

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269429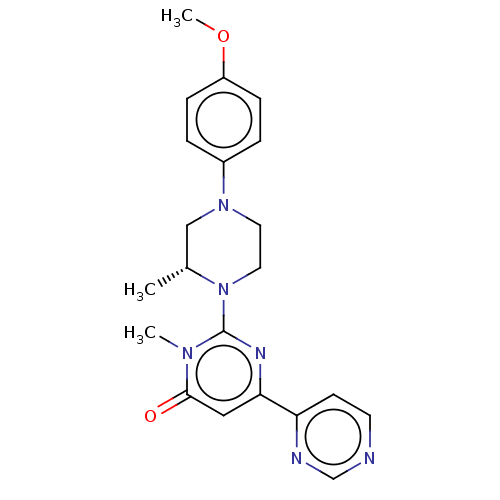 (CHEMBL4084855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269428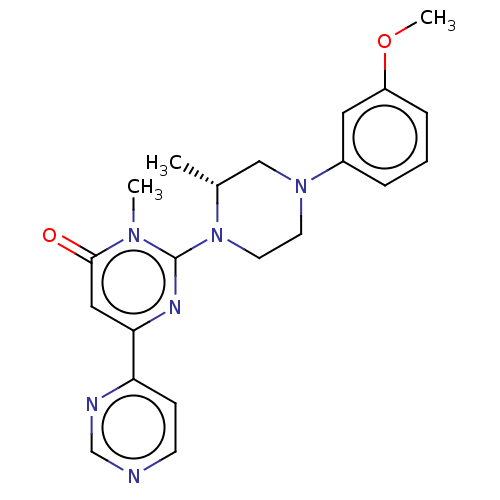 (CHEMBL4063206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269437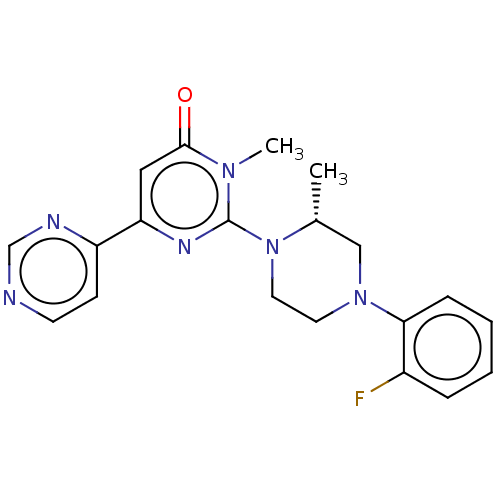 (CHEMBL4077376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269447 (CHEMBL4076186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269447 (CHEMBL4076186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269455 (CHEMBL4087402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269455 (CHEMBL4087402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269438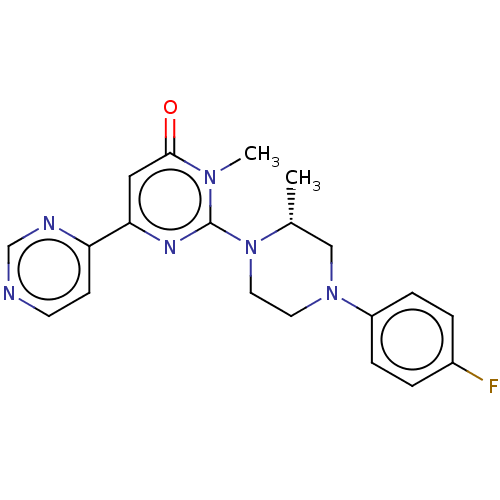 (CHEMBL4076060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269443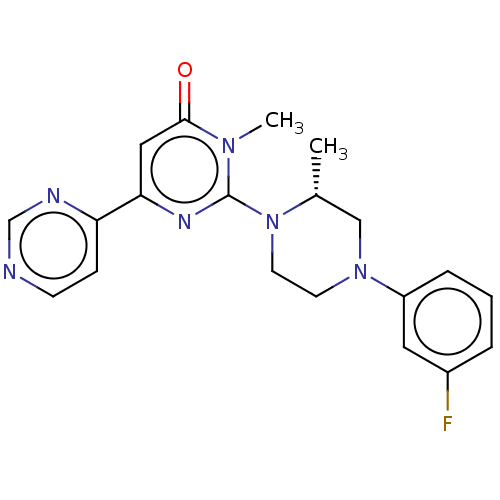 (CHEMBL4095848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269440 (CHEMBL4065818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187842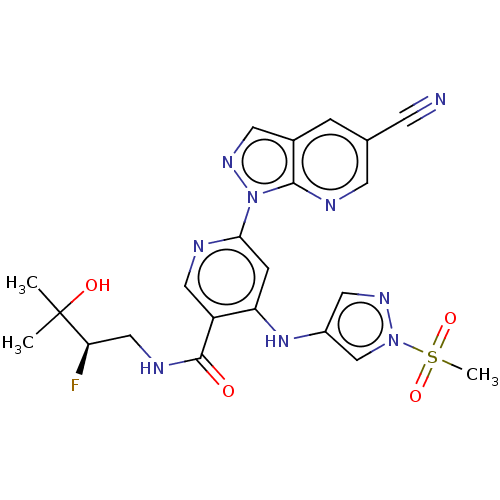 (US9169252, 385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187402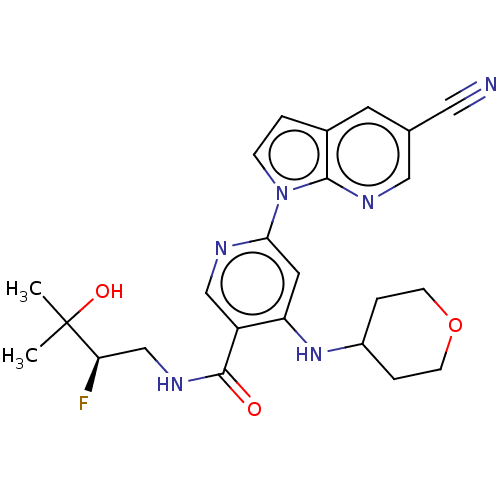 (US9169252, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438443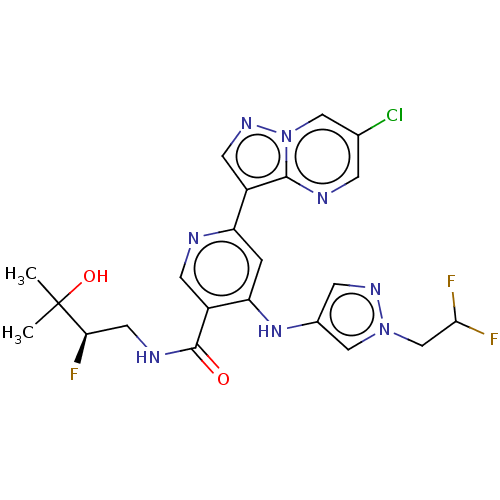 (US10618903, Example 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187896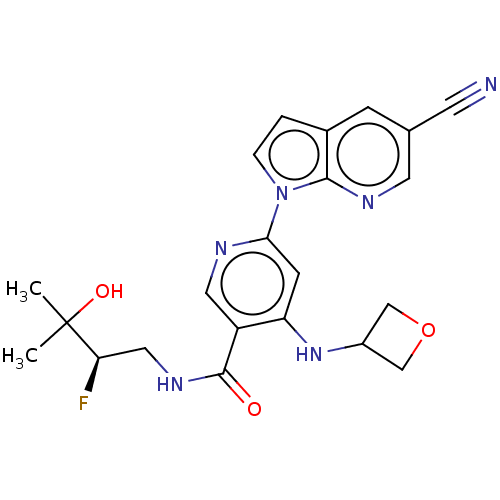 (US9169252, 437) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438484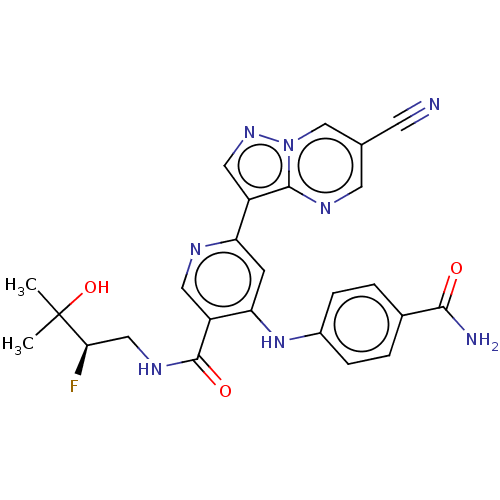 (US10618903, Example 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187405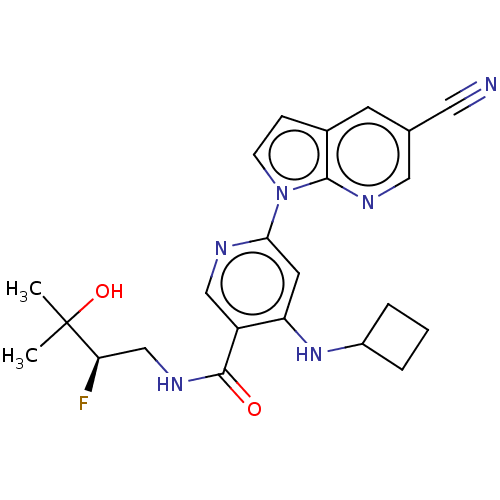 (US9169252, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187742 (US9169252, 293) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438455 (US10618903, Example 83 | US10618903, Example 84 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438672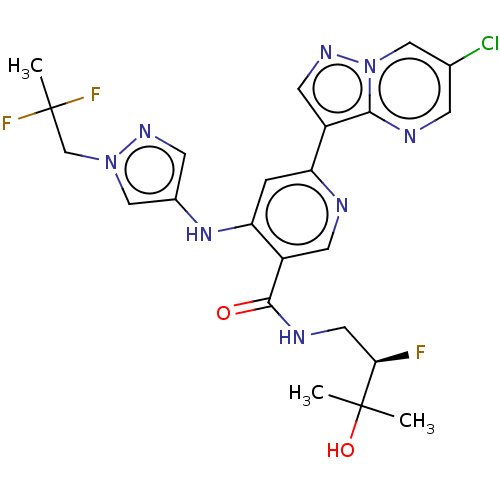 (US10618903, Example 239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438679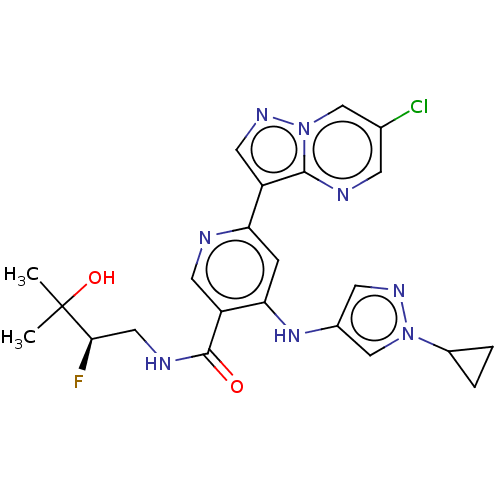 (US10618903, Example 246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438683 (US10618903, Example 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438684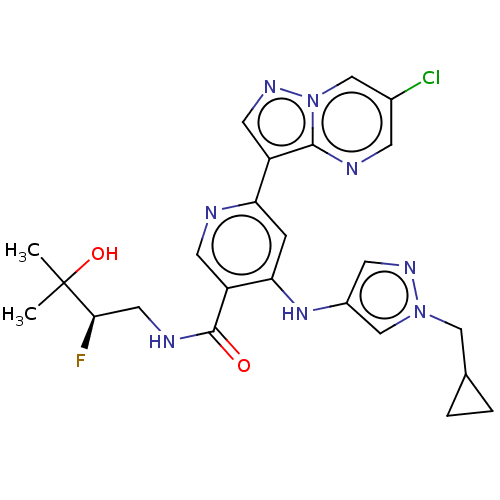 (US10618903, Example 251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438686 (US10618903, Example 253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438695 (US10618903, Example 262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438708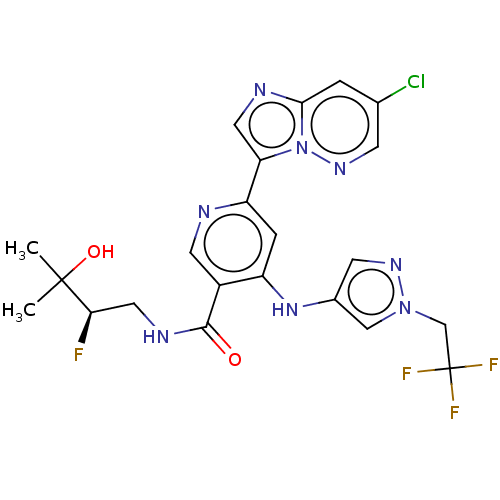 (US10618903, Example 275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438711 (US10618903, Example 278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438705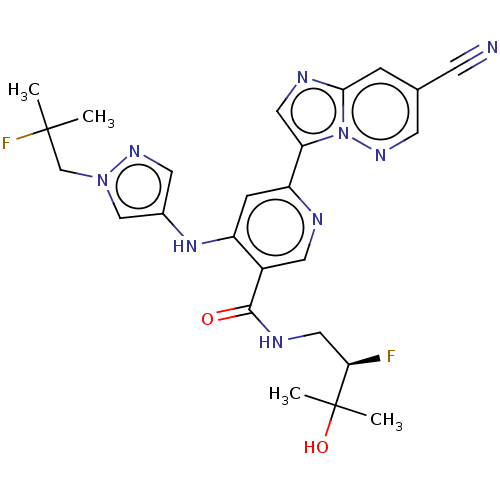 (US10618903, Example 272 | US10618903, Example 279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438631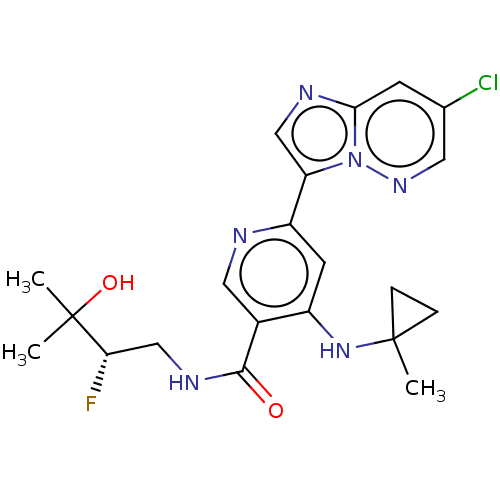 (US10618903, Example 198 | US10618903, Example 207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438664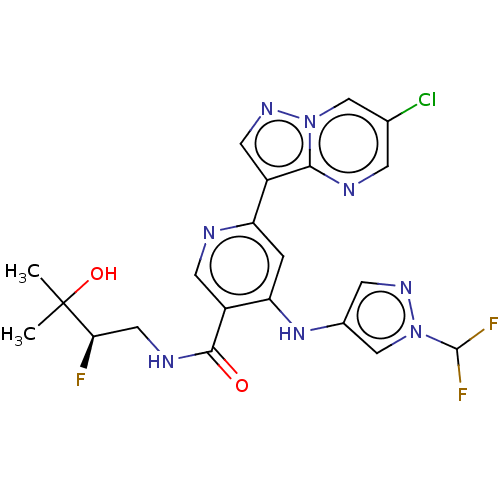 (US10618903, Example 231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438665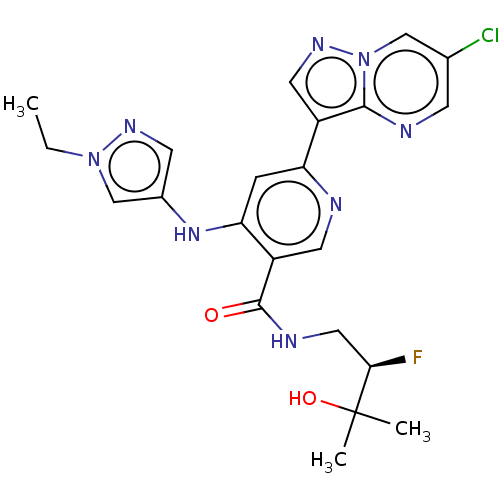 (US10618903, Example 232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438420 (US10618903, Example 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187515 (US9169252, 109 | US9169252, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187861 (US9169252, 404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM188094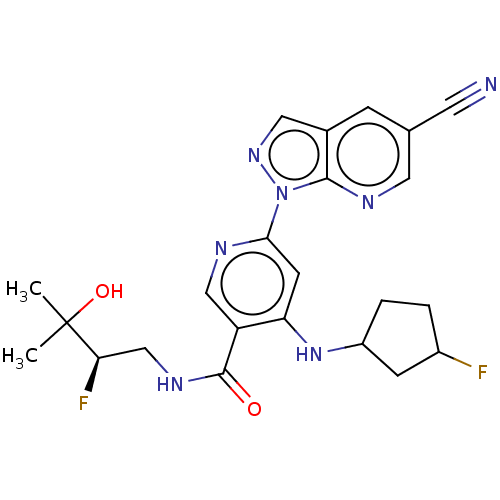 (US9169252, 625 | US9169252, 626 | US9169252, 627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269492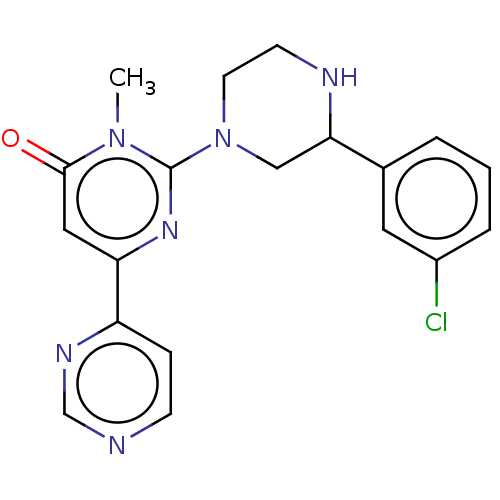 (CHEMBL4067532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187438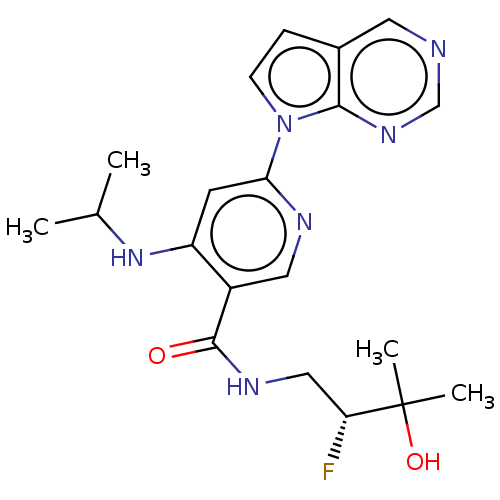 (US9169252, 39) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187480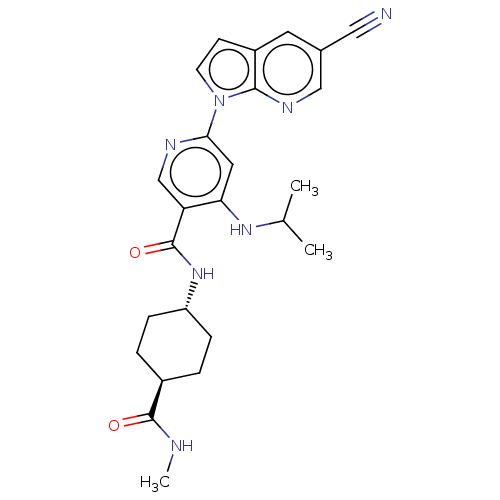 (US9169252, 80 | US9169252, 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187829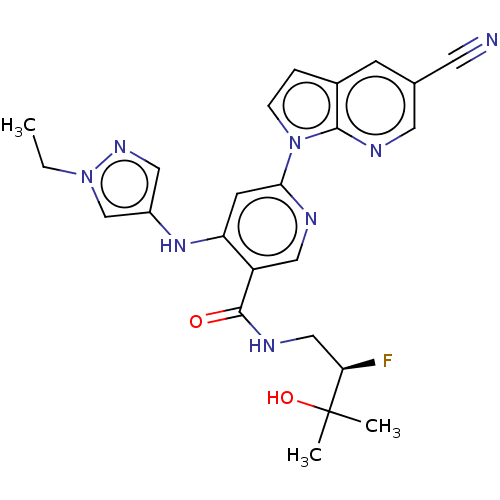 (US9169252, 372) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187415 (US9169252, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187416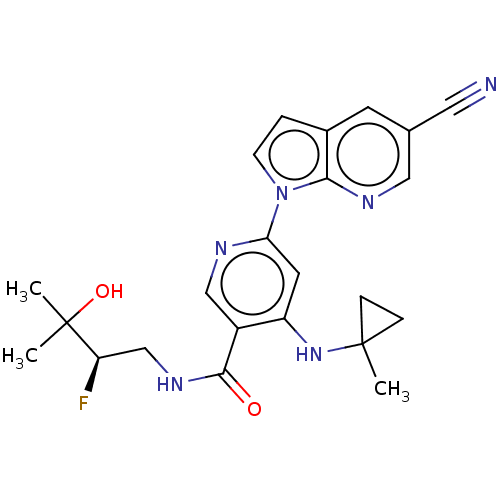 (US9169252, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269439 (CHEMBL4077048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269452 (CHEMBL4105346) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000 Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GSK-3beta using prephosphorylated GS-1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scinti... | Bioorg Med Chem Lett 27: 3726-3732 (2017) Article DOI: 10.1016/j.bmcl.2017.06.078 BindingDB Entry DOI: 10.7270/Q2T43WKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187974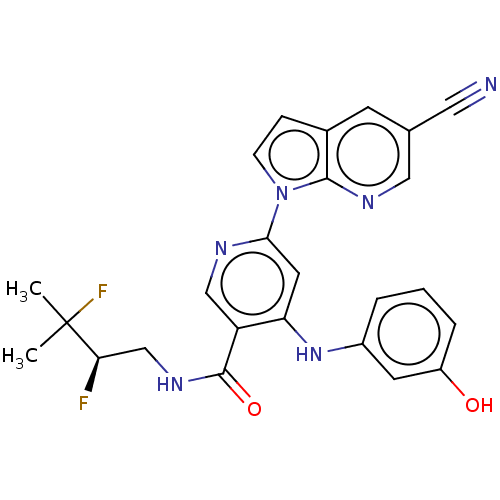 (US9169252, 517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187931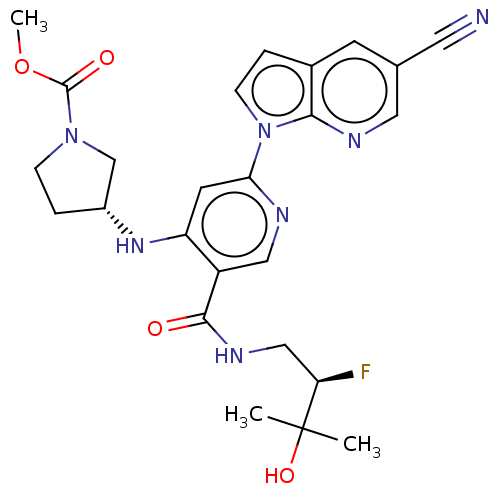 (US9169252, 474) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187943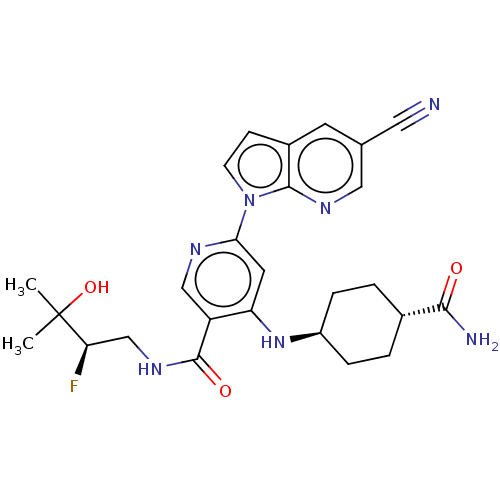 (US9169252, 486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187460 (US9169252, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187824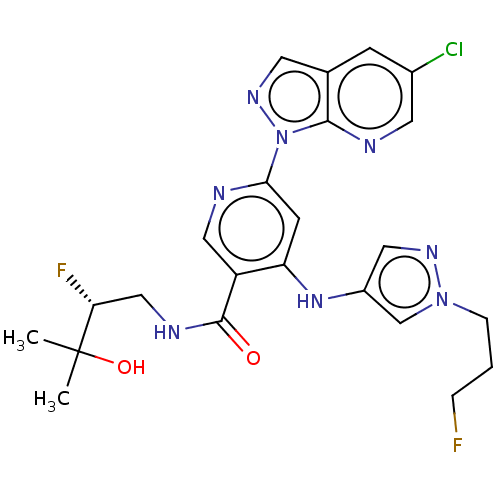 (US9169252, 367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187711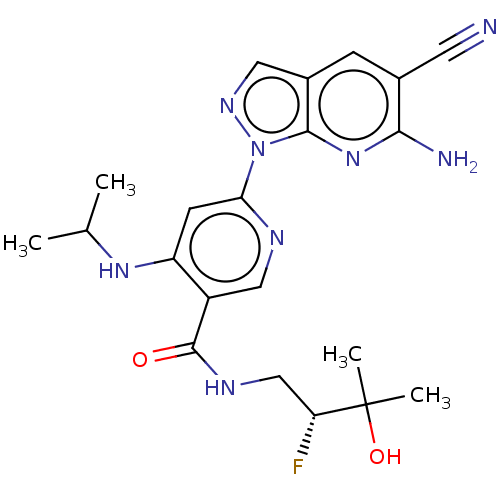 (US9169252, 262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187632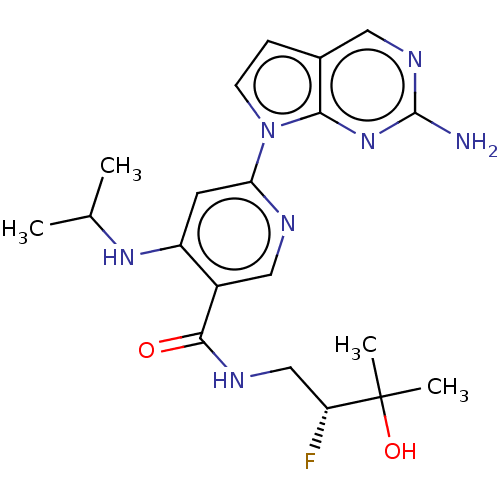 (US9169252, 186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM187468 (US9169252, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 L prepared from 15 L additions of enzyme and substrates (fluores... | US Patent US9169252 (2015) BindingDB Entry DOI: 10.7270/Q2MP5235 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1998 total ) | Next | Last >> |