 Found 1006 hits with Last Name = 'tang' and Initial = 'k'
Found 1006 hits with Last Name = 'tang' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86549
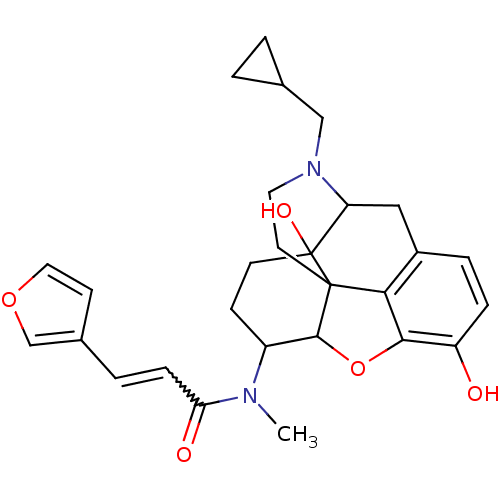
(CAS_213055 | NSC_213055 | TRK-820)Show SMILES CN(C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45)C(=O)C=Cc1ccoc1 |w:28.33,TLB:4:5:8.9.25:18.19.20,6:5:8.9.25:18.19.20| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86549

(CAS_213055 | NSC_213055 | TRK-820)Show SMILES CN(C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45)C(=O)C=Cc1ccoc1 |w:28.33,TLB:4:5:8.9.25:18.19.20,6:5:8.9.25:18.19.20| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50343621
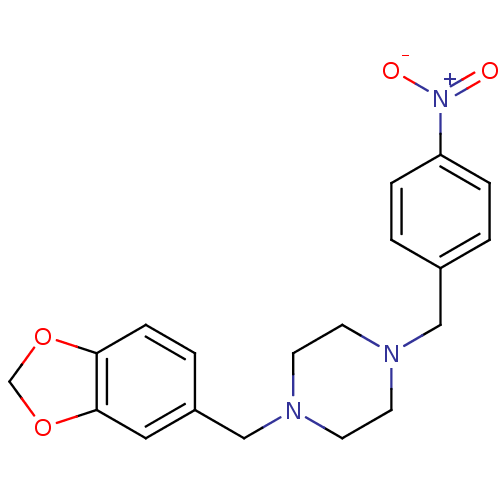
(1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-nitrobenzyl)pi...)Show SMILES [O-][N+](=O)c1ccc(CN2CCN(Cc3ccc4OCOc4c3)CC2)cc1 Show InChI InChI=1S/C19H21N3O4/c23-22(24)17-4-1-15(2-5-17)12-20-7-9-21(10-8-20)13-16-3-6-18-19(11-16)26-14-25-18/h1-6,11H,7-10,12-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50343619

(1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-bromobenzyl)pi...)Show InChI InChI=1S/C19H21BrN2O2/c20-17-4-1-15(2-5-17)12-21-7-9-22(10-8-21)13-16-3-6-18-19(11-16)24-14-23-18/h1-6,11H,7-10,12-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50343620

(1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-iodobenzyl)pip...)Show InChI InChI=1S/C19H21IN2O2/c20-17-4-1-15(2-5-17)12-21-7-9-22(10-8-21)13-16-3-6-18-19(11-16)24-14-23-18/h1-6,11H,7-10,12-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50343618
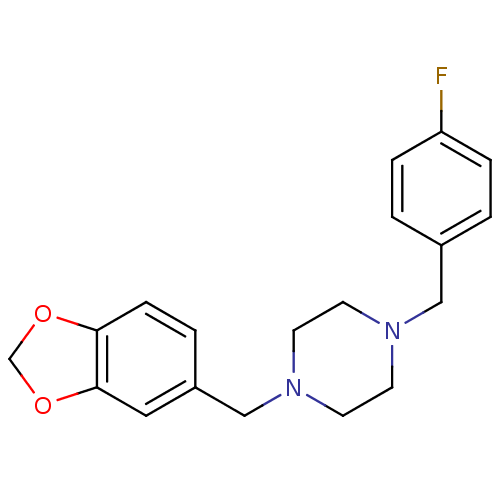
(1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-fluorobenzyl)p...)Show InChI InChI=1S/C19H21FN2O2/c20-17-4-1-15(2-5-17)12-21-7-9-22(10-8-21)13-16-3-6-18-19(11-16)24-14-23-18/h1-6,11H,7-10,12-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50343622
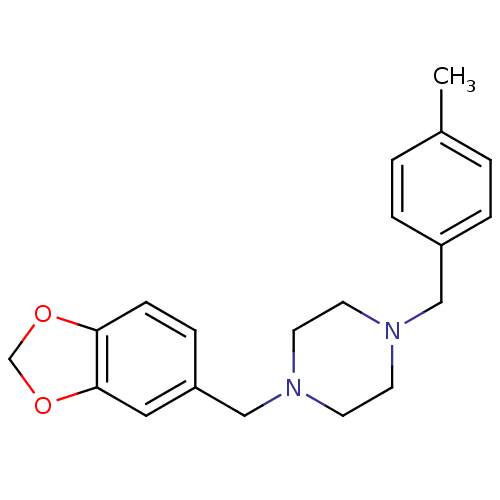
(1-(1,3-Benzodioxol-5-ylmethyl)-4-(4-methylbenzyl)-...)Show InChI InChI=1S/C20H24N2O2/c1-16-2-4-17(5-3-16)13-21-8-10-22(11-9-21)14-18-6-7-19-20(12-18)24-15-23-19/h2-7,12H,8-11,13-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in rat brain membranes by competitive binding assay |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326392

((2S)-1-({(2S,4S)-4-[2-(1,3-Dihydro-2H-isoindol-2-y...)Show SMILES FC1(F)C[C@@H](C#N)N(C1)C(=O)[C@@H]1C[C@@H](CC(=O)N2Cc3ccccc3C2)C(=O)N1 |r| Show InChI InChI=1S/C20H20F2N4O3/c21-20(22)7-15(8-23)26(11-20)19(29)16-5-14(18(28)24-16)6-17(27)25-9-12-3-1-2-4-13(12)10-25/h1-4,14-16H,5-7,9-11H2,(H,24,28)/t14-,15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase
(Homo sapiens (Human)) | BDBM50338990
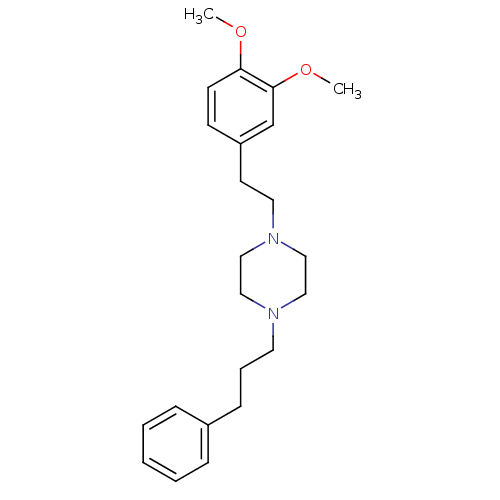
(1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...)Show InChI InChI=1S/C23H32N2O2/c1-26-22-11-10-21(19-23(22)27-2)12-14-25-17-15-24(16-18-25)13-6-9-20-7-4-3-5-8-20/h3-5,7-8,10-11,19H,6,9,12-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Key Laboratory of Radiopharmaceuticals (Beijing Normal University)
Curated by ChEMBL
| Assay Description
Binding affinity to emopamil binding protein |
Bioorg Med Chem 19: 2911-7 (2011)
Article DOI: 10.1016/j.bmc.2011.03.037
BindingDB Entry DOI: 10.7270/Q2QJ7HN1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86547
(CAS_135349 | NSC_135349 | U50,488H)Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86549

(CAS_213055 | NSC_213055 | TRK-820)Show SMILES CN(C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45)C(=O)C=Cc1ccoc1 |w:28.33,TLB:4:5:8.9.25:18.19.20,6:5:8.9.25:18.19.20| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM86546
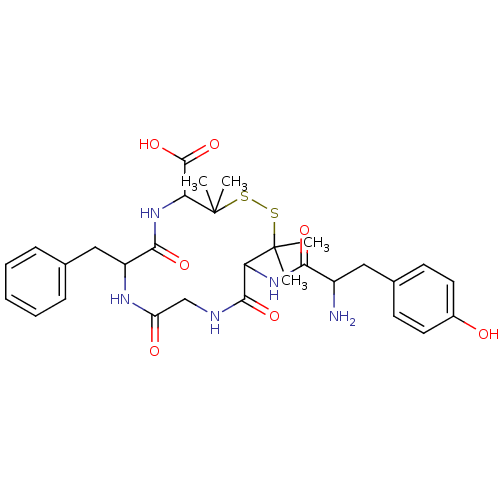
(CAS_88373-73-3 | DPDPE | NSC_104787)Show SMILES CC1(C)SSC(C)(C)C(NC(=O)C(N)Cc2ccc(O)cc2)C(=O)NCC(=O)NC(Cc2ccccc2)C(=O)NC1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Sphingosine kinase 1
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of recombinant human C-terminal His-tagged SphK1 expressed in baculovirus infected Sf21 cells using varying concentrations of ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112713
BindingDB Entry DOI: 10.7270/Q27W6GWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86548
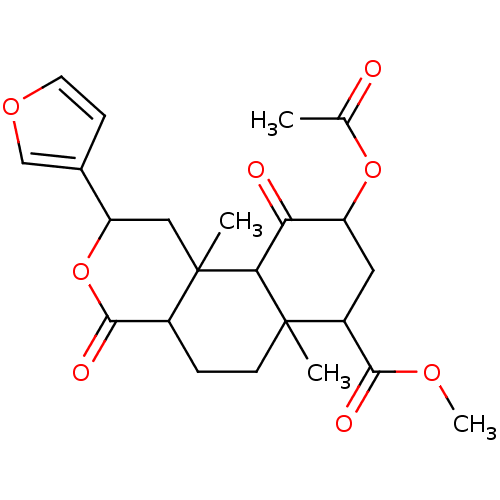
(CAS_83729-01-5 | NSC_128563 | Salvinorin A)Show SMILES COC(=O)C1CC(OC(C)=O)C(=O)C2C1(C)CCC1C(=O)OC(CC21C)c1ccoc1 Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315675
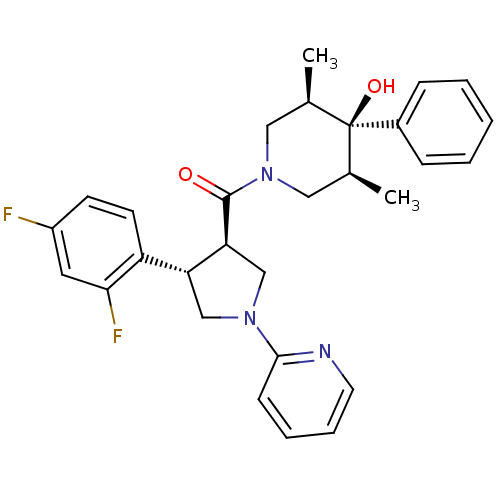
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-2-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccccn1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-8-4-3-5-9-21)28(35)25-18-33(27-10-6-7-13-32-27)17-24(25)23-12-11-22(30)14-26(23)31/h3-14,19-20,24-25,36H,15-18H2,1-2H3/t19-,20+,24-,25+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315688
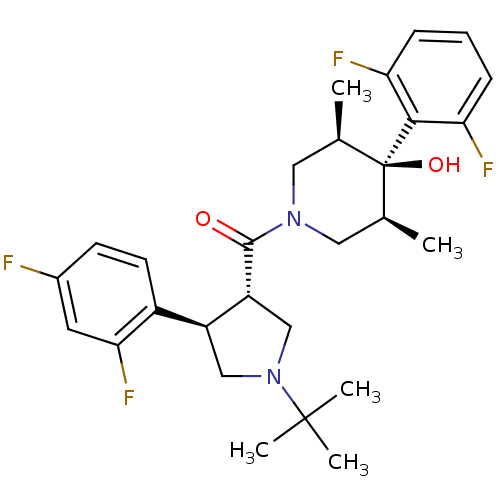
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1c(F)cccc1F)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H34F4N2O2/c1-16-12-33(13-17(2)28(16,36)25-22(30)7-6-8-23(25)31)26(35)21-15-34(27(3,4)5)14-20(21)19-10-9-18(29)11-24(19)32/h6-11,16-17,20-21,36H,12-15H2,1-5H3/t16-,17+,20-,21+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315674
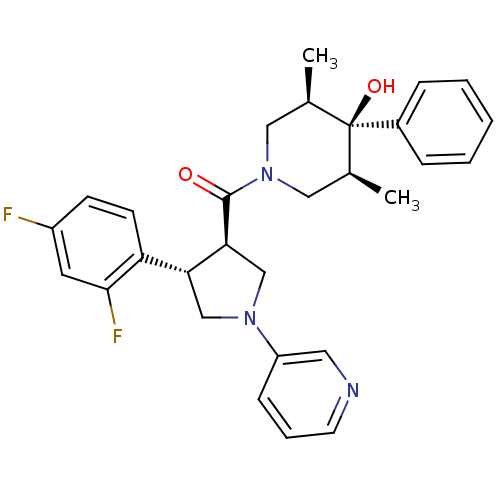
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86549

(CAS_213055 | NSC_213055 | TRK-820)Show SMILES CN(C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC1CC1)c45)C(=O)C=Cc1ccoc1 |w:28.33,TLB:4:5:8.9.25:18.19.20,6:5:8.9.25:18.19.20| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315676
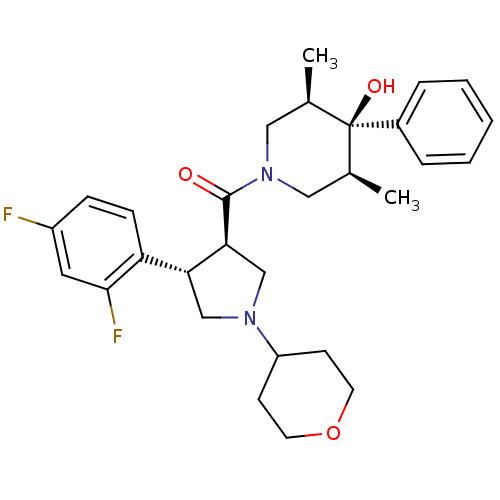
(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315686
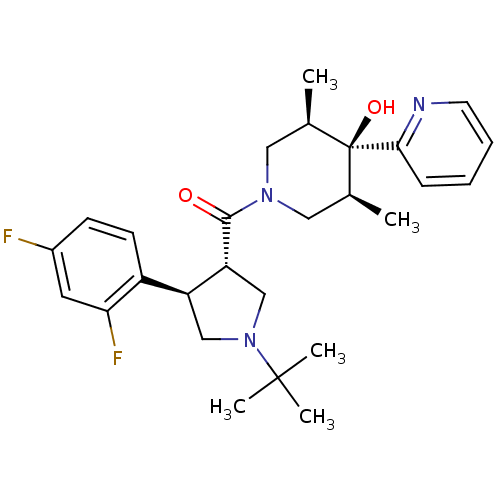
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C27H35F2N3O2/c1-17-13-31(14-18(2)27(17,34)24-8-6-7-11-30-24)25(33)22-16-32(26(3,4)5)15-21(22)20-10-9-19(28)12-23(20)29/h6-12,17-18,21-22,34H,13-16H2,1-5H3/t17-,18+,21-,22+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315680
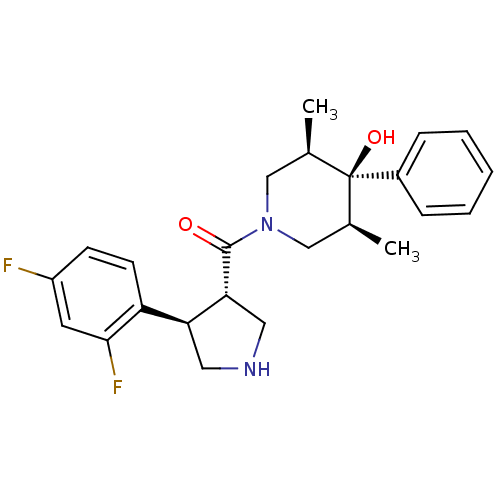
(((3S,4R)-4-(2,4-difluorophenyl)pyrrolidin-3-yl)((3...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CNC[C@H]1c1ccc(F)cc1F |r| Show InChI InChI=1S/C24H28F2N2O2/c1-15-13-28(14-16(2)24(15,30)17-6-4-3-5-7-17)23(29)21-12-27-11-20(21)19-9-8-18(25)10-22(19)26/h3-10,15-16,20-21,27,30H,11-14H2,1-2H3/t15-,16+,20-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86548

(CAS_83729-01-5 | NSC_128563 | Salvinorin A)Show SMILES COC(=O)C1CC(OC(C)=O)C(=O)C2C1(C)CCC1C(=O)OC(CC21C)c1ccoc1 Show InChI InChI=1S/C23H28O8/c1-12(24)30-16-9-15(20(26)28-4)22(2)7-5-14-21(27)31-17(13-6-8-29-11-13)10-23(14,3)19(22)18(16)25/h6,8,11,14-17,19H,5,7,9-10H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315681
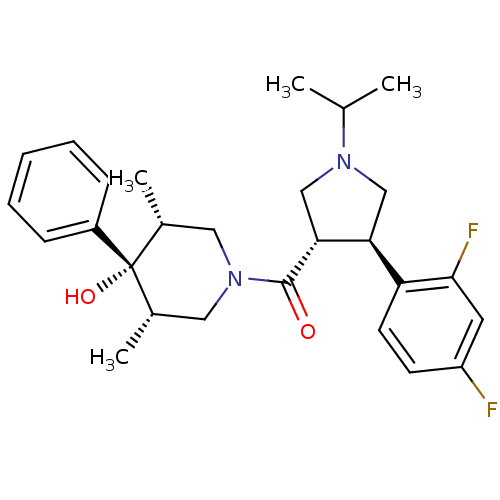
(((3S,4R)-4-(2,4-difluorophenyl)-1-isopropylpyrroli...)Show SMILES CC(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1C[C@H](C)[C@](O)([C@H](C)C1)c1ccccc1 |r| Show InChI InChI=1S/C27H34F2N2O2/c1-17(2)30-15-23(22-11-10-21(28)12-25(22)29)24(16-30)26(32)31-13-18(3)27(33,19(4)14-31)20-8-6-5-7-9-20/h5-12,17-19,23-24,33H,13-16H2,1-4H3/t18-,19+,23-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase FAP
(Homo sapiens (Human)) | BDBM50326373
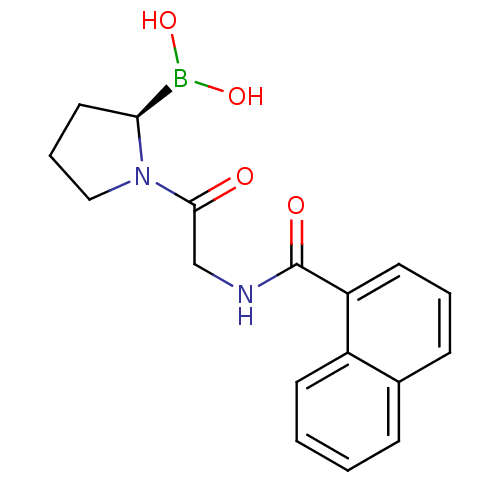
((R)-1-(2-(1-naphthamido)acetyl)pyrrolidin-2-ylboro...)Show SMILES OB(O)[C@@H]1CCCN1C(=O)CNC(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C17H19BN2O4/c21-16(20-10-4-9-15(20)18(23)24)11-19-17(22)14-8-3-6-12-5-1-2-7-13(12)14/h1-3,5-8,15,23-24H,4,9-11H2,(H,19,22)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant FAP expressed in Hi5 insect cells by Lineweaver-Burke plot analysis |
J Med Chem 53: 6572-83 (2010)
Article DOI: 10.1021/jm1002556
BindingDB Entry DOI: 10.7270/Q23F4QM9 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378743
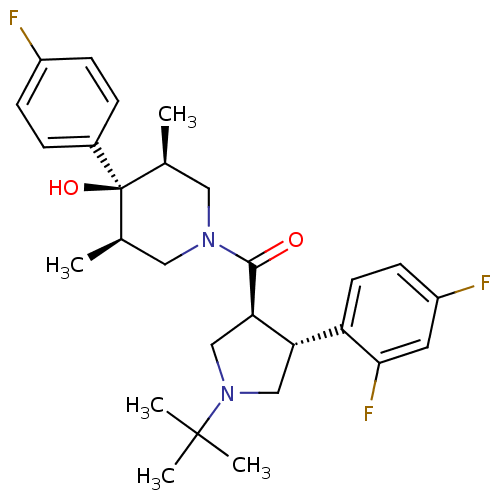
(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM86253
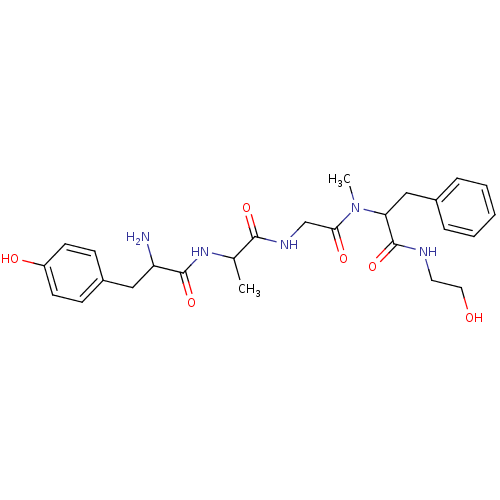
(CAS_100929-53-1 | DAMGO | NSC_104742 | US10836728,...)Show SMILES CC(NC(=O)C(N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)C(Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315679

(((3R,4S)-1-cyclopropyl-4-(2,4-difluorophenyl)pyrro...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CC1 |r| Show InChI InChI=1S/C27H32F2N2O2/c1-17-13-31(14-18(2)27(17,33)19-6-4-3-5-7-19)26(32)24-16-30(21-9-10-21)15-23(24)22-11-8-20(28)12-25(22)29/h3-8,11-12,17-18,21,23-24,33H,9-10,13-16H2,1-2H3/t17-,18+,23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378747
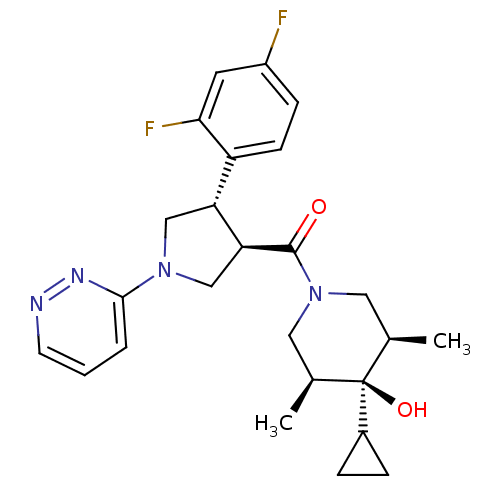
(CHEMBL1204056)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)C1CC1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H30F2N4O2/c1-15-11-31(12-16(2)25(15,33)17-5-6-17)24(32)21-14-30(23-4-3-9-28-29-23)13-20(21)19-8-7-18(26)10-22(19)27/h3-4,7-10,15-17,20-21,33H,5-6,11-14H2,1-2H3/t15-,16+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50378748
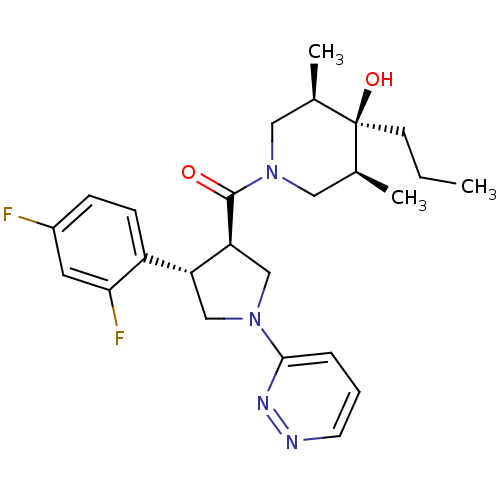
(CHEMBL1204054)Show SMILES CCC[C@]1(O)[C@@H](C)CN(C[C@H]1C)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H32F2N4O2/c1-4-9-25(33)16(2)12-31(13-17(25)3)24(32)21-15-30(23-6-5-10-28-29-23)14-20(21)19-8-7-18(26)11-22(19)27/h5-8,10-11,16-17,20-21,33H,4,9,12-15H2,1-3H3/t16-,17+,20-,21+,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315673
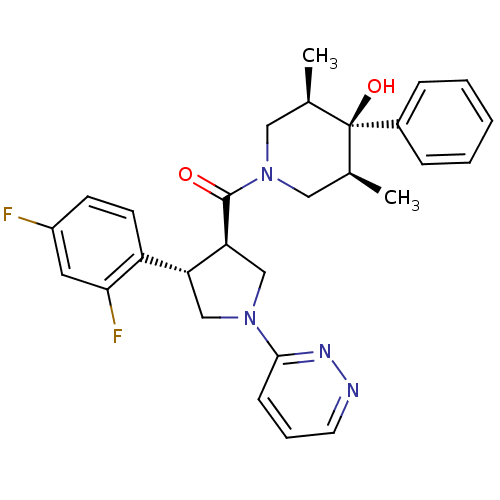
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315673

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-14-34(15-19(2)28(18,36)20-7-4-3-5-8-20)27(35)24-17-33(26-9-6-12-31-32-26)16-23(24)22-11-10-21(29)13-25(22)30/h3-13,18-19,23-24,36H,14-17H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315692
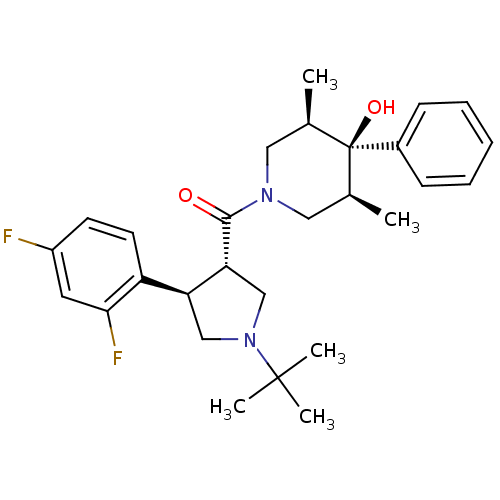
(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315691
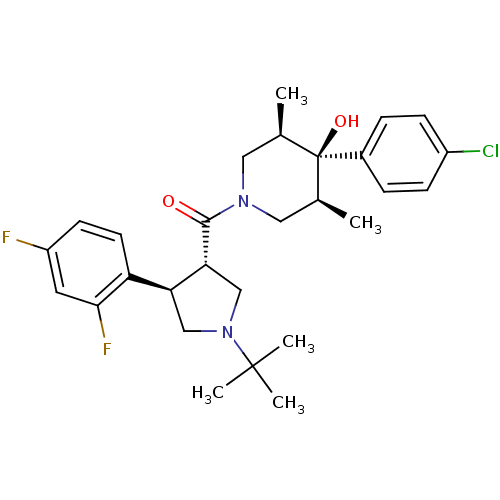
(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50315669
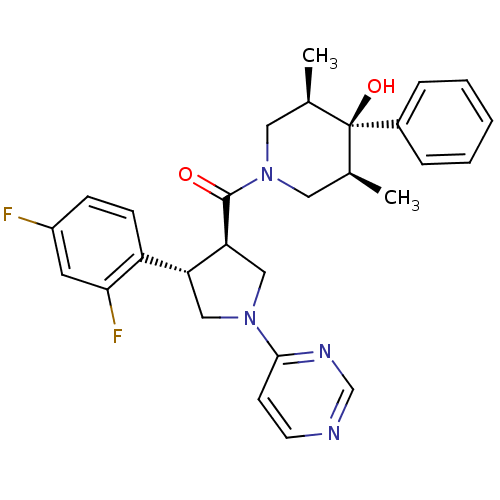
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyrimidin-4-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccncn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-13-34(14-19(2)28(18,36)20-6-4-3-5-7-20)27(35)24-16-33(26-10-11-31-17-32-26)15-23(24)22-9-8-21(29)12-25(22)30/h3-12,17-19,23-24,36H,13-16H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG expressed in CHO cells by patch clamp method |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86547

(CAS_135349 | NSC_135349 | U50,488H)Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |
DCN1-like protein 1
(Homo sapiens) | BDBM50525354
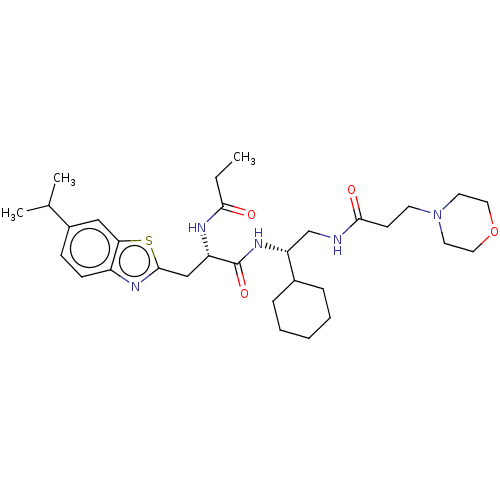
(CHEMBL4517307)Show SMILES CCC(=O)N[C@@H](Cc1nc2ccc(cc2s1)C(C)C)C(=O)N[C@H](CNC(=O)CCN1CCOCC1)C1CCCCC1 |r| Show InChI InChI=1S/C31H47N5O4S/c1-4-28(37)33-25(19-30-34-24-11-10-23(21(2)3)18-27(24)41-30)31(39)35-26(22-8-6-5-7-9-22)20-32-29(38)12-13-36-14-16-40-17-15-36/h10-11,18,21-22,25-26H,4-9,12-17,19-20H2,1-3H3,(H,32,38)(H,33,37)(H,35,39)/t25-,26+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747
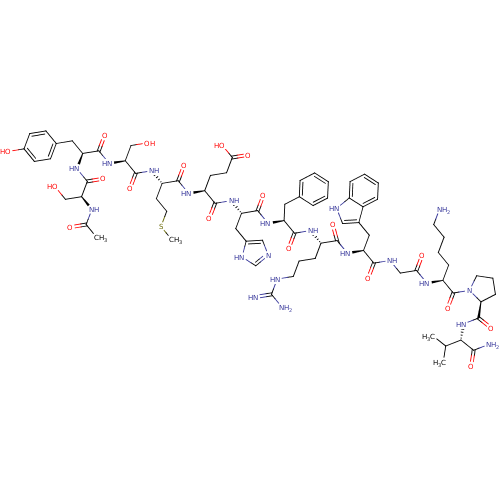
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50553786

(CHEMBL4789106)Show SMILES CC1(CN)CCN(CC1)c1cnc(Sc2cccc(Cl)c2Cl)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315692

(CHEMBL1090488 | CHEMBL1204059)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H36F2N2O2/c1-18-14-31(15-19(2)28(18,34)20-9-7-6-8-10-20)26(33)24-17-32(27(3,4)5)16-23(24)22-12-11-21(29)13-25(22)30/h6-13,18-19,23-24,34H,14-17H2,1-5H3/t18-,19+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50378743

(CHEMBL1204061)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(F)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35F3N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315676

(((3R,4S)-4-(2,4-difluorophenyl)-1-(tetrahydro-2H-p...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCOCC1 |r| Show InChI InChI=1S/C29H36F2N2O3/c1-19-15-33(16-20(2)29(19,35)21-6-4-3-5-7-21)28(34)26-18-32(23-10-12-36-13-11-23)17-25(26)24-9-8-22(30)14-27(24)31/h3-9,14,19-20,23,25-26,35H,10-13,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315691

(((3S,4R)-1-tert-butyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccc(Cl)cc1)C(=O)[C@@H]1CN(C[C@H]1c1ccc(F)cc1F)C(C)(C)C |r| Show InChI InChI=1S/C28H35ClF2N2O2/c1-17-13-32(14-18(2)28(17,35)19-6-8-20(29)9-7-19)26(34)24-16-33(27(3,4)5)15-23(24)22-11-10-21(30)12-25(22)31/h6-12,17-18,23-24,35H,13-16H2,1-5H3/t17-,18+,23-,24+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315674

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridin-3-yl)py...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnc1 |r| Show InChI InChI=1S/C29H31F2N3O2/c1-19-15-34(16-20(2)29(19,36)21-7-4-3-5-8-21)28(35)26-18-33(23-9-6-12-32-14-23)17-25(26)24-11-10-22(30)13-27(24)31/h3-14,19-20,25-26,36H,15-18H2,1-2H3/t19-,20+,25-,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315672
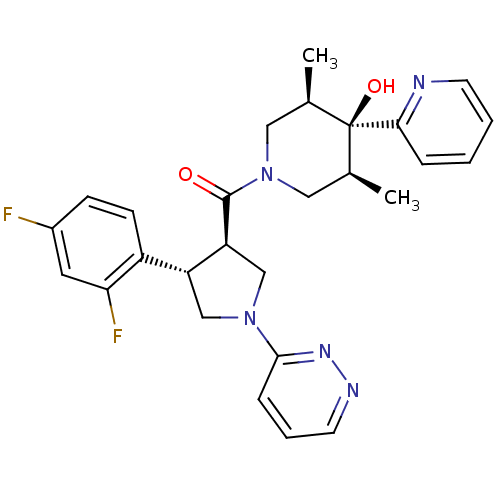
(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyridazin-3-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccn1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C27H29F2N5O2/c1-17-13-34(14-18(2)27(17,36)24-6-3-4-10-30-24)26(35)22-16-33(25-7-5-11-31-32-25)15-21(22)20-9-8-19(28)12-23(20)29/h3-12,17-18,21-22,36H,13-16H2,1-2H3/t17-,18+,21-,22+,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50378748

(CHEMBL1204054)Show SMILES CCC[C@]1(O)[C@@H](C)CN(C[C@H]1C)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1cccnn1 |r| Show InChI InChI=1S/C25H32F2N4O2/c1-4-9-25(33)16(2)12-31(13-17(25)3)24(32)21-15-30(23-6-5-10-28-29-23)14-20(21)19-8-7-18(26)11-22(19)27/h5-8,10-11,16-17,20-21,33H,4,9,12-15H2,1-3H3/t16-,17+,20-,21+,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315677

(((3R,4S)-1-cyclobutyl-4-(2,4-difluorophenyl)pyrrol...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)C1CCC1 |r| Show InChI InChI=1S/C28H34F2N2O2/c1-18-14-32(15-19(2)28(18,34)20-7-4-3-5-8-20)27(33)25-17-31(22-9-6-10-22)16-24(25)23-12-11-21(29)13-26(23)30/h3-5,7-8,11-13,18-19,22,24-25,34H,6,9-10,14-17H2,1-2H3/t18-,19+,24-,25+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50315669

(((3R,4S)-4-(2,4-difluorophenyl)-1-(pyrimidin-4-yl)...)Show SMILES C[C@H]1CN(C[C@@H](C)[C@]1(O)c1ccccc1)C(=O)[C@H]1CN(C[C@@H]1c1ccc(F)cc1F)c1ccncn1 |r| Show InChI InChI=1S/C28H30F2N4O2/c1-18-13-34(14-19(2)28(18,36)20-6-4-3-5-7-20)27(35)24-16-33(26-10-11-31-17-32-26)15-23(24)22-9-8-21(29)12-25(22)30/h3-12,17-19,23-24,36H,13-16H2,1-2H3/t18-,19+,23-,24+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H] melanocortin-2 from human recombinant MC4 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 3183-97 (2010)
Article DOI: 10.1021/jm9017866
BindingDB Entry DOI: 10.7270/Q20G3M4T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114106
BindingDB Entry DOI: 10.7270/Q26977NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50092521

(3-((3-Fluoro-phenyl)-{[(3-fluoro-phenyl)-methoxyca...)Show SMILES COC(=O)C12CN(C)CC(C(N(C)C1c1cccc(F)c1)c1cccc(F)c1)(C(=O)OC)C2=O |TLB:12:11:32:6.5.8,21:10:32:6.5.8,14:13:32:6.5.8| Show InChI InChI=1S/C25H26F2N2O5/c1-28-13-24(22(31)33-3)19(15-7-5-9-17(26)11-15)29(2)20(16-8-6-10-18(27)12-16)25(14-28,21(24)30)23(32)34-4/h5-12,19-20H,13-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 220-30 (2005)
Article DOI: 10.1124/jpet.104.073668
BindingDB Entry DOI: 10.7270/Q26T0K6G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data