 Found 344 hits with Last Name = 'rimvall' and Initial = 'k'
Found 344 hits with Last Name = 'rimvall' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146832
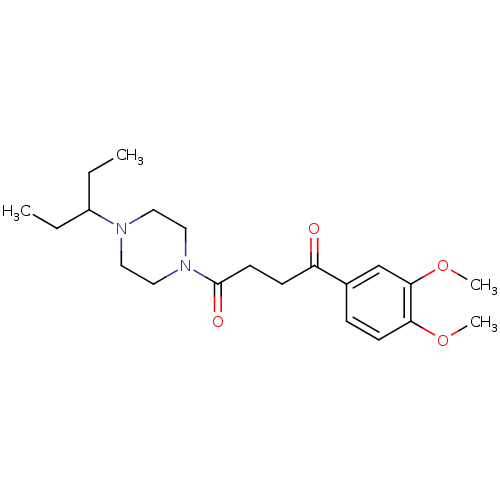
(1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C21H32N2O4/c1-5-17(6-2)22-11-13-23(14-12-22)21(25)10-8-18(24)16-7-9-19(26-3)20(15-16)27-4/h7,9,15,17H,5-6,8,10-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146832

(1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C21H32N2O4/c1-5-17(6-2)22-11-13-23(14-12-22)21(25)10-8-18(24)16-7-9-19(26-3)20(15-16)27-4/h7,9,15,17H,5-6,8,10-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370330
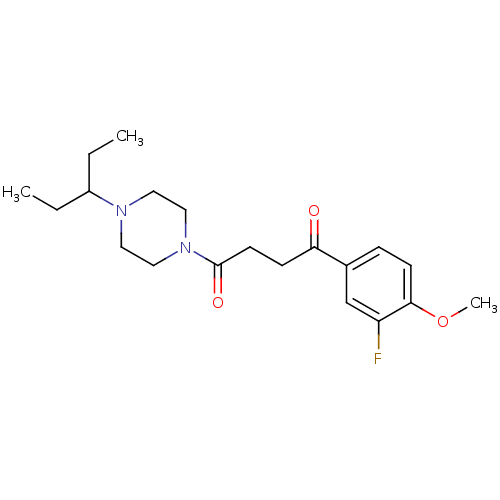
(CHEMBL1202893)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(OC)c(F)c1 Show InChI InChI=1S/C20H29FN2O3/c1-4-16(5-2)22-10-12-23(13-11-22)20(25)9-7-18(24)15-6-8-19(26-3)17(21)14-15/h6,8,14,16H,4-5,7,9-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146836
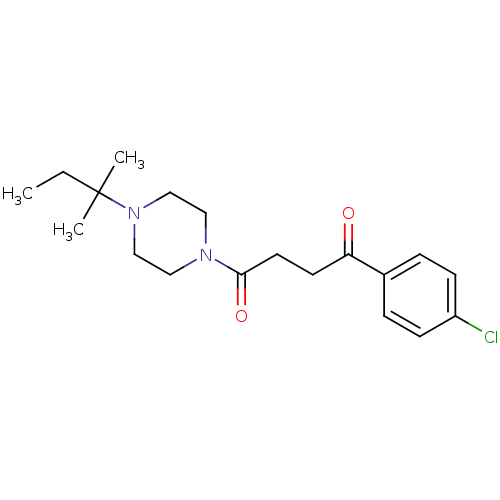
(1-(4-Chloro-phenyl)-4-[4-(1,1-dimethyl-propyl)-pip...)Show SMILES CCC(C)(C)N1CCN(CC1)C(=O)CCC(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H27ClN2O2/c1-4-19(2,3)22-13-11-21(12-14-22)18(24)10-9-17(23)15-5-7-16(20)8-6-15/h5-8H,4,9-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370328
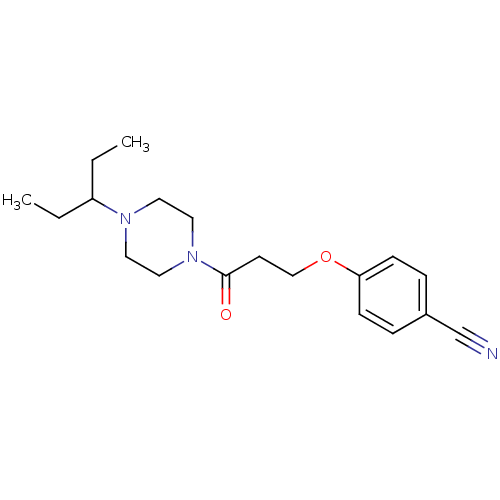
(CHEMBL1202892)Show InChI InChI=1S/C19H27N3O2/c1-3-17(4-2)21-10-12-22(13-11-21)19(23)9-14-24-18-7-5-16(15-20)6-8-18/h5-8,17H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146838
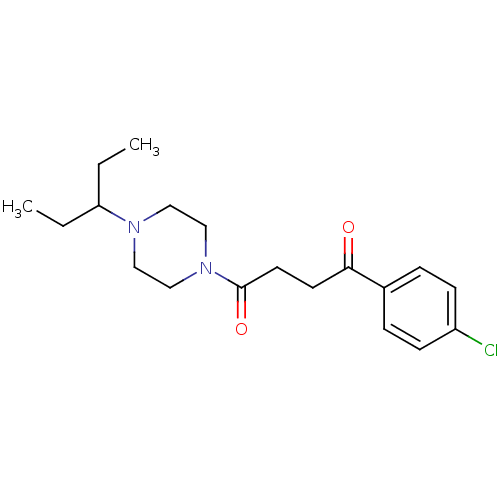
(1-(4-Chloro-phenyl)-4-[4-(1-ethyl-propyl)-piperazi...)Show InChI InChI=1S/C19H27ClN2O2/c1-3-17(4-2)21-11-13-22(14-12-21)19(24)10-9-18(23)15-5-7-16(20)8-6-15/h5-8,17H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370331
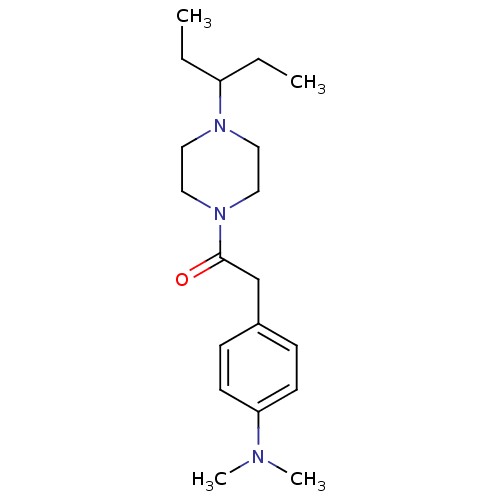
(CHEMBL1202896)Show InChI InChI=1S/C19H31N3O/c1-5-17(6-2)21-11-13-22(14-12-21)19(23)15-16-7-9-18(10-8-16)20(3)4/h7-10,17H,5-6,11-15H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159113
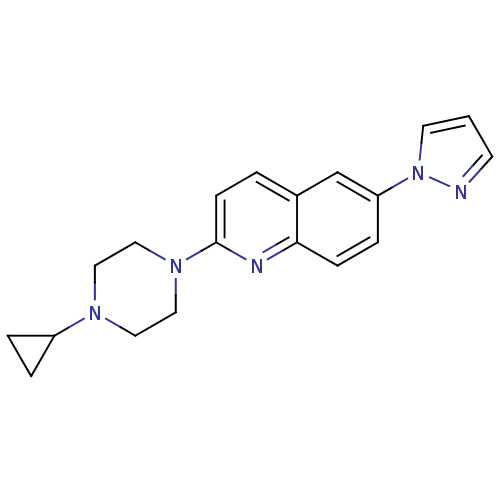
(2-(4-Cyclopropyl-piperazin-1-yl)-6-pyrazol-1-yl-qu...)Show InChI InChI=1S/C19H21N5/c1-8-20-24(9-1)17-5-6-18-15(14-17)2-7-19(21-18)23-12-10-22(11-13-23)16-3-4-16/h1-2,5-9,14,16H,3-4,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146835
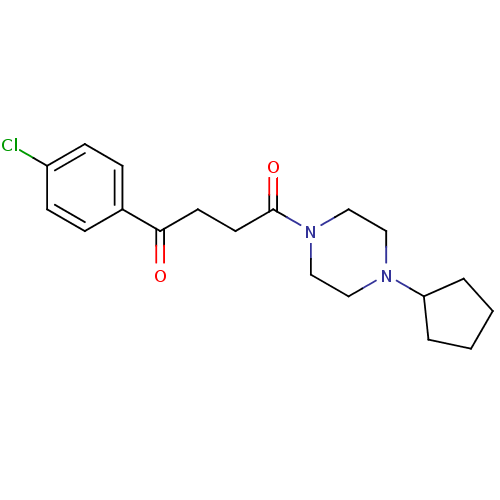
(1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...)Show InChI InChI=1S/C19H25ClN2O2/c20-16-7-5-15(6-8-16)18(23)9-10-19(24)22-13-11-21(12-14-22)17-3-1-2-4-17/h5-8,17H,1-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146826
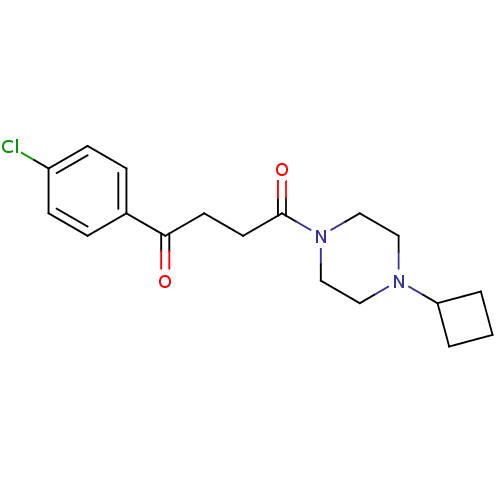
(1-(4-Chloro-phenyl)-4-(4-cyclobutyl-piperazin-1-yl...)Show InChI InChI=1S/C18H23ClN2O2/c19-15-6-4-14(5-7-15)17(22)8-9-18(23)21-12-10-20(11-13-21)16-2-1-3-16/h4-7,16H,1-3,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146840
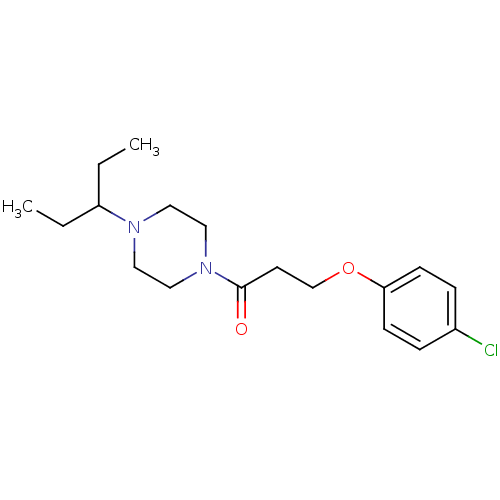
(3-(4-Chloro-phenoxy)-1-[4-(1-ethyl-propyl)-piperaz...)Show InChI InChI=1S/C18H27ClN2O2/c1-3-16(4-2)20-10-12-21(13-11-20)18(22)9-14-23-17-7-5-15(19)6-8-17/h5-8,16H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370336
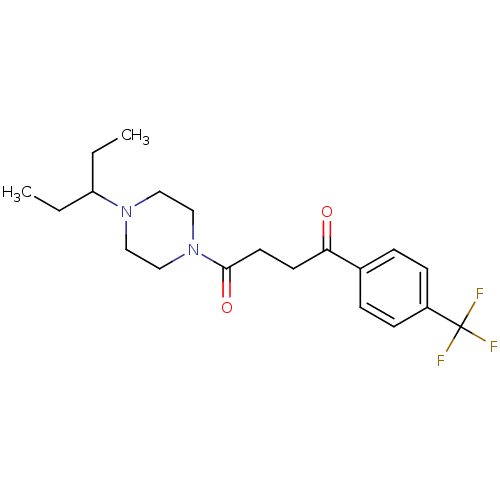
(CHEMBL1202903)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C20H27F3N2O2/c1-3-17(4-2)24-11-13-25(14-12-24)19(27)10-9-18(26)15-5-7-16(8-6-15)20(21,22)23/h5-8,17H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370338
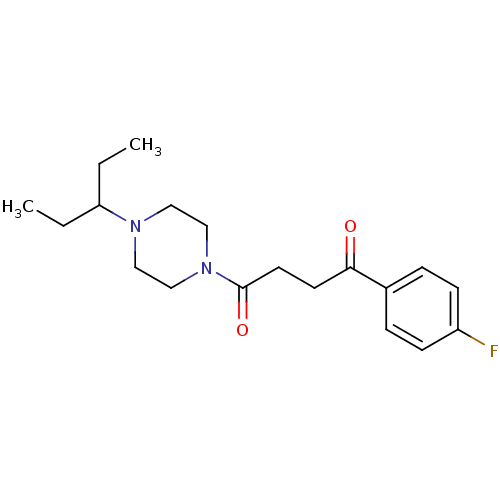
(CHEMBL1202904)Show InChI InChI=1S/C19H27FN2O2/c1-3-17(4-2)21-11-13-22(14-12-21)19(24)10-9-18(23)15-5-7-16(20)8-6-15/h5-8,17H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543
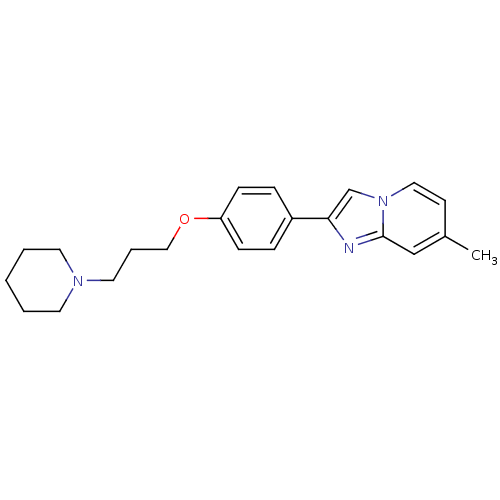
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370327

(CHEMBL1202901)Show InChI InChI=1S/C18H25F3N2O/c1-3-16(4-2)22-9-11-23(12-10-22)17(24)13-14-5-7-15(8-6-14)18(19,20)21/h5-8,16H,3-4,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370326

(CHEMBL1202900)Show InChI InChI=1S/C18H25F3N2O2/c1-3-15(4-2)22-9-11-23(12-10-22)17(24)13-14-5-7-16(8-6-14)25-18(19,20)21/h5-8,15H,3-4,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22904

((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...)Show InChI InChI=1S/C6H11N3/c1-5(7)2-6-3-8-4-9-6/h3-5H,2,7H2,1H3,(H,8,9)/t5-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370329
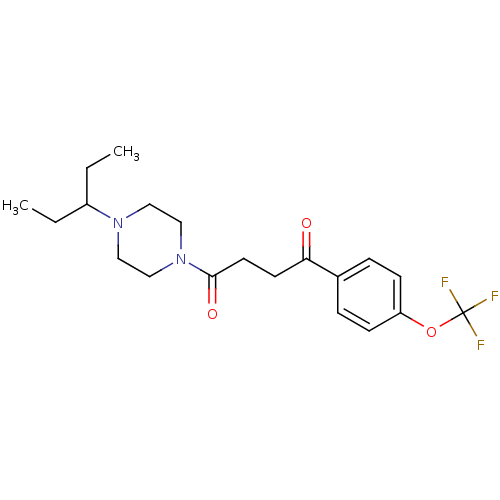
(CHEMBL1202894)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C20H27F3N2O3/c1-3-16(4-2)24-11-13-25(14-12-24)19(27)10-9-18(26)15-5-7-17(8-6-15)28-20(21,22)23/h5-8,16H,3-4,9-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159116
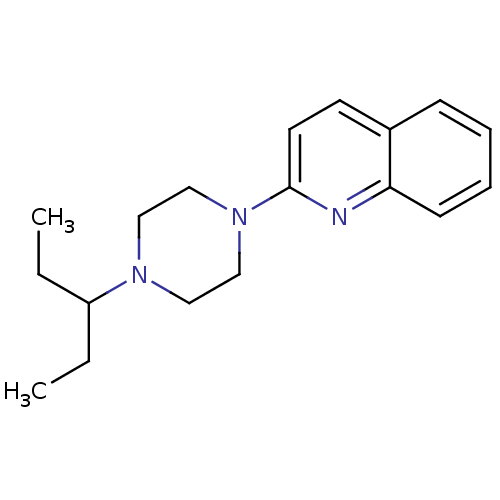
(2-[4-(1-Ethyl-propyl)-piperazin-1-yl]-quinoline | ...)Show InChI InChI=1S/C18H25N3/c1-3-16(4-2)20-11-13-21(14-12-20)18-10-9-15-7-5-6-8-17(15)19-18/h5-10,16H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370339
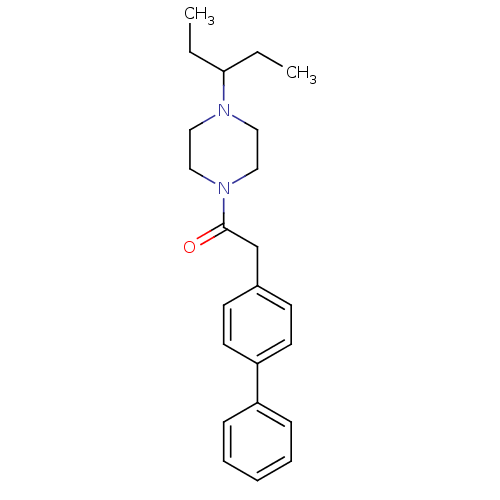
(CHEMBL1202899)Show InChI InChI=1S/C23H30N2O/c1-3-22(4-2)24-14-16-25(17-15-24)23(26)18-19-10-12-21(13-11-19)20-8-6-5-7-9-20/h5-13,22H,3-4,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159122
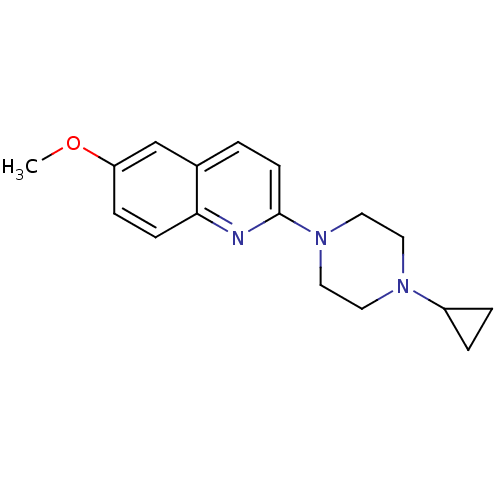
(2-(4-Cyclopropyl-piperazin-1-yl)-6-methoxy-quinoli...)Show InChI InChI=1S/C17H21N3O/c1-21-15-5-6-16-13(12-15)2-7-17(18-16)20-10-8-19(9-11-20)14-3-4-14/h2,5-7,12,14H,3-4,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146843
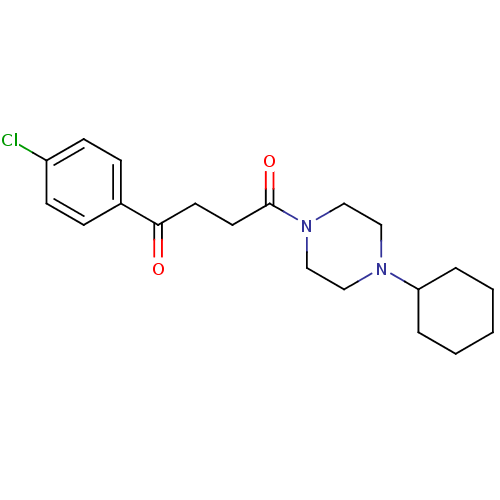
(1-(4-Chloro-phenyl)-4-(4-cyclohexyl-piperazin-1-yl...)Show InChI InChI=1S/C20H27ClN2O2/c21-17-8-6-16(7-9-17)19(24)10-11-20(25)23-14-12-22(13-15-23)18-4-2-1-3-5-18/h6-9,18H,1-5,10-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193204
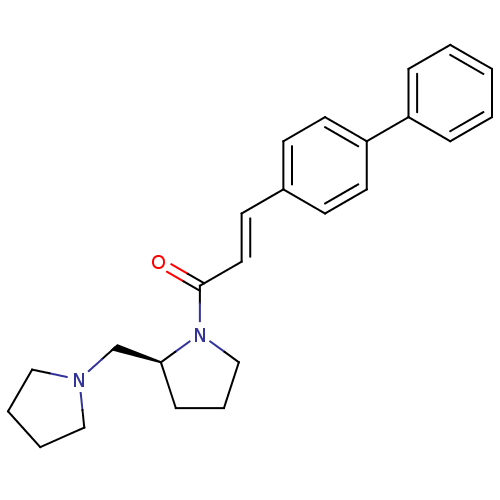
(3-biphenyl-4-yl-1-((S)-2-pyrrolidin-1-ylmethyl-pyr...)Show SMILES O=C(\C=C\c1ccc(cc1)-c1ccccc1)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C24H28N2O/c27-24(26-18-6-9-23(26)19-25-16-4-5-17-25)15-12-20-10-13-22(14-11-20)21-7-2-1-3-8-21/h1-3,7-8,10-15,23H,4-6,9,16-19H2/b15-12+/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185383
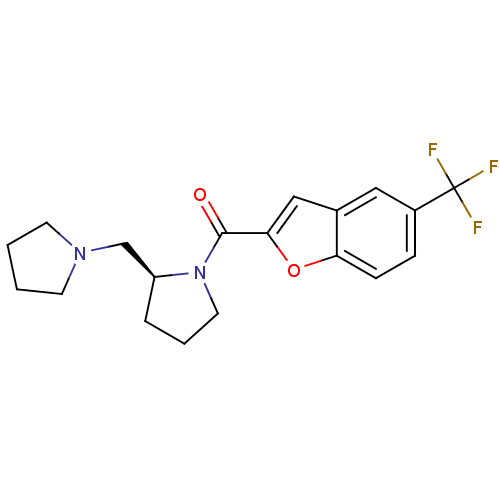
((S)-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)(5-(...)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C19H21F3N2O2/c20-19(21,22)14-5-6-16-13(10-14)11-17(26-16)18(25)24-9-3-4-15(24)12-23-7-1-2-8-23/h5-6,10-11,15H,1-4,7-9,12H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185376
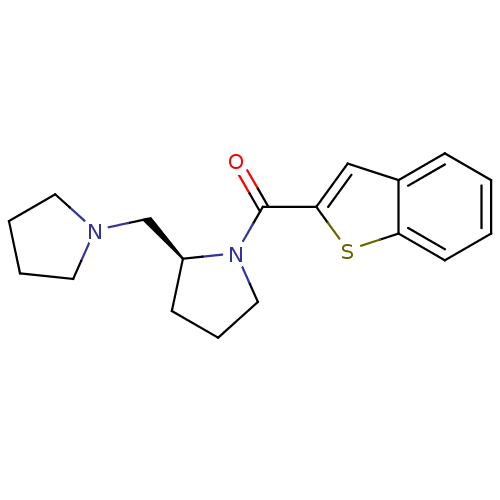
((S)-benzo[b]thiophen-2-yl(2-(pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C18H22N2OS/c21-18(17-12-14-6-1-2-8-16(14)22-17)20-11-5-7-15(20)13-19-9-3-4-10-19/h1-2,6,8,12,15H,3-5,7,9-11,13H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146839

(1-(4-Chloro-phenyl)-4-(4-isopropyl-piperazin-1-yl)...)Show InChI InChI=1S/C17H23ClN2O2/c1-13(2)19-9-11-20(12-10-19)17(22)8-7-16(21)14-3-5-15(18)6-4-14/h3-6,13H,7-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193199
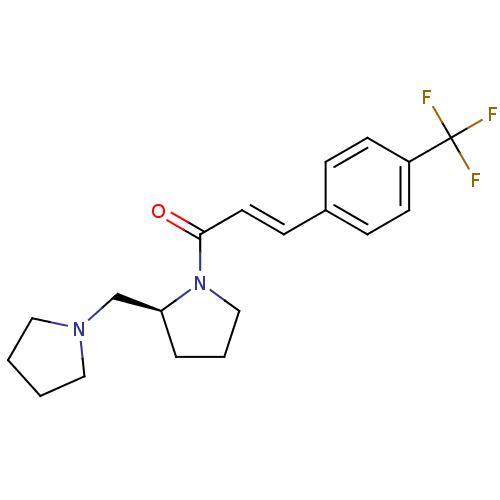
((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 |r| Show InChI InChI=1S/C19H23F3N2O/c20-19(21,22)16-8-5-15(6-9-16)7-10-18(25)24-13-3-4-17(24)14-23-11-1-2-12-23/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159121

(2-(4-Cyclopropyl-piperazin-1-yl)-quinoline-6-carbo...)Show InChI InChI=1S/C17H18N4/c18-12-13-1-5-16-14(11-13)2-6-17(19-16)21-9-7-20(8-10-21)15-3-4-15/h1-2,5-6,11,15H,3-4,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185378
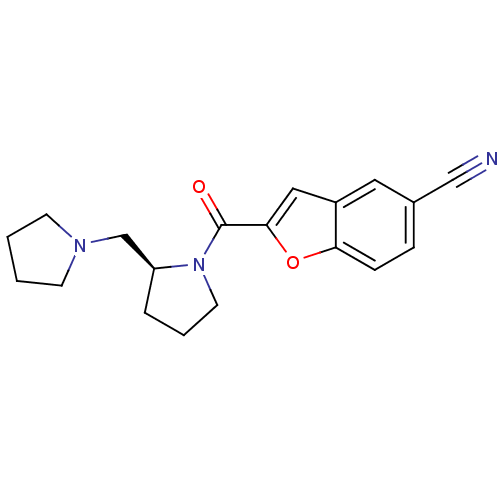
((S)-2-(2-(pyrrolidin-1-ylmethyl)pyrrolidine-1-carb...)Show InChI InChI=1S/C19H21N3O2/c20-12-14-5-6-17-15(10-14)11-18(24-17)19(23)22-9-3-4-16(22)13-21-7-1-2-8-21/h5-6,10-11,16H,1-4,7-9,13H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185371
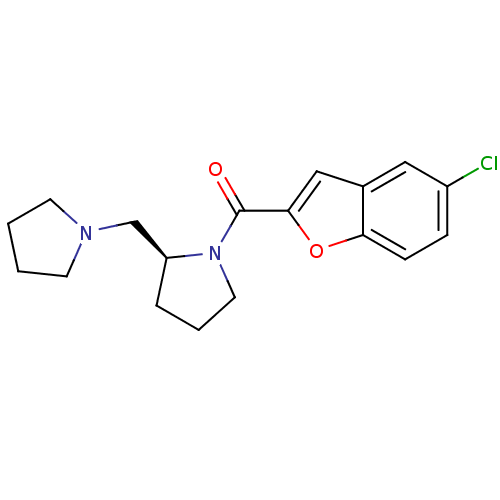
((S)-(5-chlorobenzofuran-2-yl)(2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C18H21ClN2O2/c19-14-5-6-16-13(10-14)11-17(23-16)18(22)21-9-3-4-15(21)12-20-7-1-2-8-20/h5-6,10-11,15H,1-4,7-9,12H2/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159120
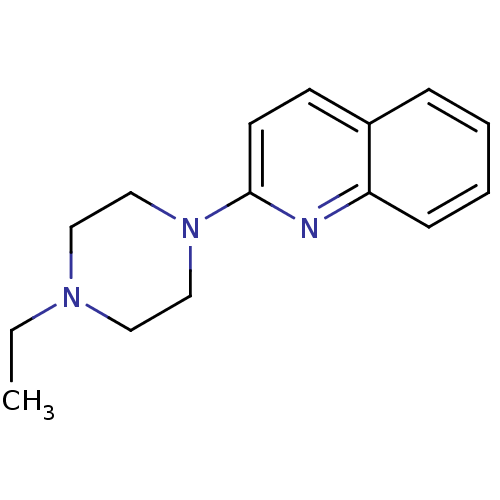
(2-(4-Propyl-piperazin-1-yl)-quinoline | CHEMBL1804...)Show InChI InChI=1S/C15H19N3/c1-2-17-9-11-18(12-10-17)15-8-7-13-5-3-4-6-14(13)16-15/h3-8H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159108
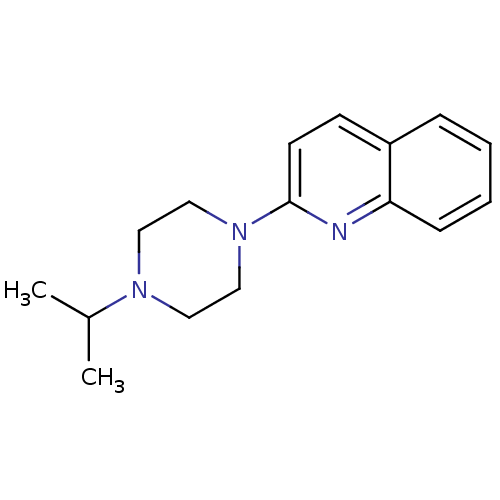
(2-(4-Isopropyl-piperazin-1-yl)-quinoline | CHEMBL3...)Show InChI InChI=1S/C16H21N3/c1-13(2)18-9-11-19(12-10-18)16-8-7-14-5-3-4-6-15(14)17-16/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185370
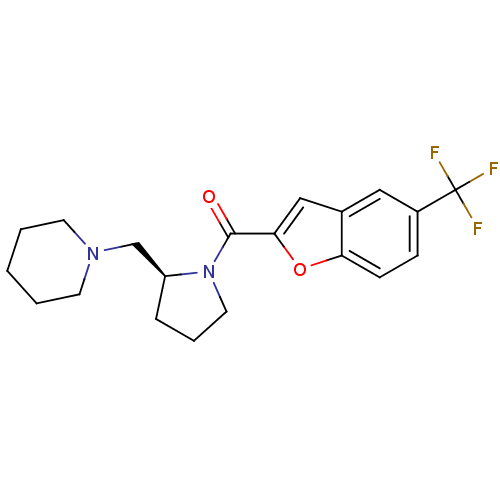
((S)-(2-(piperidin-1-ylmethyl)pyrrolidin-1-yl)(5-(t...)Show SMILES FC(F)(F)c1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCCC1 Show InChI InChI=1S/C20H23F3N2O2/c21-20(22,23)15-6-7-17-14(11-15)12-18(27-17)19(26)25-10-4-5-16(25)13-24-8-2-1-3-9-24/h6-7,11-12,16H,1-5,8-10,13H2/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185382
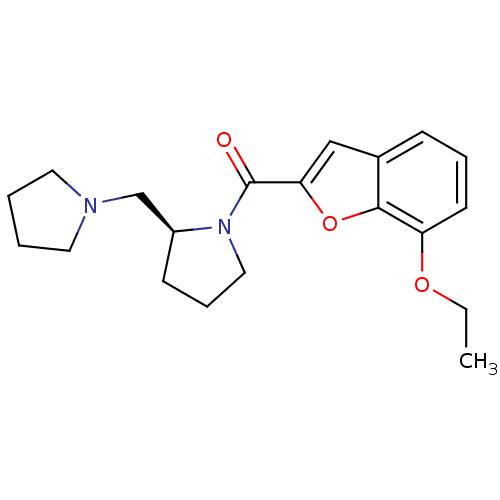
((S)-(7-ethoxybenzofuran-2-yl)(2-(pyrrolidin-1-ylme...)Show InChI InChI=1S/C20H26N2O3/c1-2-24-17-9-5-7-15-13-18(25-19(15)17)20(23)22-12-6-8-16(22)14-21-10-3-4-11-21/h5,7,9,13,16H,2-4,6,8,10-12,14H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146820
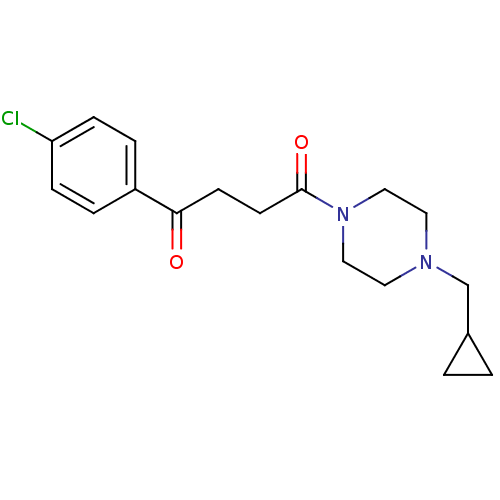
(1-(4-Chloro-phenyl)-4-(4-cyclopropylmethyl-piperaz...)Show InChI InChI=1S/C18H23ClN2O2/c19-16-5-3-15(4-6-16)17(22)7-8-18(23)21-11-9-20(10-12-21)13-14-1-2-14/h3-6,14H,1-2,7-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50370337
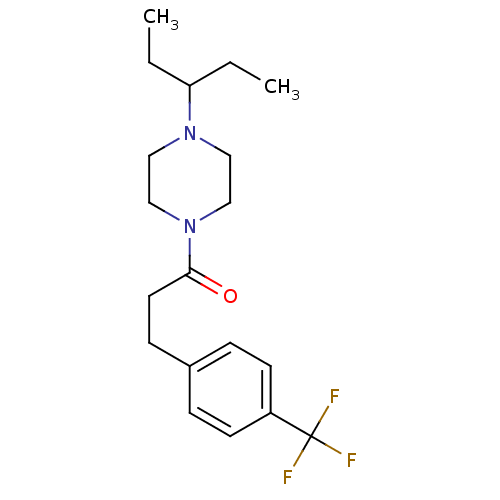
(CHEMBL1202902)Show InChI InChI=1S/C19H27F3N2O/c1-3-17(4-2)23-11-13-24(14-12-23)18(25)10-7-15-5-8-16(9-6-15)19(20,21)22/h5-6,8-9,17H,3-4,7,10-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146818
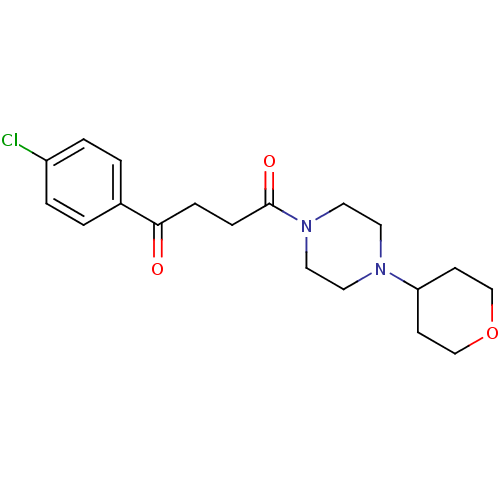
(1-(4-Chloro-phenyl)-4-[4-(tetrahydro-pyran-4-yl)-p...)Show InChI InChI=1S/C19H25ClN2O3/c20-16-3-1-15(2-4-16)18(23)5-6-19(24)22-11-9-21(10-12-22)17-7-13-25-14-8-17/h1-4,17H,5-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185377
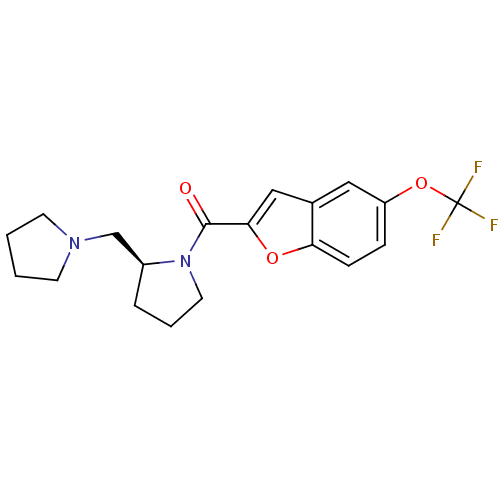
((S)-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)(5-(...)Show SMILES FC(F)(F)Oc1ccc2oc(cc2c1)C(=O)N1CCC[C@H]1CN1CCCC1 Show InChI InChI=1S/C19H21F3N2O3/c20-19(21,22)27-15-5-6-16-13(10-15)11-17(26-16)18(25)24-9-3-4-14(24)12-23-7-1-2-8-23/h5-6,10-11,14H,1-4,7-9,12H2/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193175
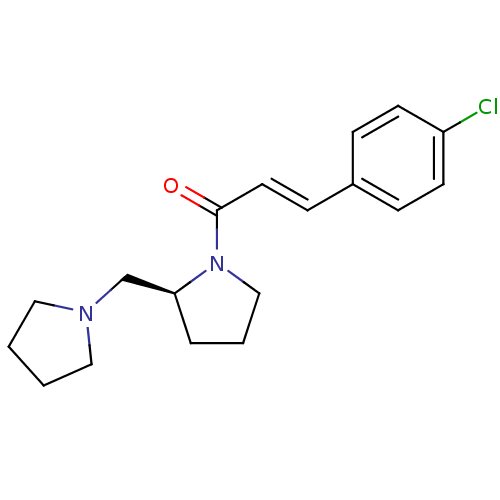
((S)-3-(4-chlorophenyl)-1-(2-(pyrrolidin-1-ylmethyl...)Show InChI InChI=1S/C18H23ClN2O/c19-16-8-5-15(6-9-16)7-10-18(22)21-13-3-4-17(21)14-20-11-1-2-12-20/h5-10,17H,1-4,11-14H2/b10-7+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185381
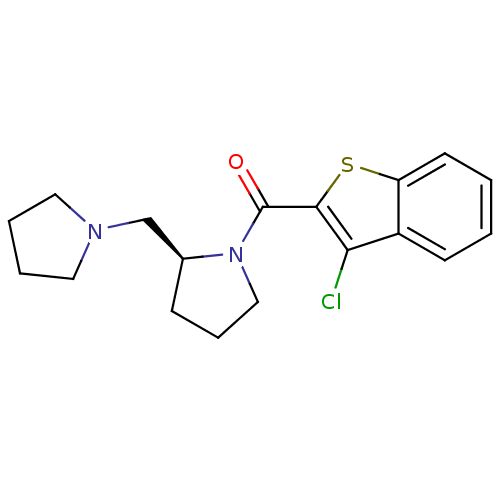
((S)-(3-chlorobenzo[b]thiophen-2-yl)(2-(pyrrolidin-...)Show InChI InChI=1S/C18H21ClN2OS/c19-16-14-7-1-2-8-15(14)23-17(16)18(22)21-11-5-6-13(21)12-20-9-3-4-10-20/h1-2,7-8,13H,3-6,9-12H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159109
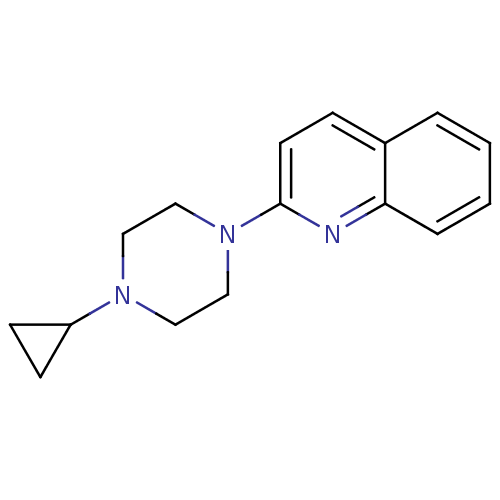
(2-(4-Cyclopropyl-piperazin-1-yl)-quinoline | CHEMB...)Show InChI InChI=1S/C16H19N3/c1-2-4-15-13(3-1)5-8-16(17-15)19-11-9-18(10-12-19)14-6-7-14/h1-5,8,14H,6-7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146829

(1-(4-Chloro-phenyl)-4-(4-cyclopropyl-piperazin-1-y...)Show InChI InChI=1S/C17H21ClN2O2/c18-14-3-1-13(2-4-14)16(21)7-8-17(22)20-11-9-19(10-12-20)15-5-6-15/h1-4,15H,5-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human histamine H3 receptor expressed in CHO cells was determined by GTPgamma-S-assay |
J Med Chem 47: 2833-8 (2004)
Article DOI: 10.1021/jm031028z
BindingDB Entry DOI: 10.7270/Q2T154CW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193185

((S)-1-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl)-3...)Show SMILES FC(F)(F)Oc1ccc(\C=C\C(=O)N2CCC[C@H]2CN2CCCC2)cc1 Show InChI InChI=1S/C19H23F3N2O2/c20-19(21,22)26-17-8-5-15(6-9-17)7-10-18(25)24-13-3-4-16(24)14-23-11-1-2-12-23/h5-10,16H,1-4,11-14H2/b10-7+/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50185372
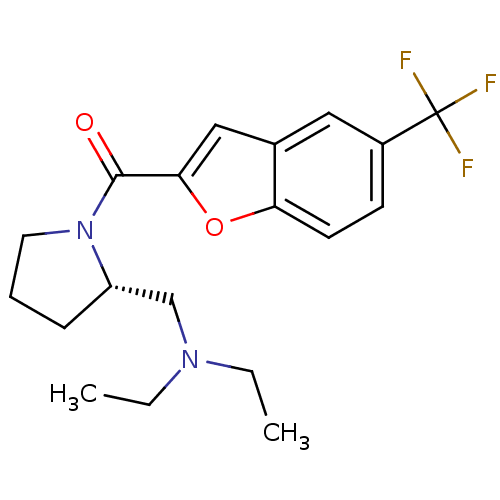
((S)-(2-((diethylamino)methyl)pyrrolidin-1-yl)(5-(t...)Show SMILES CCN(CC)C[C@@H]1CCCN1C(=O)c1cc2cc(ccc2o1)C(F)(F)F Show InChI InChI=1S/C19H23F3N2O2/c1-3-23(4-2)12-15-6-5-9-24(15)18(25)17-11-13-10-14(19(20,21)22)7-8-16(13)26-17/h7-8,10-11,15H,3-6,9,12H2,1-2H3/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor by [35S]GTPgammaS assay |
Bioorg Med Chem Lett 16: 3162-5 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.074
BindingDB Entry DOI: 10.7270/Q2T1537P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50193189

(CHEMBL218834 | [4-(3-Aza-bicyclo[3.2.2]nonane-3-ca...)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CCC(CC1)C(=O)N1CC2CCC(CC2)C1 |(-10.27,-12.49,;-8.93,-13.25,;-8.92,-14.79,;-7.6,-12.47,;-7.61,-10.93,;-6.29,-10.15,;-4.94,-10.9,;-4.93,-12.44,;-6.26,-13.23,;-3.62,-10.12,;-3.64,-8.58,;-2.28,-10.87,;-2.27,-12.41,;-.93,-13.16,;.4,-12.38,;.38,-10.84,;-.96,-10.08,;1.74,-13.14,;1.75,-14.68,;3.07,-12.37,;4.64,-12.83,;5.99,-11.86,;7.33,-12.56,;6.6,-11.37,;4.98,-10.51,;5.01,-9.04,;6.04,-10.28,;4.29,-11.83,)| Show InChI InChI=1S/C22H38N4O2/c1-17(2)23-11-13-25(14-12-23)22(28)24-9-7-20(8-10-24)21(27)26-15-18-3-4-19(16-26)6-5-18/h17-20H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity at histamine H3 receptor by hH3-[35S]GTPgamma[S] binding assay |
Bioorg Med Chem Lett 16: 5303-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.093
BindingDB Entry DOI: 10.7270/Q2RV0NBD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data