

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against Dopamine receptor D2 binding site using radioligand [3H]spiperone | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against 5-hydroxytryptamine 1A receptor binding site using radioligand [3H]8-OH-DPAT | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017514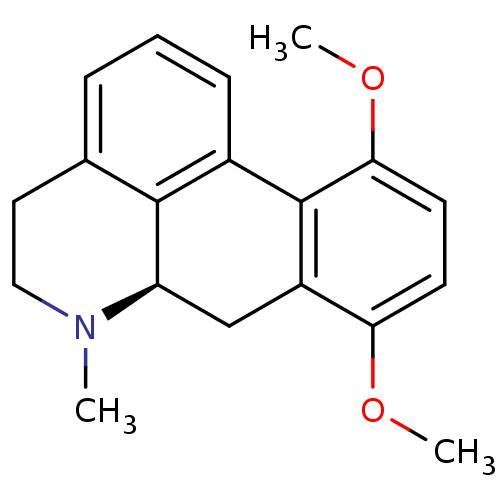 (8,11-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dib...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against 5-hydroxytryptamine 1A receptor binding site using radioligand [3H]8-OH-DPAT | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against 5-hydroxytryptamine 1A receptor binding site using radioligand [3H]8-OH-DPAT | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017514 (8,11-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against Dopamine receptor D2 binding site using radioligand [3H]spiperone | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017513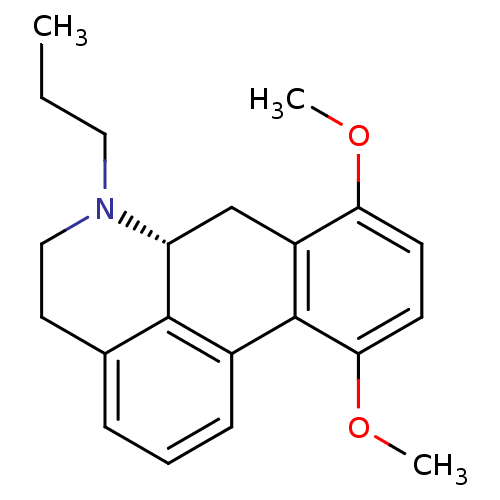 (8,11-Dimethoxy-6-propyl-5,6,6a,7-tetrahydro-4H-dib...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against 5-hydroxytryptamine 1A receptor binding site using radioligand [3H]8-OH-DPAT | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017513 (8,11-Dimethoxy-6-propyl-5,6,6a,7-tetrahydro-4H-dib...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against Dopamine receptor D2 binding site using radioligand [3H]spiperone | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
College of Pharmacy University of Iowa Curated by ChEMBL | Assay Description Binding affinity of compound against Dopamine receptor D2 binding site using radioligand [3H]spiperone | J Med Chem 32: 1959-62 (1989) BindingDB Entry DOI: 10.7270/Q237799C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50368271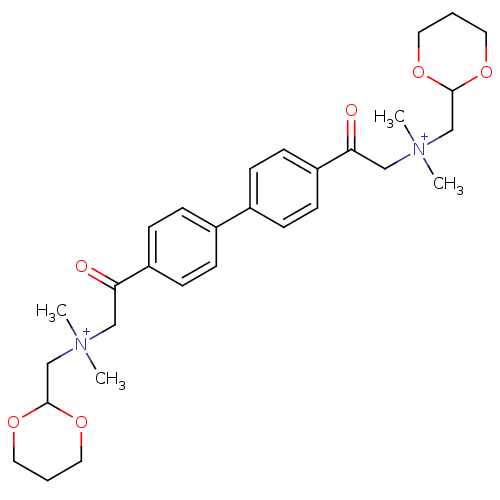 (CHEMBL1204112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of Acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50313079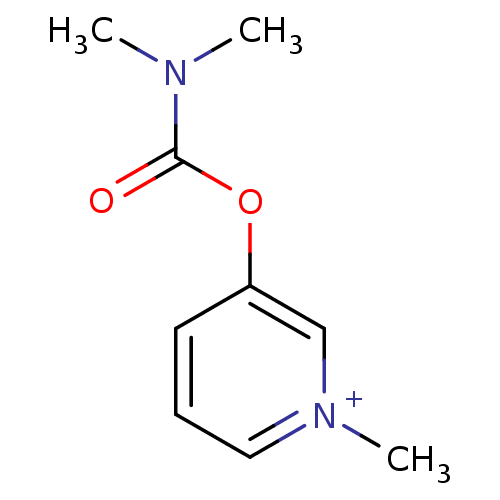 (3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50224035 (79907 | Lergotrile) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50223956 (CHEMBL555412) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50368269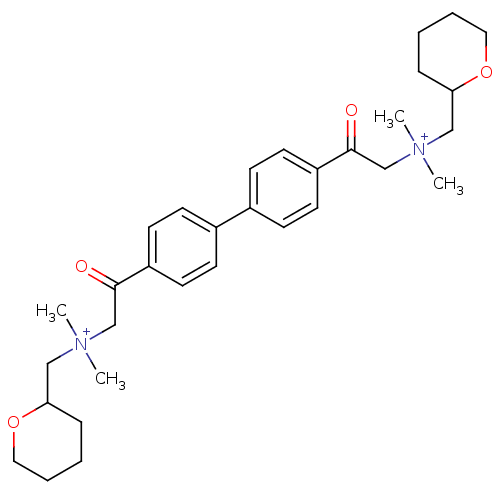 (CHEMBL1202156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536287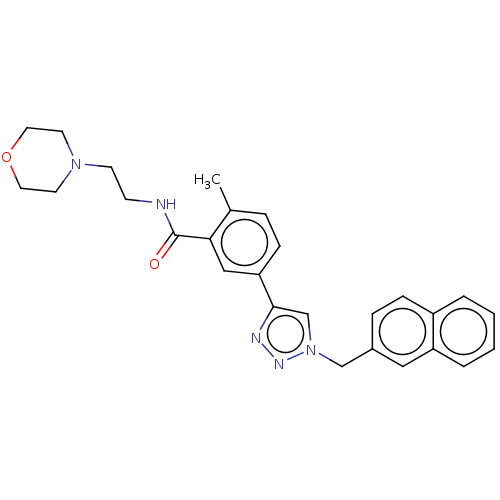 (CHEMBL4581664) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]spiperone as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50368268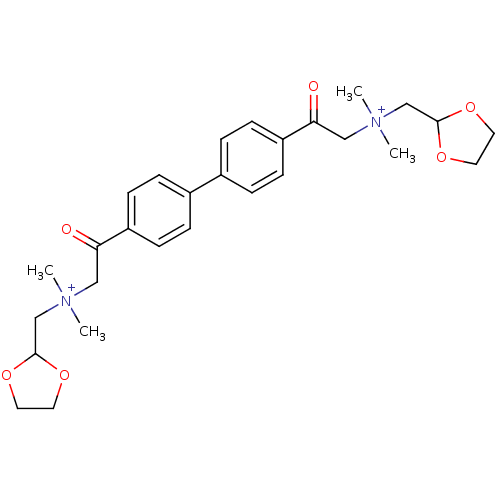 (CHEMBL1202155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50223901 (CHEMBL544695) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50223957 (CHEMBL544931) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]ADTN as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50107876 ((5,6-Dimethoxy-indan-2-yl)-dipropyl-amine | CHEMBL...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]spiperone as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536290 (CHEMBL4525497) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536298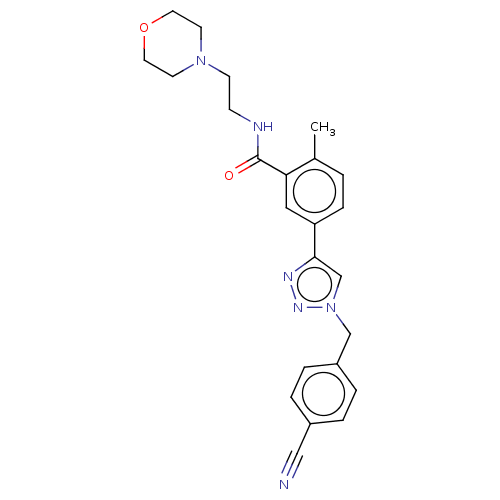 (CHEMBL4551175) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50368272 (CHEMBL1202157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536299 (CHEMBL4525580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50290378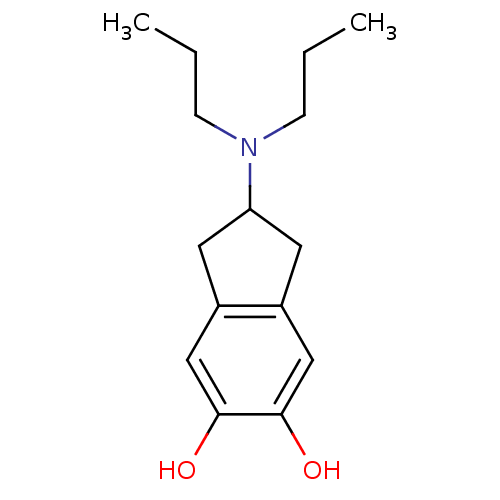 (2-Dipropylamino-indan-5,6-diol | CHEMBL277743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]spiperone as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50012004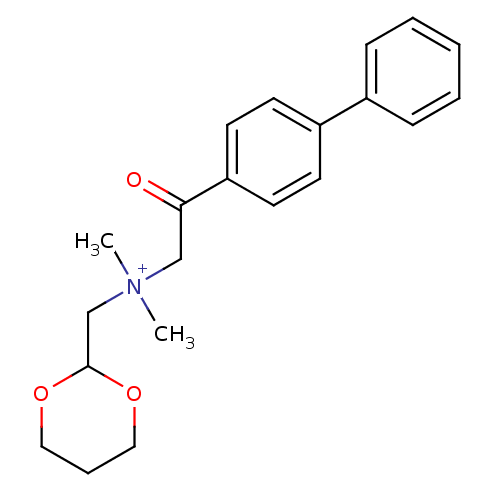 ((2-Biphenyl-4-yl-2-oxo-ethyl)-[1,3]dioxan-2-ylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536288 (CHEMBL4561751) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536294 (CHEMBL4581887) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536296 (CHEMBL4578074) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50224035 (79907 | Lergotrile) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]spiperone as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536291 (CHEMBL4513605) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50368270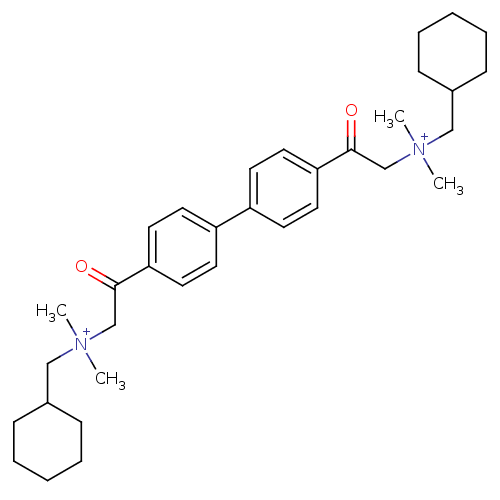 (CHEMBL1202154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa Curated by ChEMBL | Assay Description Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% | J Med Chem 34: 1582-4 (1991) BindingDB Entry DOI: 10.7270/Q26D5TK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536306 (CHEMBL4513836) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536293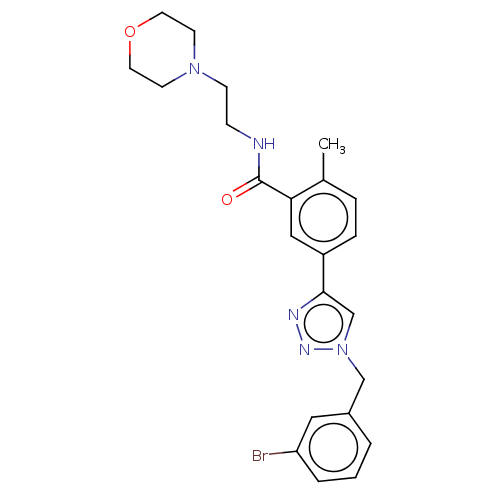 (CHEMBL4524162) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(2) dopamine receptor (BOVINE) | BDBM50223959 (CHEMBL538227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory binding activity against dopamine receptor using [3H]spiperone as the radioligand in striatal tissue of calf brain. | J Med Chem 25: 1442-6 (1982) BindingDB Entry DOI: 10.7270/Q2XD13WD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536292 (CHEMBL4536296) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536295 (CHEMBL4516485) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536285 (CHEMBL4521556) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536286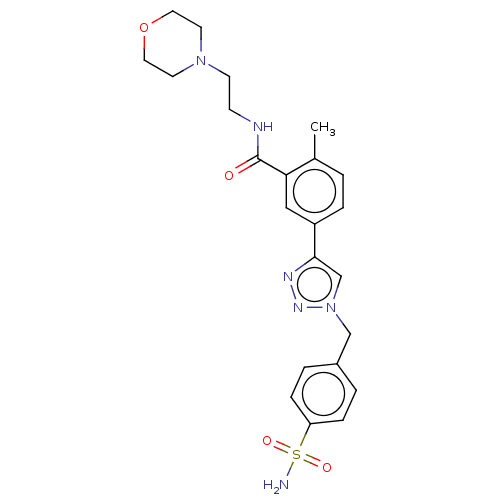 (CHEMBL4522818) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536307 (CHEMBL4541730) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536309 (CHEMBL4576458) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536303 (CHEMBL4531267) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536305 (CHEMBL4584351) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536307 (CHEMBL4541730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50536292 (CHEMBL4536296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE preincubated for 6 mins followed by addition of S-butyrylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50536304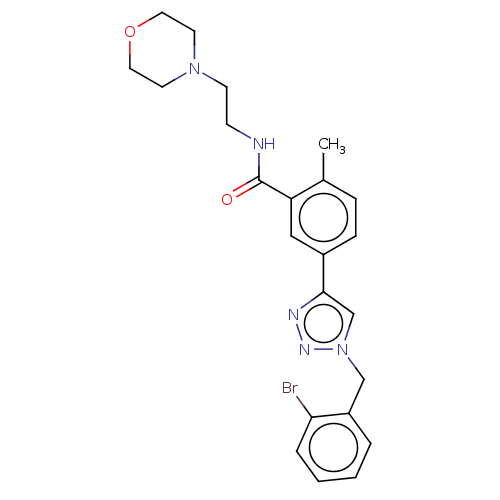 (CHEMBL4530986) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan Curated by ChEMBL | Assay Description Inhibition of electric eel AChE preincubated for 6 mins followed by addition of acetylthiocholine iodide by Ellman's method | Bioorg Med Chem Lett 26: 3881-5 (2016) Article DOI: 10.1016/j.bmcl.2016.07.017 BindingDB Entry DOI: 10.7270/Q2KW5KJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 103 total ) | Next | Last >> |