incubated for 20 hrs |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198044

(CHEMBL3903187)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cc3)-c3nnn[nH]3)c2)C(=O)N1C Show InChI InChI=1S/C25H22N8OS/c1-3-16-4-8-20(9-5-16)28-25-33(2)24(34)21(35-25)14-17-12-13-26-22(15-17)27-19-10-6-18(7-11-19)23-29-31-32-30-23/h4-15H,3H2,1-2H3,(H,26,27)(H,29,30,31,32)/b21-14-,28-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50502759
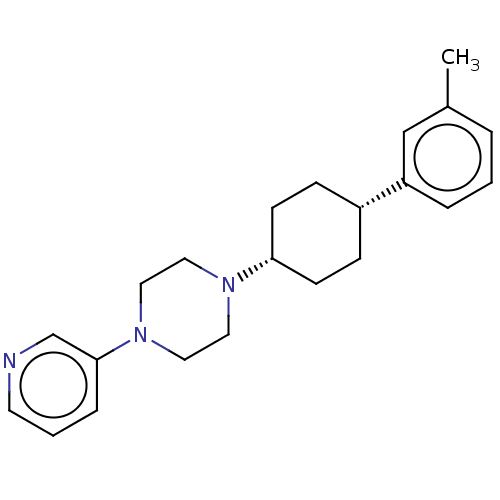
(CHEMBL4435596)Show SMILES Cc1cccc(c1)[C@@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccnc1 |r,wU:10.14,7.7,(75.17,-12.91,;75.14,-14.45,;76.48,-15.26,;76.44,-16.8,;75.09,-17.54,;73.77,-16.74,;73.79,-15.2,;72.43,-17.48,;71.1,-16.68,;69.74,-17.42,;69.73,-18.96,;71.04,-19.77,;72.39,-19.02,;68.39,-19.71,;68.36,-21.25,;67.02,-22,;65.7,-21.2,;65.7,-19.66,;67.06,-18.91,;64.36,-21.96,;64.35,-23.5,;63.01,-24.26,;61.67,-23.47,;61.69,-21.93,;63.03,-21.17,)| Show InChI InChI=1S/C22H29N3/c1-18-4-2-5-20(16-18)19-7-9-21(10-8-19)24-12-14-25(15-13-24)22-6-3-11-23-17-22/h2-6,11,16-17,19,21H,7-10,12-15H2,1H3/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550080
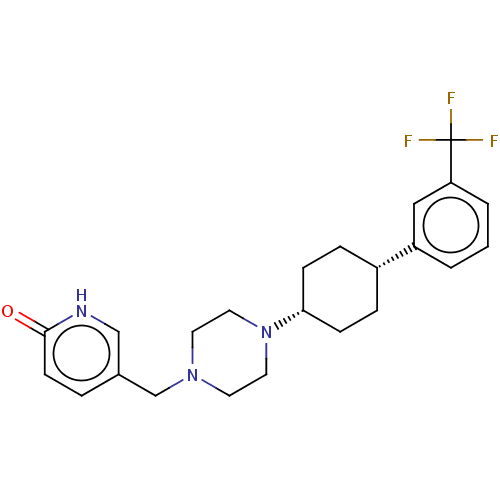
(CHEMBL4796516)Show SMILES FC(F)(F)c1cccc(c1)[C@@H]1CC[C@@H](CC1)N1CCN(Cc2ccc(=O)[nH]c2)CC1 |r,wU:13.17,10.10,(22.93,-9.55,;21.61,-8.77,;21.62,-7.24,;22.93,-7.99,;20.27,-9.53,;20.26,-11.08,;18.92,-11.83,;17.6,-11.05,;17.62,-9.52,;18.95,-8.76,;16.3,-8.74,;16.31,-7.19,;14.98,-6.41,;13.65,-7.17,;13.63,-8.71,;14.95,-9.5,;12.32,-6.39,;10.97,-7.15,;9.64,-6.38,;9.66,-4.83,;8.32,-4.05,;6.98,-4.82,;5.65,-4.03,;4.32,-4.82,;4.32,-6.36,;2.98,-7.13,;5.65,-7.12,;6.98,-6.36,;10.99,-4.06,;12.33,-4.85,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550078

(CHEMBL4777551)Show SMILES O[C@]1(CC[C@@H](CC1)N1CCN(Cc2ccc(=O)[nH]c2)CC1)c1cccc(c1)C(F)(F)F |r,wU:4.7,1.23,(66.55,-8.01,;65.22,-8.79,;65.24,-7.25,;63.91,-6.46,;62.58,-7.22,;62.56,-8.77,;63.88,-9.55,;61.24,-6.45,;59.9,-7.21,;58.57,-6.43,;58.58,-4.88,;57.25,-4.1,;55.91,-4.87,;54.57,-4.09,;53.25,-4.87,;53.25,-6.42,;51.91,-7.19,;54.57,-7.18,;55.91,-6.42,;59.91,-4.11,;61.25,-4.9,;66.55,-9.57,;66.53,-11.1,;67.85,-11.88,;69.19,-11.13,;69.2,-9.58,;67.87,-8.81,;70.53,-8.82,;71.86,-9.6,;70.54,-7.29,;71.85,-8.04,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550077
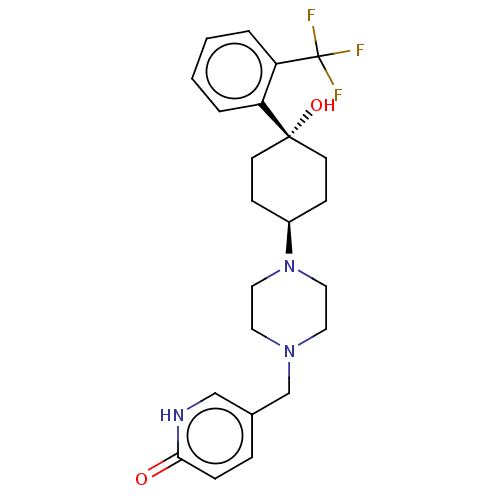
(CHEMBL4789936)Show SMILES O[C@]1(CC[C@@H](CC1)N1CCN(Cc2ccc(=O)[nH]c2)CC1)c1ccccc1C(F)(F)F |r,wU:4.7,1.23,(18.72,-20.47,;17.4,-21.24,;17.41,-19.7,;16.08,-18.91,;14.75,-19.68,;14.73,-21.22,;16.05,-22,;13.42,-18.9,;12.07,-19.66,;10.74,-18.89,;10.76,-17.33,;9.42,-16.55,;8.08,-17.32,;6.75,-16.54,;5.42,-17.32,;5.42,-18.87,;4.09,-19.64,;6.75,-19.63,;8.08,-18.87,;12.09,-16.57,;13.43,-17.35,;18.72,-22.03,;18.7,-23.56,;20.02,-24.34,;21.36,-23.58,;21.37,-22.04,;20.05,-21.26,;20.05,-19.73,;21.39,-18.96,;18.73,-18.95,;20.04,-18.19,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198045
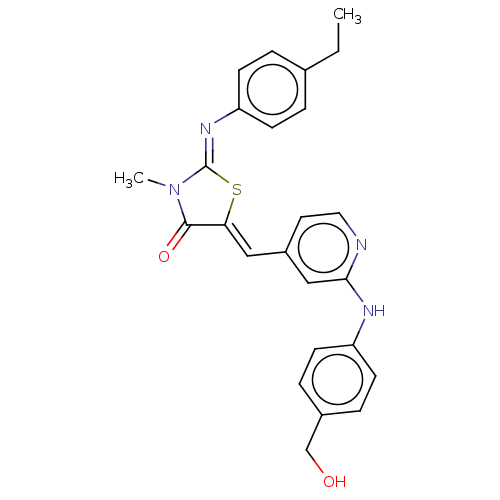
(CHEMBL3975102)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(CO)cc3)c2)C(=O)N1C Show InChI InChI=1S/C25H24N4O2S/c1-3-17-4-8-21(9-5-17)28-25-29(2)24(31)22(32-25)14-19-12-13-26-23(15-19)27-20-10-6-18(16-30)7-11-20/h4-15,30H,3,16H2,1-2H3,(H,26,27)/b22-14-,28-25- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Natural resistance-associated macrophage protein 2
(Homo sapiens (Human)) | BDBM50387726

(CHEMBL2058878)Show InChI InChI=1S/C16H16N4OS2/c17-15(18)22-7-9-3-1-5-11-12-6-2-4-10(8-23-16(19)20)14(12)21-13(9)11/h1-6H,7-8H2,(H3,17,18)(H3,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DMT1 expressed in Xenopus oocytes assessed as inhibition of channel currents at -50 mV holding potential by two-electrode voltage... |
Citation and Details
Article DOI: 10.1039/d0md00085j
BindingDB Entry DOI: 10.7270/Q2J96B1J |
More data for this
Ligand-Target Pair | |
Aurora kinase B/Inner centromere protein
(Homo sapiens (Human)) | BDBM50198027

(CHEMBL3921246)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccc(cn3)C(O)=O)c2)C(=O)N1C Show InChI InChI=1S/C24H21N5O3S/c1-3-15-4-7-18(8-5-15)27-24-29(2)22(30)19(33-24)12-16-10-11-25-21(13-16)28-20-9-6-17(14-26-20)23(31)32/h4-14H,3H2,1-2H3,(H,31,32)(H,25,26,28)/b19-12-,27-24- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B/INCENP (unknown origin) using biotinylated STK2 substrate incubated for 30 mins by HTRF assay |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550081
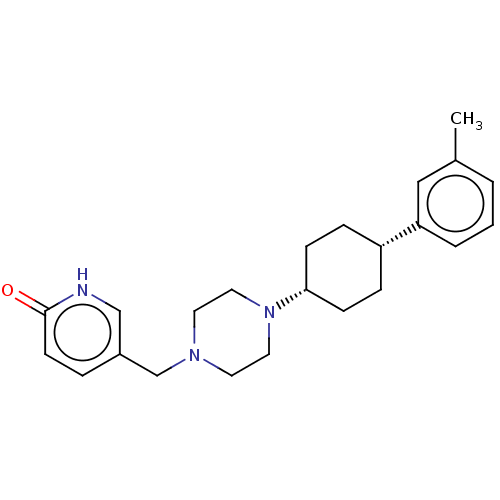
(CHEMBL4740003)Show SMILES Cc1cccc(c1)[C@@H]1CC[C@@H](CC1)N1CCN(Cc2ccc(=O)[nH]c2)CC1 |r,wU:10.14,7.7,(68.54,-46.75,;67.21,-47.51,;67.2,-49.06,;65.86,-49.81,;64.54,-49.03,;64.56,-47.5,;65.88,-46.74,;63.24,-46.72,;63.25,-45.18,;61.92,-44.39,;60.59,-45.16,;60.57,-46.7,;61.89,-47.48,;59.25,-44.38,;57.91,-45.14,;56.58,-44.36,;56.59,-42.81,;55.26,-42.03,;53.92,-42.8,;52.58,-42.02,;51.26,-42.8,;51.26,-44.35,;49.92,-45.12,;52.58,-45.11,;53.92,-44.35,;57.92,-42.04,;59.26,-42.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550082
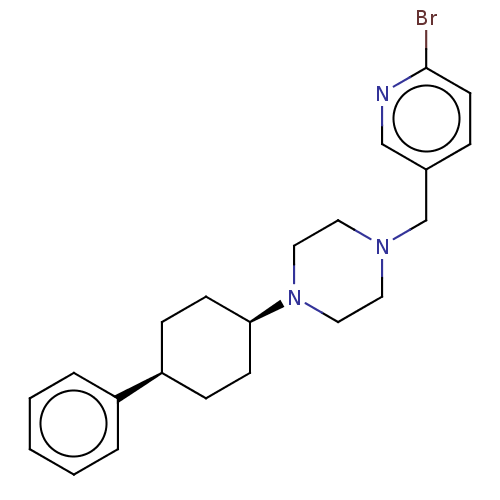
(CHEMBL4760109)Show SMILES Brc1ccc(CN2CCN(CC2)[C@H]2CC[C@H](CC2)c2ccccc2)cn1 |r,wU:12.12,15.19,(23.99,-43.45,;25.32,-42.69,;25.32,-41.14,;26.66,-40.37,;27.99,-41.15,;29.33,-40.38,;30.66,-41.16,;30.65,-42.71,;31.98,-43.48,;33.32,-42.72,;33.33,-41.18,;31.99,-40.39,;34.65,-43.5,;35.98,-42.74,;37.31,-43.52,;37.29,-45.06,;35.95,-45.82,;34.63,-45.04,;38.61,-45.84,;38.59,-47.37,;39.91,-48.15,;41.25,-47.39,;41.26,-45.85,;39.94,-45.08,;28,-42.69,;26.66,-43.46,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198078

(CHEMBL3941377)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(N)c2)C(=O)N1C Show InChI InChI=1S/C18H18N4OS/c1-3-12-4-6-14(7-5-12)21-18-22(2)17(23)15(24-18)10-13-8-9-20-16(19)11-13/h4-11H,3H2,1-2H3,(H2,19,20)/b15-10-,21-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198079
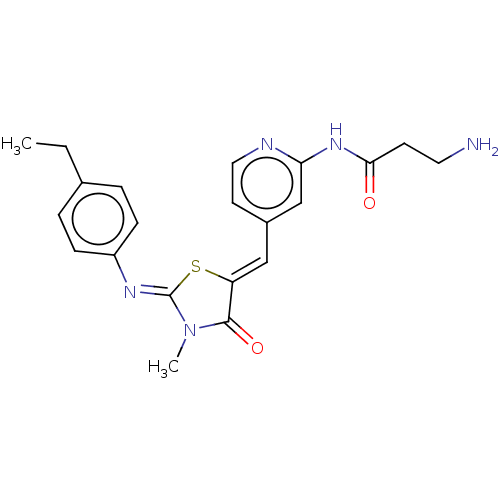
(CHEMBL3924918)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(NC(=O)CCN)c2)C(=O)N1C Show InChI InChI=1S/C21H23N5O2S/c1-3-14-4-6-16(7-5-14)24-21-26(2)20(28)17(29-21)12-15-9-11-23-18(13-15)25-19(27)8-10-22/h4-7,9,11-13H,3,8,10,22H2,1-2H3,(H,23,25,27)/b17-12-,24-21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
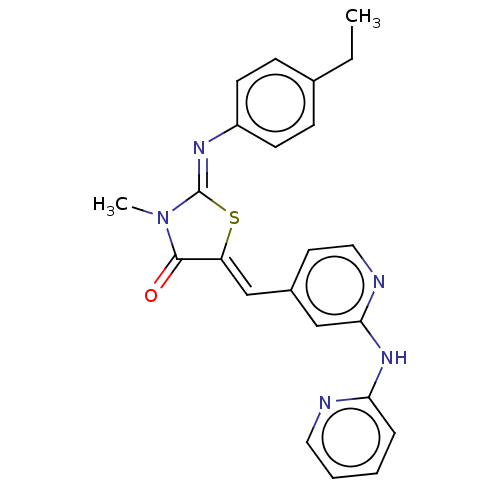
(Homo sapiens (Human)) | BDBM50198084
(CHEMBL3913242)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccnc(Nc3ccccn3)c2)C(=O)N1C Show InChI InChI=1S/C23H21N5OS/c1-3-16-7-9-18(10-8-16)26-23-28(2)22(29)19(30-23)14-17-11-13-25-21(15-17)27-20-6-4-5-12-24-20/h4-15H,3H2,1-2H3,(H,24,25,27)/b19-14-,26-23- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Fucose-binding lectin PA-IIL
(Pseudomonas aeruginosa) | BDBM50509063
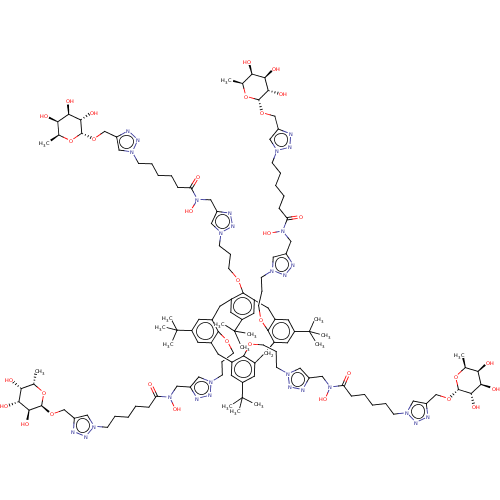
(CHEMBL4515649)Show SMILES C[C@@H]1O[C@@H](OCc2cn(CCCCCC(=O)N(O)Cc3cn(CCCOc4c5Cc6cc(cc(Cc7cc(cc(Cc8cc(cc(Cc4cc(c5)C(C)(C)C)c8OCCCn4cc(CN(O)C(=O)CCCCCn5cc(CO[C@@H]8O[C@@H](C)[C@@H](O)[C@@H](O)[C@@H]8O)nn5)nn4)C(C)(C)C)c7OCCCn4cc(CN(O)C(=O)CCCCCn5cc(CO[C@@H]7O[C@@H](C)[C@@H](O)[C@@H](O)[C@@H]7O)nn5)nn4)C(C)(C)C)c6OCCCn4cc(CN(O)C(=O)CCCCCn5cc(CO[C@@H]6O[C@@H](C)[C@@H](O)[C@@H](O)[C@@H]6O)nn5)nn4)C(C)(C)C)nn3)nn2)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C128H188N28O32/c1-77-105(161)109(165)113(169)121(185-77)181-73-97-65-145(141-133-97)37-25-17-21-33-101(157)153(173)69-93-61-149(137-129-93)41-29-45-177-117-81-49-83-55-90(126(8,9)10)57-85(118(83)178-46-30-42-150-62-94(130-138-150)70-154(174)102(158)34-22-18-26-38-146-66-98(134-142-146)74-182-122-114(170)110(166)106(162)78(2)186-122)51-87-59-92(128(14,15)16)60-88(120(87)180-48-32-44-152-64-96(132-140-152)72-156(176)104(160)36-24-20-28-40-148-68-100(136-144-148)76-184-124-116(172)112(168)108(164)80(4)188-124)52-86-58-91(127(11,12)13)56-84(50-82(117)54-89(53-81)125(5,6)7)119(86)179-47-31-43-151-63-95(131-139-151)71-155(175)103(159)35-23-19-27-39-147-67-99(135-143-147)75-183-123-115(171)111(167)107(163)79(3)187-123/h53-68,77-80,105-116,121-124,161-176H,17-52,69-76H2,1-16H3/t77-,78-,79-,80-,105+,106+,107+,108+,109+,110+,111+,112+,113-,114-,115-,116-,121+,122+,123+,124+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay |
J Med Chem 62: 7722-7738 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00481
BindingDB Entry DOI: 10.7270/Q2DB8554 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50175305

(CHEMBL3600873)Show SMILES OC(=O)[C@@]1(Cc2cccc(Nc3nccs3)n2)CC[C@@H](CC1)Oc1cccc(Cl)c1F |r,wU:3.2,wD:19.24,(2.4,1.65,;1.33,2.27,;1.34,3.5,;,1.54,;-1.33,2.27,;-2.67,1.5,;-2.64,-.04,;-3.96,-.84,;-5.31,-.09,;-5.34,1.45,;-6.69,2.19,;-8,1.39,;-8.11,-.13,;-9.61,-.48,;-10.41,.83,;-9.4,2,;-4.02,2.24,;1.33,.77,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;0,-3.08,;1.34,-3.85,;2.67,-3.07,;4.01,-3.84,;4.01,-5.38,;2.68,-6.15,;2.68,-7.39,;1.34,-5.39,;.28,-6.01,)| Show InChI InChI=1S/C22H21ClFN3O3S/c23-16-4-2-5-17(19(16)24)30-15-7-9-22(10-8-15,20(28)29)13-14-3-1-6-18(26-14)27-21-25-11-12-31-21/h1-6,11-12,15H,7-10,13H2,(H,28,29)(H,25,26,27)/t15-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase autophosphorylation at Thr288 in human HeLa Kyoto cells incubated for 20 hrs |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198077
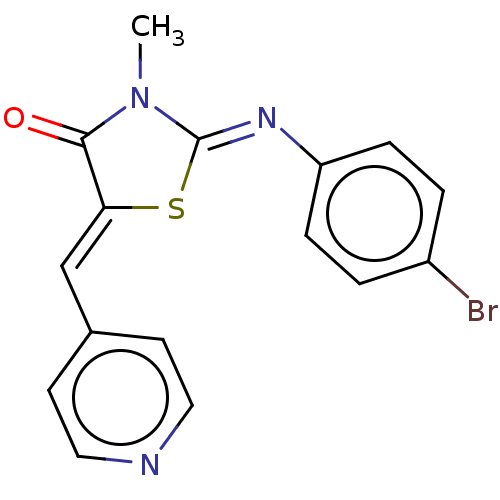
(CHEMBL3951166)Show InChI InChI=1S/C16H12BrN3OS/c1-20-15(21)14(10-11-6-8-18-9-7-11)22-16(20)19-13-4-2-12(17)3-5-13/h2-10H,1H3/b14-10-,19-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Excitatory amino acid transporter 3
(Homo sapiens (Human)) | BDBM50327884

((2S,3S)-2-amino-3-(3-(4-(trifluoromethyl)benzamido...)Show SMILES N[C@@H]([C@H](OCc1cccc(NC(=O)c2ccc(cc2)C(F)(F)F)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H17F3N2O6/c20-19(21,22)12-6-4-11(5-7-12)16(25)24-13-3-1-2-10(8-13)9-30-15(18(28)29)14(23)17(26)27/h1-8,14-15H,9,23H2,(H,24,25)(H,26,27)(H,28,29)/t14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of human EAAC1 expressed in Xenopus laevis Oocytes assessed as reduction of [3H]-glutamate uptake after 10 mins by scintillation counting |
J Med Chem 53: 7236-50 (2010)
Article DOI: 10.1021/jm100959g
BindingDB Entry DOI: 10.7270/Q27081NR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50502759

(CHEMBL4435596)Show SMILES Cc1cccc(c1)[C@@H]1CC[C@@H](CC1)N1CCN(CC1)c1cccnc1 |r,wU:10.14,7.7,(75.17,-12.91,;75.14,-14.45,;76.48,-15.26,;76.44,-16.8,;75.09,-17.54,;73.77,-16.74,;73.79,-15.2,;72.43,-17.48,;71.1,-16.68,;69.74,-17.42,;69.73,-18.96,;71.04,-19.77,;72.39,-19.02,;68.39,-19.71,;68.36,-21.25,;67.02,-22,;65.7,-21.2,;65.7,-19.66,;67.06,-18.91,;64.36,-21.96,;64.35,-23.5,;63.01,-24.26,;61.67,-23.47,;61.69,-21.93,;63.03,-21.17,)| Show InChI InChI=1S/C22H29N3/c1-18-4-2-5-20(16-18)19-7-9-21(10-8-19)24-12-14-25(15-13-24)22-6-3-11-23-17-22/h2-6,11,16-17,19,21H,7-10,12-15H2,1H3/t19-,21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bern
Curated by ChEMBL
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction of Cd2+ influx preincubated for 5 mins followed by CdCl2 addition by calciu... |
ACS Med Chem Lett 10: 1341-1345 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00298
BindingDB Entry DOI: 10.7270/Q2ZG6WGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Natural resistance-associated macrophage protein 2
(Homo sapiens (Human)) | BDBM50553222
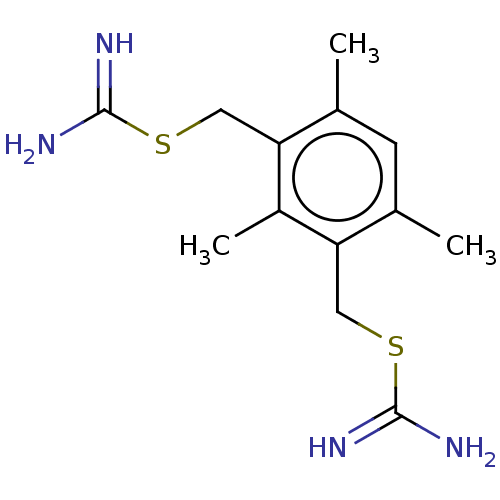
(CHEMBL1197758) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DMT1 expressed in HEK293T cells assessed as inhibition of radiolabeled 55Fe2+ uptake preincubated with compound for 5 mins follow... |
Citation and Details
Article DOI: 10.1039/d0md00085j
BindingDB Entry DOI: 10.7270/Q2J96B1J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550085
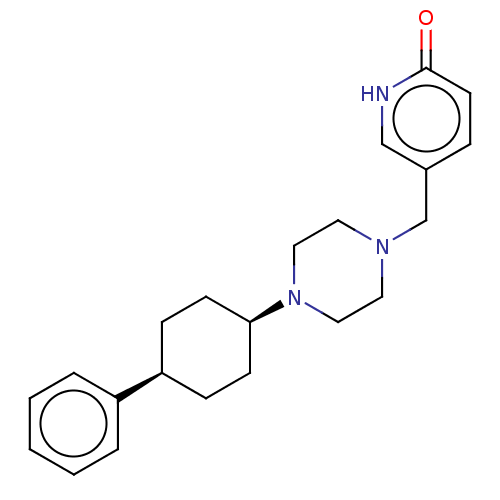
(CHEMBL4762249)Show SMILES O=c1ccc(CN2CCN(CC2)[C@H]2CC[C@H](CC2)c2ccccc2)c[nH]1 |r,wU:12.12,15.19,(4.26,-30.95,;5.59,-30.18,;5.59,-28.64,;6.91,-27.85,;8.24,-28.64,;9.58,-27.87,;10.92,-28.64,;10.9,-30.19,;12.23,-30.96,;13.57,-30.21,;13.58,-28.67,;12.24,-27.88,;14.9,-30.98,;16.23,-30.22,;17.56,-31.01,;17.54,-32.55,;16.2,-33.31,;14.88,-32.52,;18.87,-33.33,;18.84,-34.86,;20.16,-35.63,;21.5,-34.88,;21.51,-33.34,;20.19,-32.57,;8.24,-30.18,;6.91,-30.94,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198076
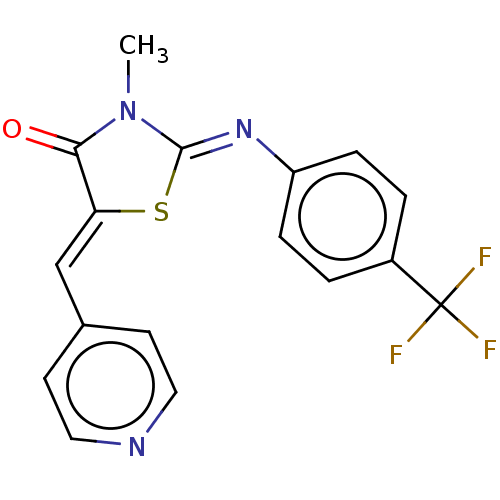
(CHEMBL3939478)Show SMILES CN1\C(S\C(=C/c2ccncc2)C1=O)=N\c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C17H12F3N3OS/c1-23-15(24)14(10-11-6-8-21-9-7-11)25-16(23)22-13-4-2-12(3-5-13)17(18,19)20/h2-10H,1H3/b14-10-,22-16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198042
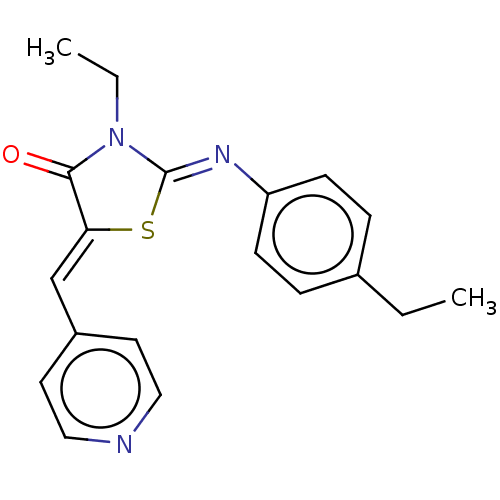
(CHEMBL3935395)Show SMILES CCN1\C(S\C(=C/c2ccncc2)C1=O)=N\c1ccc(CC)cc1 Show InChI InChI=1S/C19H19N3OS/c1-3-14-5-7-16(8-6-14)21-19-22(4-2)18(23)17(24-19)13-15-9-11-20-12-10-15/h5-13H,3-4H2,1-2H3/b17-13-,21-19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198041
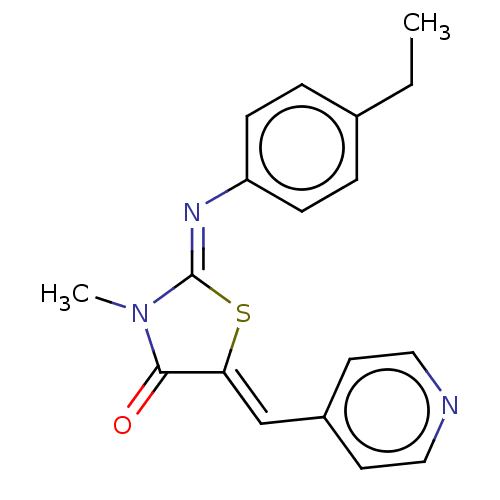
(CHEMBL3984254)Show InChI InChI=1S/C18H17N3OS/c1-3-13-4-6-15(7-5-13)20-18-21(2)17(22)16(23-18)12-14-8-10-19-11-9-14/h4-12H,3H2,1-2H3/b16-12-,20-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 4
(Homo sapiens) | BDBM96504
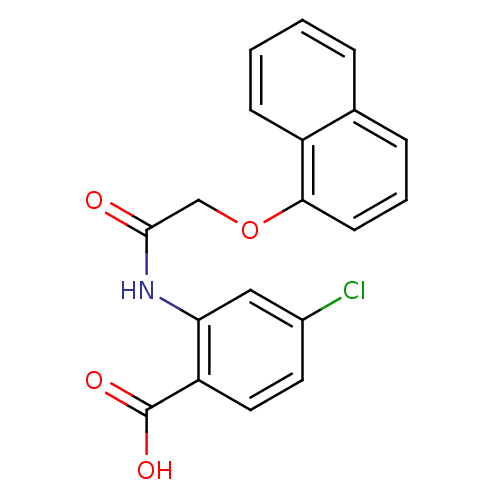
(4-chloranyl-2-(2-naphthalen-1-yloxyethanoylamino)b...)Show InChI InChI=1S/C19H14ClNO4/c20-13-8-9-15(19(23)24)16(10-13)21-18(22)11-25-17-7-3-5-12-4-1-2-6-14(12)17/h1-10H,11H2,(H,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged TRPM4 (unknown origin) expressed in HEK293 cells assessed as reduction in intracellular Na+ influx after 45 mins by ANG2 dy... |
Eur J Med Chem 166: 167-177 (2019)
Article DOI: 10.1016/j.ejmech.2019.01.048
BindingDB Entry DOI: 10.7270/Q2GH9NCB |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198152
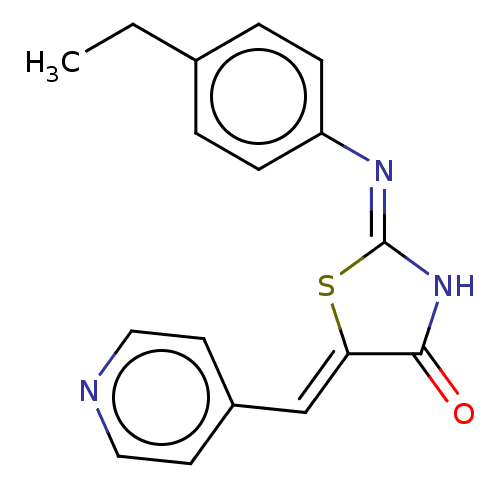
(CHEMBL3923442)Show InChI InChI=1S/C17H15N3OS/c1-2-12-3-5-14(6-4-12)19-17-20-16(21)15(22-17)11-13-7-9-18-10-8-13/h3-11H,2H2,1H3,(H,19,20,21)/b15-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 6
(Homo sapiens (Human)) | BDBM50550087
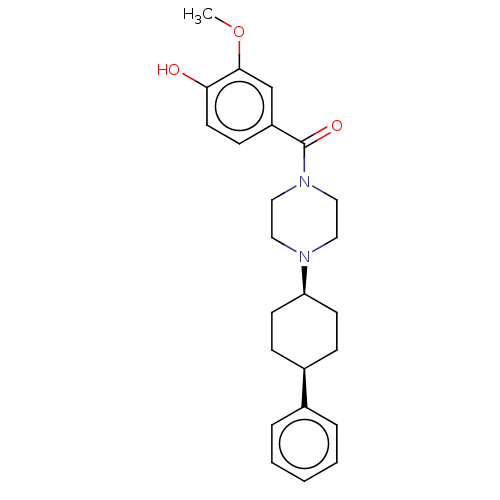
(CHEMBL4761058)Show SMILES COc1cc(ccc1O)C(=O)N1CCN(CC1)[C@H]1CC[C@H](CC1)c1ccccc1 |r,wU:17.18,20.25,(26.41,-17.33,;27.75,-16.57,;29.08,-17.34,;30.42,-16.58,;31.75,-17.36,;31.75,-18.89,;30.42,-19.66,;29.08,-18.88,;27.74,-19.66,;33.09,-16.59,;33.09,-15.06,;34.42,-17.36,;34.41,-18.91,;35.72,-19.69,;37.07,-18.93,;37.07,-17.39,;35.74,-16.61,;38.4,-19.71,;39.73,-18.94,;41.06,-19.73,;41.05,-21.27,;39.7,-22.03,;38.38,-21.25,;42.37,-22.05,;42.36,-23.6,;43.68,-24.38,;45.02,-23.62,;45.03,-22.07,;43.7,-21.3,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TRPV6 expressed in HEK293 cells assessed as reduction in Cd2+ influx by calcium-5 fluorescence dye-based FLIPR assay |
Citation and Details
Article DOI: 10.1039/d0md00145g
BindingDB Entry DOI: 10.7270/Q2FF3X0F |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50198048
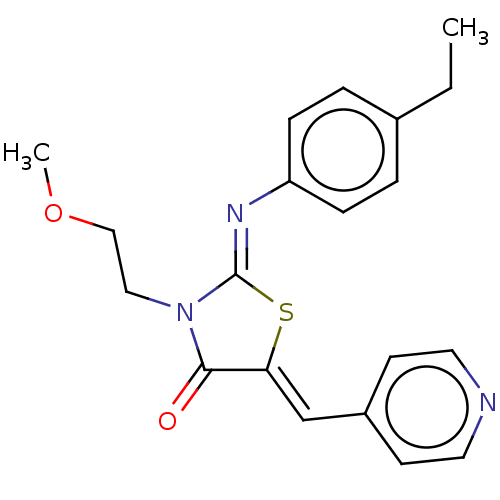
(CHEMBL3941909)Show SMILES CCc1ccc(cc1)\N=C1/S\C(=C/c2ccncc2)C(=O)N1CCOC Show InChI InChI=1S/C20H21N3O2S/c1-3-15-4-6-17(7-5-15)22-20-23(12-13-25-2)19(24)18(26-20)14-16-8-10-21-11-9-16/h4-11,14H,3,12-13H2,1-2H3/b18-14-,22-20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Berne
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human Aurora A kinase (122 to 40 residues) expressed in Escherichia coli BL21 (DE3) Rosetta cells using biotinylated STK2 su... |
J Med Chem 59: 7188-211 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00709
BindingDB Entry DOI: 10.7270/Q2JM2CM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fucose-binding lectin PA-IIL
(Pseudomonas aeruginosa) | BDBM50509060
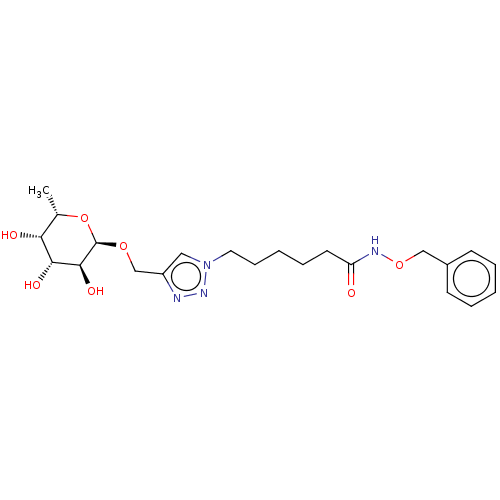
(CHEMBL4515315)Show SMILES C[C@@H]1O[C@@H](OCc2cn(CCCCCC(=O)NOCc3ccccc3)nn2)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C22H32N4O7/c1-15-19(28)20(29)21(30)22(33-15)31-14-17-12-26(25-23-17)11-7-3-6-10-18(27)24-32-13-16-8-4-2-5-9-16/h2,4-5,8-9,12,15,19-22,28-30H,3,6-7,10-11,13-14H2,1H3,(H,24,27)/t15-,19+,20+,21-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universit£ de Picardie Jules Verne
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LecB by fluorescence polarization assay |
J Med Chem 62: 7722-7738 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00481
BindingDB Entry DOI: 10.7270/Q2DB8554 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data