 Found 68 hits with Last Name = 'hoffmaster' and Initial = 'k'
Found 68 hits with Last Name = 'hoffmaster' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50227631
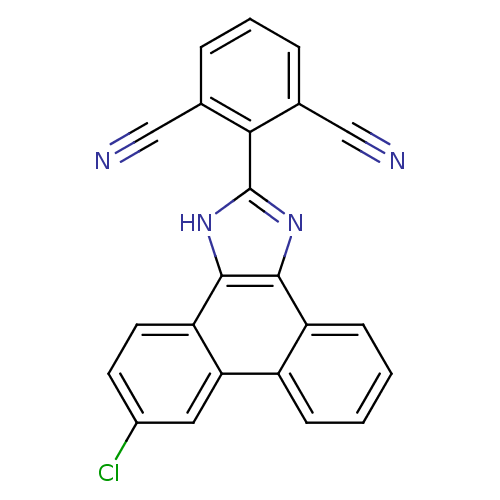
(2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...)Show SMILES Clc1ccc2c3[nH]c(nc3c3ccccc3c2c1)-c1c(cccc1C#N)C#N Show InChI InChI=1S/C23H11ClN4/c24-15-8-9-18-19(10-15)16-6-1-2-7-17(16)21-22(18)28-23(27-21)20-13(11-25)4-3-5-14(20)12-26/h1-10H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50168761
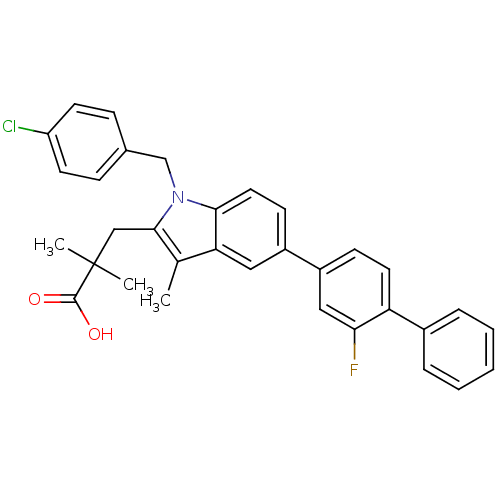
(3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...)Show SMILES Cc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(cc12)-c1ccc(c(F)c1)-c1ccccc1 Show InChI InChI=1S/C33H29ClFNO2/c1-21-28-17-24(25-11-15-27(29(35)18-25)23-7-5-4-6-8-23)12-16-30(28)36(20-22-9-13-26(34)14-10-22)31(21)19-33(2,3)32(37)38/h4-18H,19-20H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425313
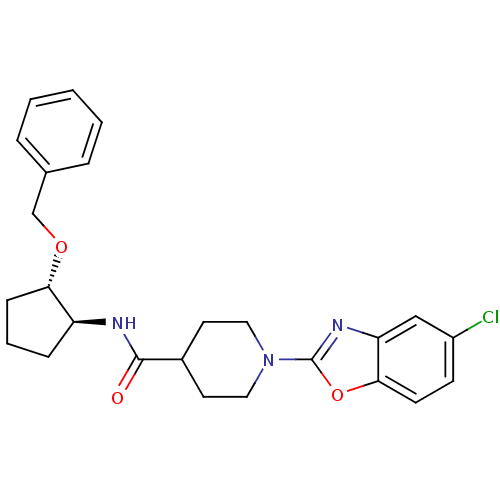
(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAP (unknown origin) |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425317

(CHEMBL2315856)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1COc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-22(15-19)28-25(32-23)29-13-11-17(12-14-29)24(30)27-21-8-4-5-18(21)16-31-20-6-2-1-3-7-20/h1-3,6-7,9-10,15,17-18,21H,4-5,8,11-14,16H2,(H,27,30)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425313

(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425315
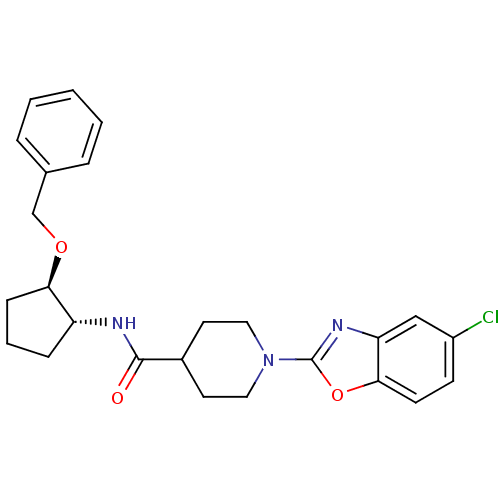
(CHEMBL2315854)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@@H]1CCC[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425320

(CHEMBL2315857)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@@H]1CCC[C@@H]1COc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-22(15-19)28-25(32-23)29-13-11-17(12-14-29)24(30)27-21-8-4-5-18(21)16-31-20-6-2-1-3-7-20/h1-3,6-7,9-10,15,17-18,21H,4-5,8,11-14,16H2,(H,27,30)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465930

(CHEMBL4292990)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2ccccc2cc1O Show InChI InChI=1S/C24H30N4O/c1-23(2)14-18(15-24(3,4)27-23)28(5)22-11-10-20(25-26-22)19-12-16-8-6-7-9-17(16)13-21(19)29/h6-13,18,27,29H,14-15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425316
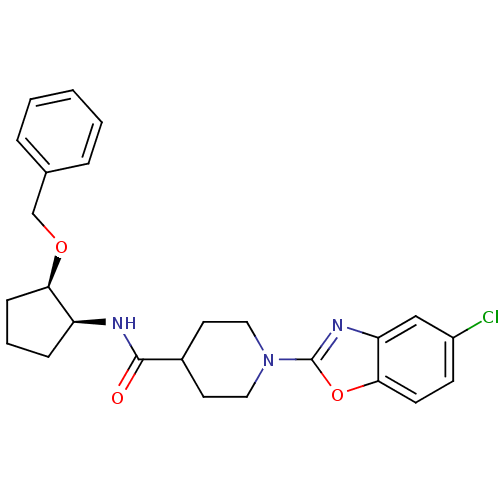
(CHEMBL2315855)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425317

(CHEMBL2315856)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1COc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-22(15-19)28-25(32-23)29-13-11-17(12-14-29)24(30)27-21-8-4-5-18(21)16-31-20-6-2-1-3-7-20/h1-3,6-7,9-10,15,17-18,21H,4-5,8,11-14,16H2,(H,27,30)/t18-,21+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465915

(CHEMBL4281315)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2ccccc2s1 Show InChI InChI=1S/C22H28N4S/c1-21(2)13-16(14-22(3,4)25-21)26(5)20-11-10-17(23-24-20)19-12-15-8-6-7-9-18(15)27-19/h6-12,16,25H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425323

(CHEMBL2315862)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@@H]1CCC[C@H]1OCC1CCCC1 |r| Show InChI InChI=1S/C24H32ClN3O3/c25-18-8-9-22-20(14-18)27-24(31-22)28-12-10-17(11-13-28)23(29)26-19-6-3-7-21(19)30-15-16-4-1-2-5-16/h8-9,14,16-17,19,21H,1-7,10-13,15H2,(H,26,29)/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465921
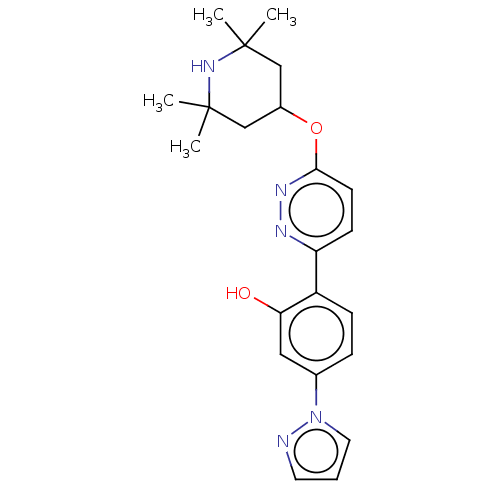
(CHEMBL4288439)Show SMILES CC1(C)CC(CC(C)(C)N1)Oc1ccc(nn1)-c1ccc(cc1O)-n1cccn1 Show InChI InChI=1S/C22H27N5O2/c1-21(2)13-16(14-22(3,4)26-21)29-20-9-8-18(24-25-20)17-7-6-15(12-19(17)28)27-11-5-10-23-27/h5-12,16,26,28H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425328
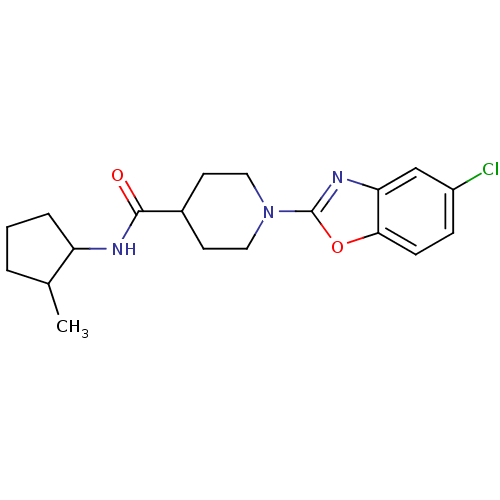
(CHEMBL2315867)Show InChI InChI=1S/C19H24ClN3O2/c1-12-3-2-4-15(12)21-18(24)13-7-9-23(10-8-13)19-22-16-11-14(20)5-6-17(16)25-19/h5-6,11-13,15H,2-4,7-10H2,1H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465926
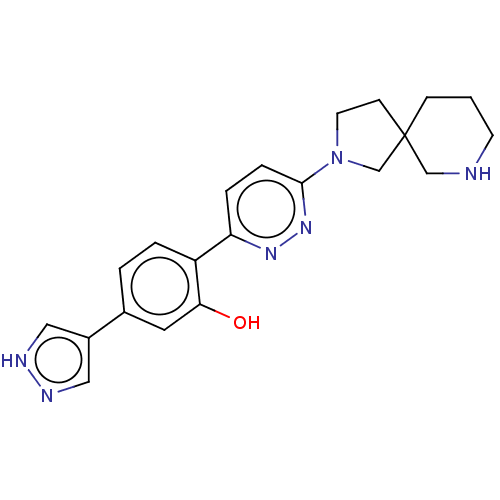
(CHEMBL4294614)Show SMILES Oc1cc(ccc1-c1ccc(nn1)N1CCC2(C1)CCCNC2)-c1cn[nH]c1 Show InChI InChI=1S/C21H24N6O/c28-19-10-15(16-11-23-24-12-16)2-3-17(19)18-4-5-20(26-25-18)27-9-7-21(14-27)6-1-8-22-13-21/h2-5,10-12,22,28H,1,6-9,13-14H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425327
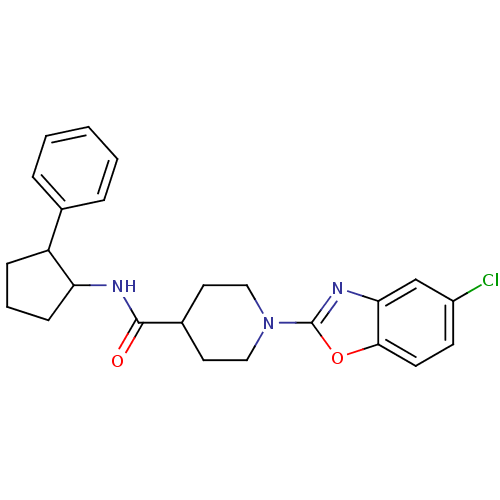
(CHEMBL2315866)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)NC1CCCC1c1ccccc1 Show InChI InChI=1S/C24H26ClN3O2/c25-18-9-10-22-21(15-18)27-24(30-22)28-13-11-17(12-14-28)23(29)26-20-8-4-7-19(20)16-5-2-1-3-6-16/h1-3,5-6,9-10,15,17,19-20H,4,7-8,11-14H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425315

(CHEMBL2315854)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@@H]1CCC[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 296 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465919
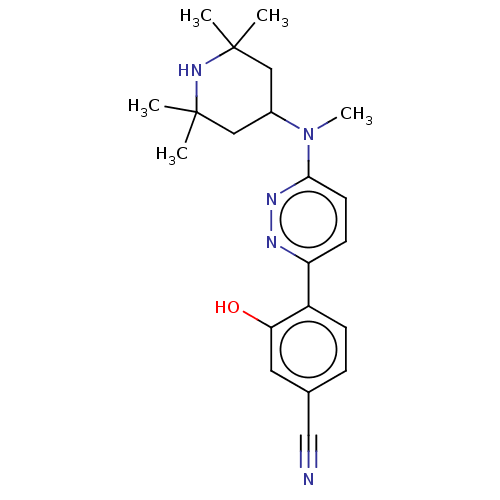
(CHEMBL4283397)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)C#N Show InChI InChI=1S/C21H27N5O/c1-20(2)11-15(12-21(3,4)25-20)26(5)19-9-8-17(23-24-19)16-7-6-14(13-22)10-18(16)27/h6-10,15,25,27H,11-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465920

(CHEMBL4280558)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)-n1cccn1 Show InChI InChI=1S/C23H30N6O/c1-22(2)14-17(15-23(3,4)27-22)28(5)21-10-9-19(25-26-21)18-8-7-16(13-20(18)30)29-12-6-11-24-29/h6-13,17,27,30H,14-15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425318
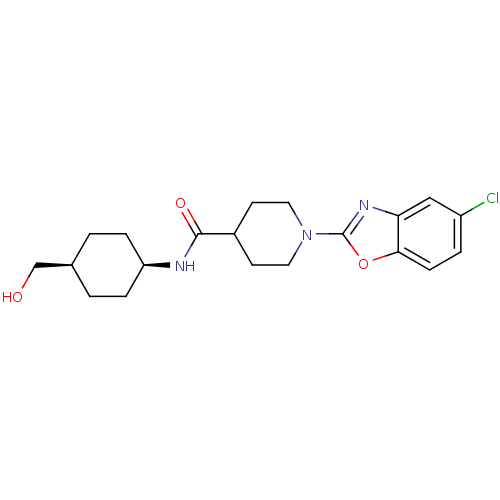
(CHEMBL2315861)Show SMILES OC[C@H]1CC[C@H](CC1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r,wD:5.8,2.1,(21.18,-14.42,;20.41,-13.09,;18.87,-13.08,;18.1,-11.75,;16.56,-11.75,;15.8,-13.08,;16.57,-14.42,;18.1,-14.42,;14.27,-13.08,;13.49,-11.75,;14.26,-10.42,;11.95,-11.75,;11.18,-13.09,;9.65,-13.09,;8.88,-11.77,;9.63,-10.43,;11.18,-10.42,;7.34,-11.77,;6.44,-13.03,;4.97,-12.56,;3.63,-13.34,;2.3,-12.57,;.96,-13.34,;2.3,-11.02,;3.63,-10.25,;4.96,-11.02,;6.43,-10.53,)| Show InChI InChI=1S/C20H26ClN3O3/c21-15-3-6-18-17(11-15)23-20(27-18)24-9-7-14(8-10-24)19(26)22-16-4-1-13(12-25)2-5-16/h3,6,11,13-14,16,25H,1-2,4-5,7-10,12H2,(H,22,26)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465916
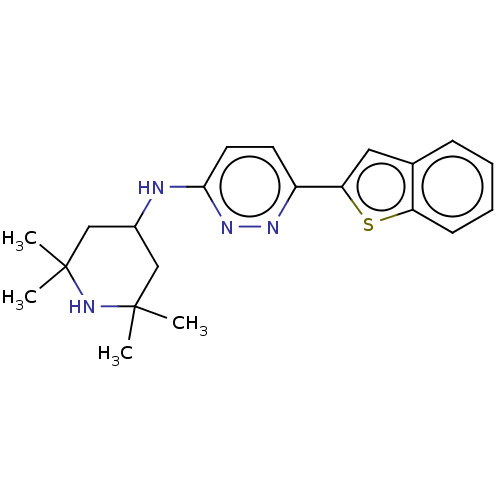
(CHEMBL4289183)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1ccc(nn1)-c1cc2ccccc2s1 Show InChI InChI=1S/C21H26N4S/c1-20(2)12-15(13-21(3,4)25-20)22-19-10-9-16(23-24-19)18-11-14-7-5-6-8-17(14)26-18/h5-11,15,25H,12-13H2,1-4H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425316

(CHEMBL2315855)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 618 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425322

(CHEMBL2315859)Show SMILES OC[C@H]1CCC[C@H]1NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C19H24ClN3O3/c20-14-4-5-17-16(10-14)22-19(26-17)23-8-6-12(7-9-23)18(25)21-15-3-1-2-13(15)11-24/h4-5,10,12-13,15,24H,1-3,6-9,11H2,(H,21,25)/t13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425329
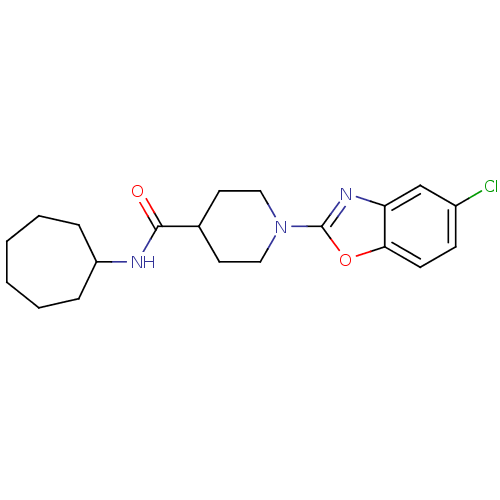
(CHEMBL2315868)Show InChI InChI=1S/C20H26ClN3O2/c21-15-7-8-18-17(13-15)23-20(26-18)24-11-9-14(10-12-24)19(25)22-16-5-3-1-2-4-6-16/h7-8,13-14,16H,1-6,9-12H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 821 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425324

(CHEMBL2315864)Show SMILES CCCCO[C@@H]1CCC[C@H]1NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C22H30ClN3O3/c1-2-3-13-28-19-6-4-5-17(19)24-21(27)15-9-11-26(12-10-15)22-25-18-14-16(23)7-8-20(18)29-22/h7-8,14-15,17,19H,2-6,9-13H2,1H3,(H,24,27)/t17-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 826 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425314
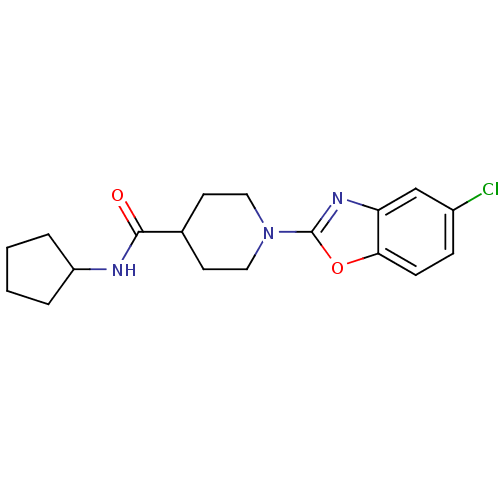
(CHEMBL2315877)Show InChI InChI=1S/C18H22ClN3O2/c19-13-5-6-16-15(11-13)21-18(24-16)22-9-7-12(8-10-22)17(23)20-14-3-1-2-4-14/h5-6,11-12,14H,1-4,7-10H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 853 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425313

(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 934 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as LPS-induced PGE2 production incubated 15 mins prior to LPS challenge measured after 24 hrs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425314

(CHEMBL2315877)Show InChI InChI=1S/C18H22ClN3O2/c19-13-5-6-16-15(11-13)21-18(24-16)22-9-7-12(8-10-22)17(23)20-14-3-1-2-4-14/h5-6,11-12,14H,1-4,7-10H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425337
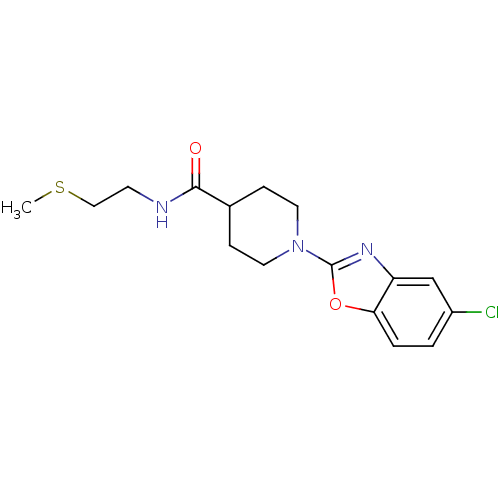
(CHEMBL2315876)Show InChI InChI=1S/C16H20ClN3O2S/c1-23-9-6-18-15(21)11-4-7-20(8-5-11)16-19-13-10-12(17)2-3-14(13)22-16/h2-3,10-11H,4-9H2,1H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Canis familiaris) | BDBM50425313

(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in dog whole blood assessed as LPS-induced PGE2 production |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425318

(CHEMBL2315861)Show SMILES OC[C@H]1CC[C@H](CC1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r,wD:5.8,2.1,(21.18,-14.42,;20.41,-13.09,;18.87,-13.08,;18.1,-11.75,;16.56,-11.75,;15.8,-13.08,;16.57,-14.42,;18.1,-14.42,;14.27,-13.08,;13.49,-11.75,;14.26,-10.42,;11.95,-11.75,;11.18,-13.09,;9.65,-13.09,;8.88,-11.77,;9.63,-10.43,;11.18,-10.42,;7.34,-11.77,;6.44,-13.03,;4.97,-12.56,;3.63,-13.34,;2.3,-12.57,;.96,-13.34,;2.3,-11.02,;3.63,-10.25,;4.96,-11.02,;6.43,-10.53,)| Show InChI InChI=1S/C20H26ClN3O3/c21-15-3-6-18-17(11-15)23-20(27-18)24-9-7-14(8-10-24)19(26)22-16-4-1-13(12-25)2-5-16/h3,6,11,13-14,16,25H,1-2,4-5,7-10,12H2,(H,22,26)/t13-,16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465927

(CHEMBL4285798)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1ccc(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H28N4/c1-22(2)14-19(15-23(3,4)27-22)24-21-12-11-20(25-26-21)18-10-9-16-7-5-6-8-17(16)13-18/h5-13,19,27H,14-15H2,1-4H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425330
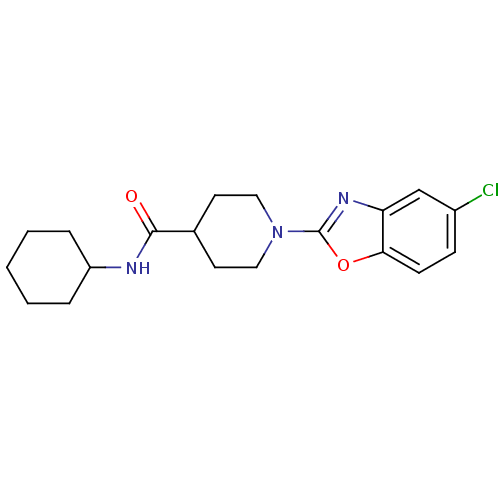
(CHEMBL2315870)Show InChI InChI=1S/C19H24ClN3O2/c20-14-6-7-17-16(12-14)22-19(25-17)23-10-8-13(9-11-23)18(24)21-15-4-2-1-3-5-15/h6-7,12-13,15H,1-5,8-11H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465918

(CHEMBL4282391)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(F)cc1O Show InChI InChI=1S/C20H27FN4O/c1-19(2)11-14(12-20(3,4)24-19)25(5)18-9-8-16(22-23-18)15-7-6-13(21)10-17(15)26/h6-10,14,24,26H,11-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465929

(CHEMBL4286715)Show SMILES [H][C@@]12CNC[C@]1([H])CN(C2)c1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 |r| Show InChI InChI=1S/C19H20N6O/c26-18-5-12(13-8-21-22-9-13)1-2-16(18)17-3-4-19(24-23-17)25-10-14-6-20-7-15(14)11-25/h1-5,8-9,14-15,20,26H,6-7,10-11H2,(H,21,22)/t14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425331
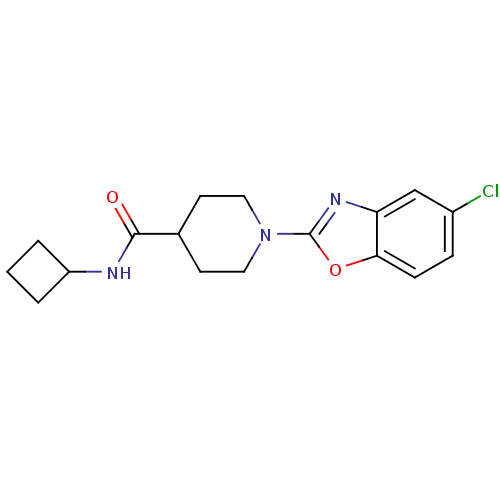
(CHEMBL2315869)Show InChI InChI=1S/C17H20ClN3O2/c18-12-4-5-15-14(10-12)20-17(23-15)21-8-6-11(7-9-21)16(22)19-13-2-1-3-13/h4-5,10-11,13H,1-3,6-9H2,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425321
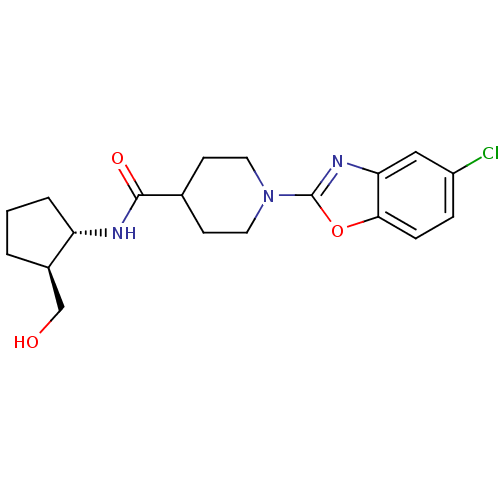
(CHEMBL2315858)Show SMILES OC[C@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C19H24ClN3O3/c20-14-4-5-17-16(10-14)22-19(26-17)23-8-6-12(7-9-23)18(25)21-15-3-1-2-13(15)11-24/h4-5,10,12-13,15,24H,1-3,6-9,11H2,(H,21,25)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465922

(CHEMBL4277023)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2cccnc2cc1O Show InChI InChI=1S/C23H29N5O/c1-22(2)13-16(14-23(3,4)27-22)28(5)21-9-8-18(25-26-21)17-11-15-7-6-10-24-19(15)12-20(17)29/h6-12,16,27,29H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465925

(CHEMBL4283367)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 Show InChI InChI=1S/C23H30N6O/c1-22(2)11-17(12-23(3,4)28-22)29(5)21-9-8-19(26-27-21)18-7-6-15(10-20(18)30)16-13-24-25-14-16/h6-10,13-14,17,28,30H,11-12H2,1-5H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465931

(CHEMBL4290774)Show InChI InChI=1S/C18H19N5O2/c24-17-12-13(23-11-1-8-20-23)2-3-15(17)16-4-5-18(22-21-16)25-14-6-9-19-10-7-14/h1-5,8,11-12,14,19,24H,6-7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465914
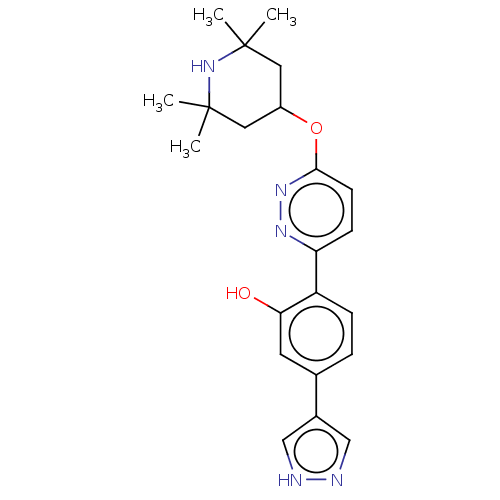
(Branaplam | LMI-070 | LMI070)Show SMILES CC1(C)CC(CC(C)(C)N1)Oc1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N5O2/c1-21(2)10-16(11-22(3,4)27-21)29-20-8-7-18(25-26-20)17-6-5-14(9-19(17)28)15-12-23-24-13-15/h5-9,12-13,16,27-28H,10-11H2,1-4H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425326

(CHEMBL2315865)Show SMILES OC(=O)C1CCCC1NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 Show InChI InChI=1S/C19H22ClN3O4/c20-12-4-5-16-15(10-12)22-19(27-16)23-8-6-11(7-9-23)17(24)21-14-3-1-2-13(14)18(25)26/h4-5,10-11,13-14H,1-3,6-9H2,(H,21,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425313

(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as PGE2 level incubated for 15 mins prior to substrate addition measured after 10 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425325
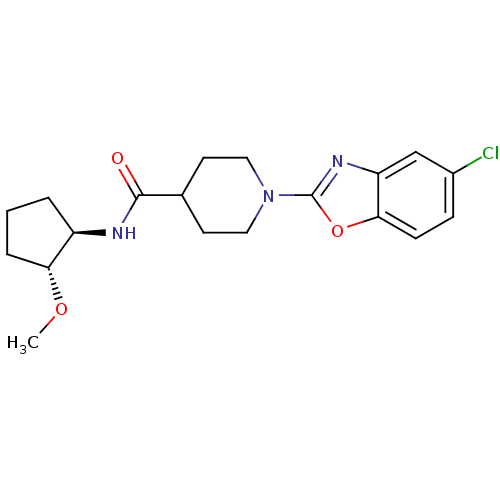
(CHEMBL2315863)Show SMILES CO[C@@H]1CCC[C@H]1NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C19H24ClN3O3/c1-25-16-4-2-3-14(16)21-18(24)12-7-9-23(10-8-12)19-22-15-11-13(20)5-6-17(15)26-19/h5-6,11-12,14,16H,2-4,7-10H2,1H3,(H,21,24)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50425313

(CHEMBL2315853)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)C(=O)N[C@H]1CCC[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C25H28ClN3O3/c26-19-9-10-23-21(15-19)28-25(32-23)29-13-11-18(12-14-29)24(30)27-20-7-4-8-22(20)31-16-17-5-2-1-3-6-17/h1-3,5-6,9-10,15,18,20,22H,4,7-8,11-14,16H2,(H,27,30)/t20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PGDS |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50425319
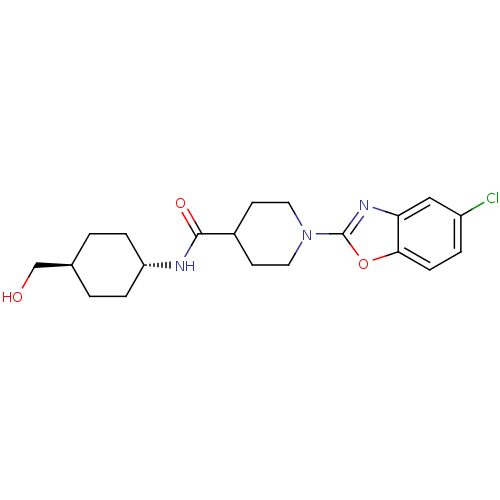
(CHEMBL2315860)Show SMILES OC[C@H]1CC[C@@H](CC1)NC(=O)C1CCN(CC1)c1nc2cc(Cl)ccc2o1 |r,wU:5.8,wD:2.1,(41.91,-13.61,;41.14,-12.28,;39.6,-12.28,;38.83,-10.94,;37.29,-10.94,;36.53,-12.27,;37.29,-13.61,;38.83,-13.61,;34.99,-12.28,;34.22,-10.94,;34.99,-9.61,;32.68,-10.95,;31.91,-12.28,;30.38,-12.29,;29.61,-10.96,;30.36,-9.62,;31.91,-9.62,;28.07,-10.97,;27.17,-12.22,;25.7,-11.75,;24.36,-12.53,;23.02,-11.76,;21.69,-12.53,;23.03,-10.22,;24.35,-9.45,;25.69,-10.21,;27.15,-9.72,)| Show InChI InChI=1S/C20H26ClN3O3/c21-15-3-6-18-17(11-15)23-20(27-18)24-9-7-14(8-10-24)19(26)22-16-4-1-13(12-25)2-5-16/h3,6,11,13-14,16,25H,1-2,4-5,7-10,12H2,(H,22,26)/t13-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human fetal fibroblast assessed as inhibition of IL-1beta-mediated PGE2 production after 50 mins by ELISA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425334
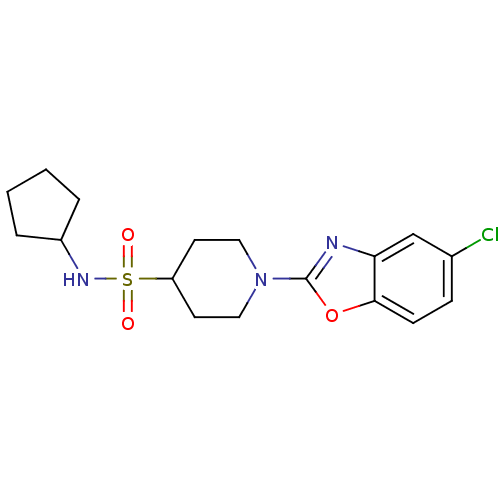
(CHEMBL2315874)Show SMILES Clc1ccc2oc(nc2c1)N1CCC(CC1)S(=O)(=O)NC1CCCC1 Show InChI InChI=1S/C17H22ClN3O3S/c18-12-5-6-16-15(11-12)19-17(24-16)21-9-7-14(8-10-21)25(22,23)20-13-3-1-2-4-13/h5-6,11,13-14,20H,1-4,7-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50425335
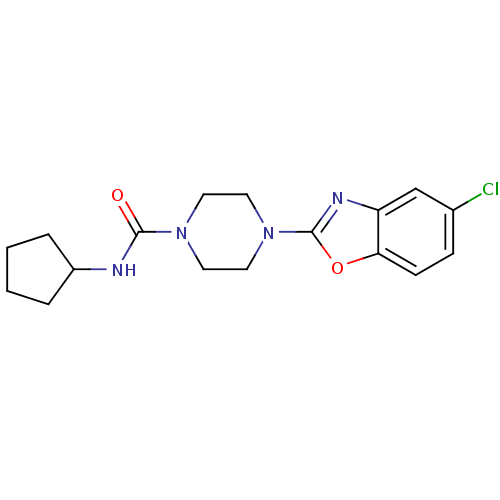
(CHEMBL2315873)Show InChI InChI=1S/C17H21ClN4O2/c18-12-5-6-15-14(11-12)20-17(24-15)22-9-7-21(8-10-22)16(23)19-13-3-1-2-4-13/h5-6,11,13H,1-4,7-10H2,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA |
Bioorg Med Chem Lett 23: 907-11 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.040
BindingDB Entry DOI: 10.7270/Q2Z3210H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data