

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118462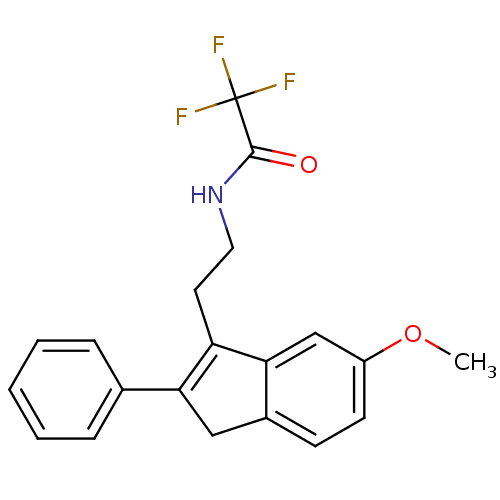 (2,2,2-Trifluoro-N-[2-(6-methoxy-2-phenyl-3H-inden-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118435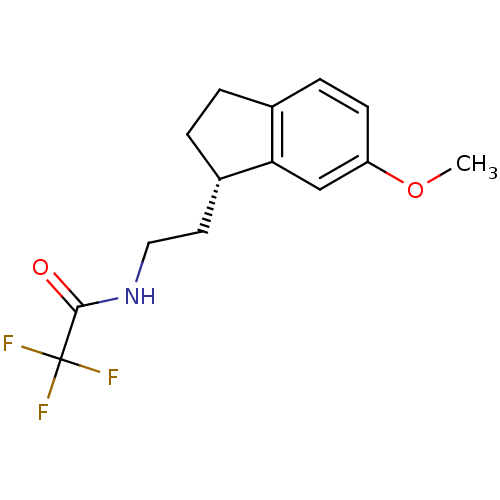 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470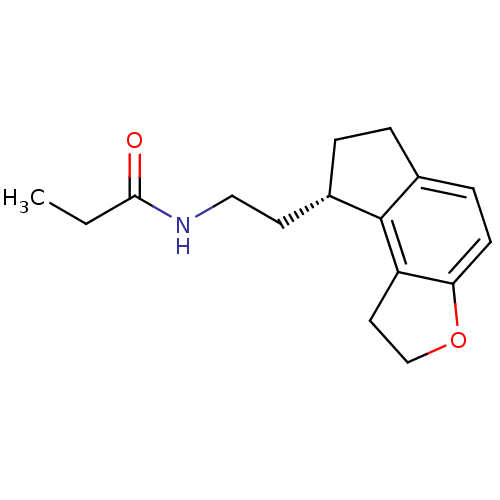 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.0138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity against human Melatonin receptor type 1A (MT1) | J Med Chem 45: 4222-39 (2002) BindingDB Entry DOI: 10.7270/Q2D799S6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534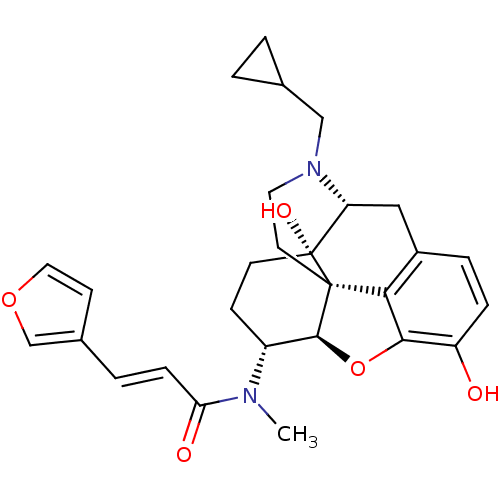 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118446 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118456 (CHEMBL334645 | N-[2-(6-Methoxy-3H-inden-1-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50596292 (CHEMBL5185211) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118453 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-butyramide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50596293 (CHEMBL5188658) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118440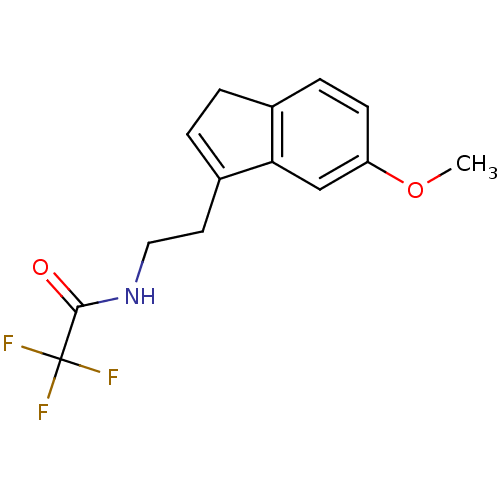 (2,2,2-Trifluoro-N-[2-(6-methoxy-3H-inden-1-yl)-eth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0408 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118430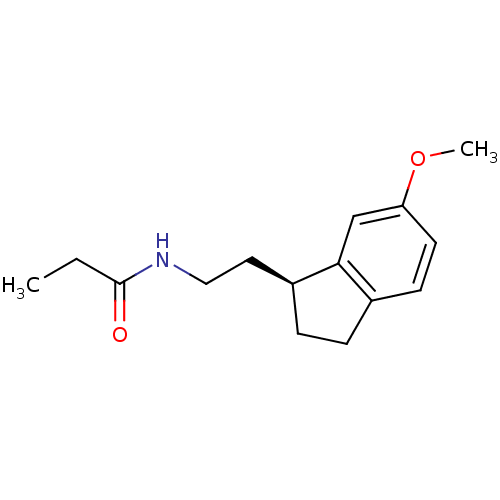 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-propionamid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50118470 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity against human Melatonin receptor type 1B (MT2) | J Med Chem 45: 4222-39 (2002) BindingDB Entry DOI: 10.7270/Q2D799S6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118463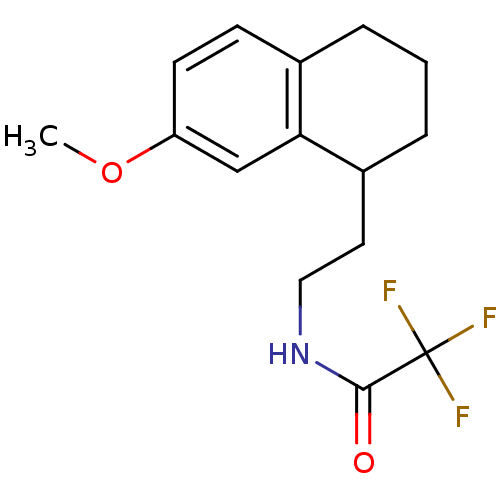 (2,2,2-Trifluoro-N-[2-(7-methoxy-1,2,3,4-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118450 (CHEMBL335437 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118458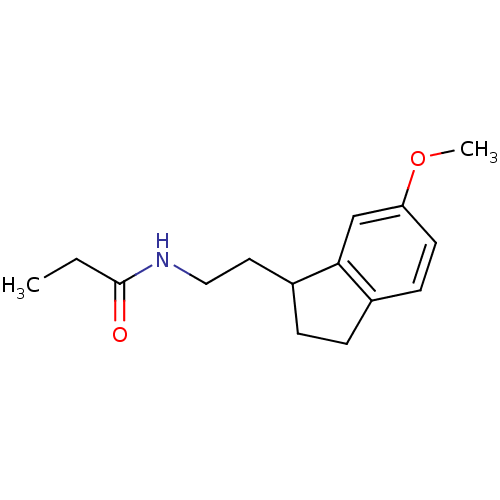 (CHEMBL337837 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0728 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118460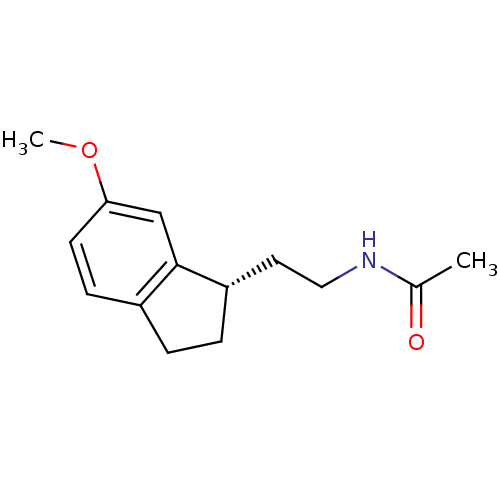 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-acetamide |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0733 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM9019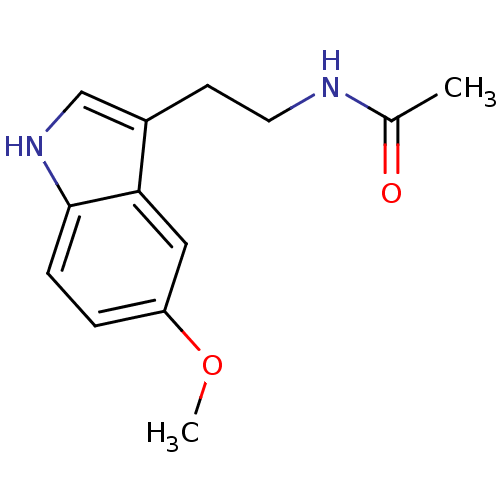 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.0823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity against human Melatonin receptor type 1A (MT1) | J Med Chem 45: 4222-39 (2002) BindingDB Entry DOI: 10.7270/Q2D799S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.0823 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118436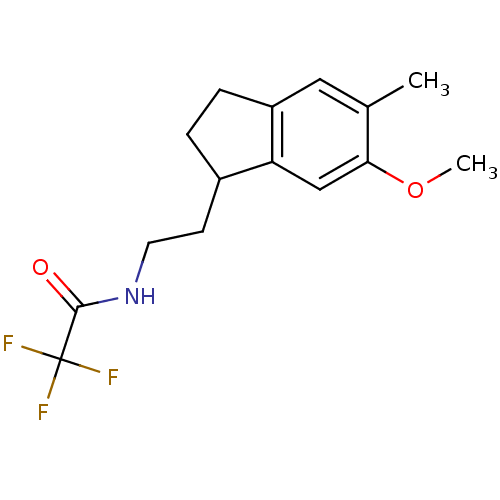 (2,2,2-Trifluoro-N-[2-(6-methoxy-5-methyl-indan-1-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0984 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118447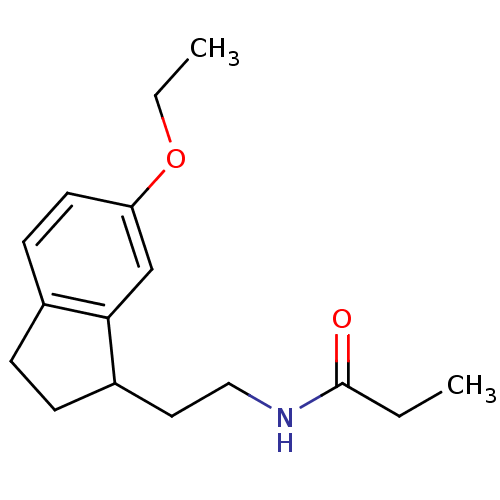 (CHEMBL134878 | N-[2-(6-Ethoxy-indan-1-yl)-ethyl]-p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118441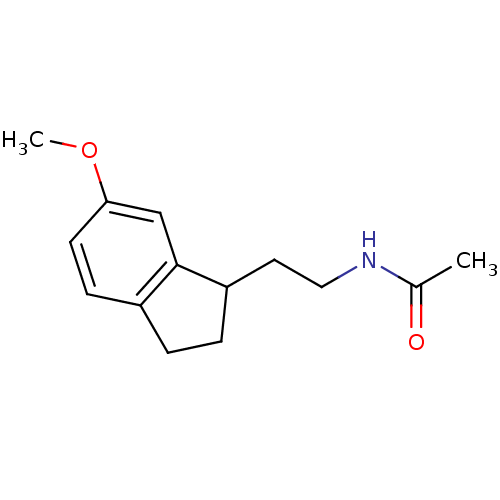 (CHEMBL134171 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50596292 (CHEMBL5185211) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50454212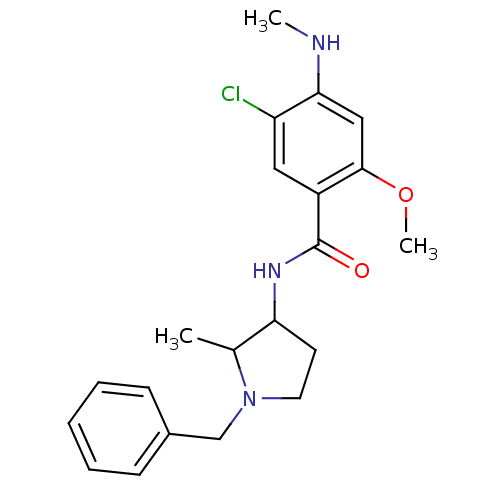 (CHEBI:64217 | Emonapride | Nemonapride) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50454212 (CHEBI:64217 | Emonapride | Nemonapride) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity against human Melatonin receptor type 1B (MT2) | J Med Chem 45: 4222-39 (2002) BindingDB Entry DOI: 10.7270/Q2D799S6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118438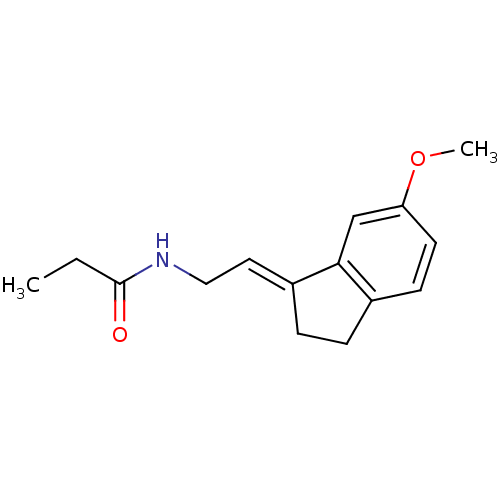 ((E) N-[2-(6-Methoxy-indan-1-ylidene)-ethyl]-propio...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052196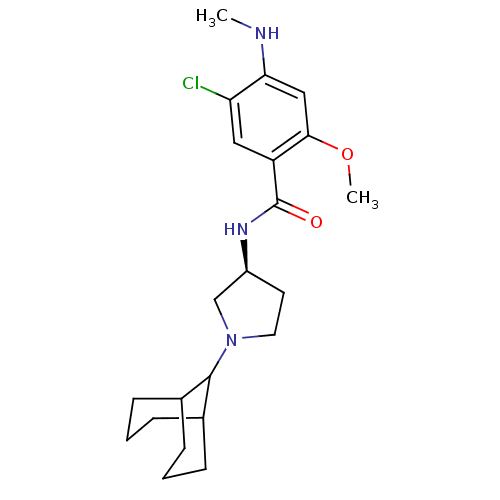 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118445 (CHEMBL134832 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22542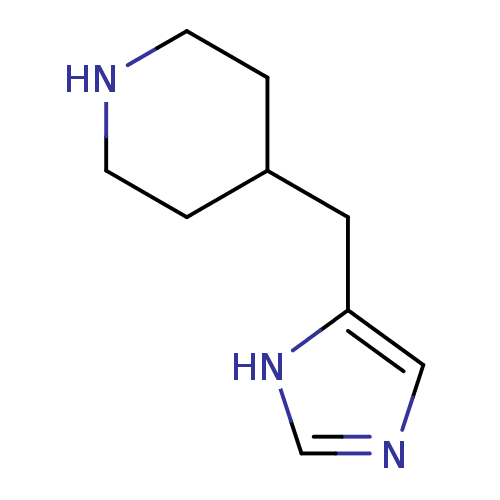 (4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118452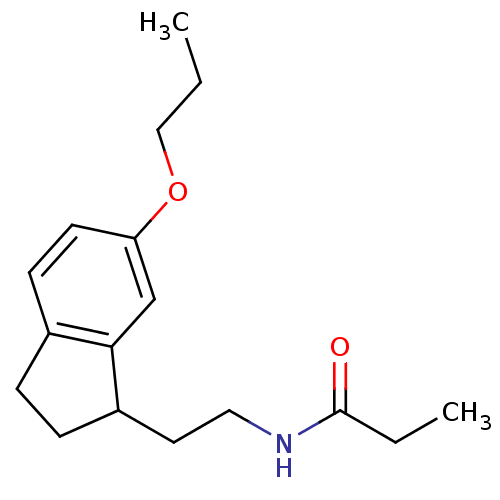 (CHEMBL134933 | N-[2-(6-Propoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118444 (2,2,2-Trifluoro-N-[3-(6-methoxy-indan-1-yl)-propyl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.526 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052194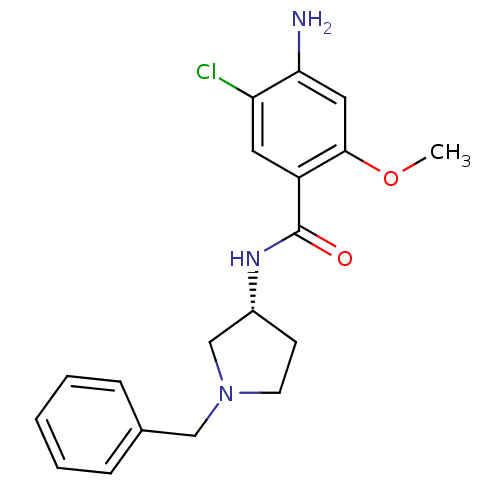 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22911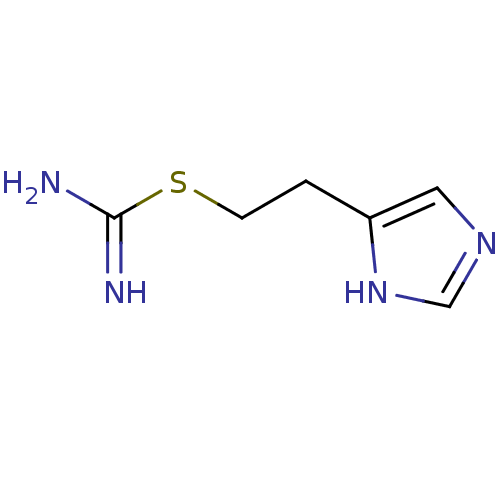 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound to rat Dopamine receptor D3 expressed in CHO cells was determined using [125 I] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50325534 (CHEMBL267495 | nalfurafine) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM22910 (4-(1H-imidazol-5-ylmethyl)pyridine | Immethridine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 53: 6445-56 (2010) Article DOI: 10.1021/jm100643t BindingDB Entry DOI: 10.7270/Q2QF8T3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052196 (CHEMBL319597 | N-((S)-1-Bicyclo[3.3.1]non-9-yl-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317868 (4-(2-(4-tert-Butylphenylamino)ethyl)-1H-imidazole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118437 ((Z) N-[2-(6-Methoxy-indan-1-ylidene)-ethyl]-propio...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50317865 (2-(2-(4-tert-Butylphenylthio)ethyl)-1H-imidazole |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant histamine H4 receptor expressed in CHO cells | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50317864 (4-(2-(4-Trifluoromethylphenylamino)ethyl)-1H-imida...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd. Curated by ChEMBL | Assay Description Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co... | J Med Chem 53: 3840-4 (2010) Article DOI: 10.1021/jm901890s BindingDB Entry DOI: 10.7270/Q21Z44KC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50052191 (4-Amino-N-((S)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to Human Dopamine receptor D4 expressed in CHO cells was determined using [3H]- nemonapride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063477 ((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) | J Med Chem 41: 902-12 (1998) Article DOI: 10.1021/jm970568r BindingDB Entry DOI: 10.7270/Q2GH9H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50052194 (4-Amino-N-((R)-1-benzyl-pyrrolidin-3-yl)-5-chloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity to rat Dopamine receptor D2 expressesd in CHO cells was determined using [125 I ] iodosulpride as radioligand | J Med Chem 39: 2764-72 (1996) Article DOI: 10.1021/jm9601720 BindingDB Entry DOI: 10.7270/Q2SB44VK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1131 total ) | Next | Last >> |