 Found 335 hits with Last Name = 'aldegheri' and Initial = 'l'
Found 335 hits with Last Name = 'aldegheri' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331382
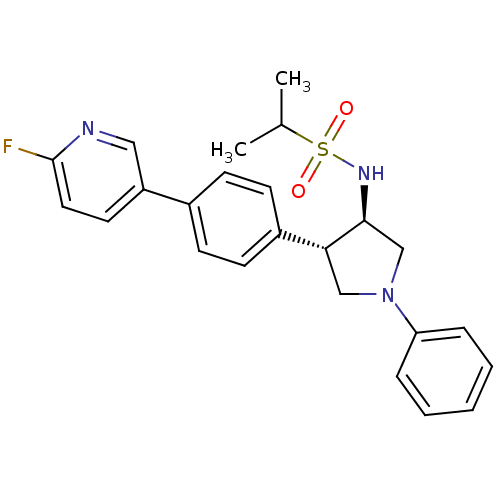
(CHEMBL1289063 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1)c1ccccc1 |r| Show InChI InChI=1S/C24H26FN3O2S/c1-17(2)31(29,30)27-23-16-28(21-6-4-3-5-7-21)15-22(23)19-10-8-18(9-11-19)20-12-13-24(25)26-14-20/h3-14,17,22-23,27H,15-16H2,1-2H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334942

(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C16H15F3N2O/c1-10(22)11-6-8-12(9-7-11)21-14-5-3-2-4-13(14)15(20-21)16(17,18)19/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334936
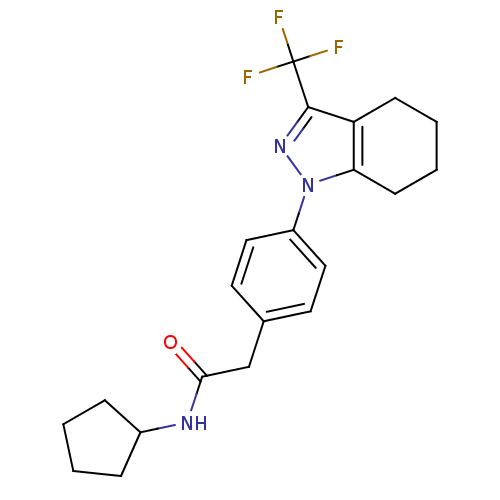
(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334935
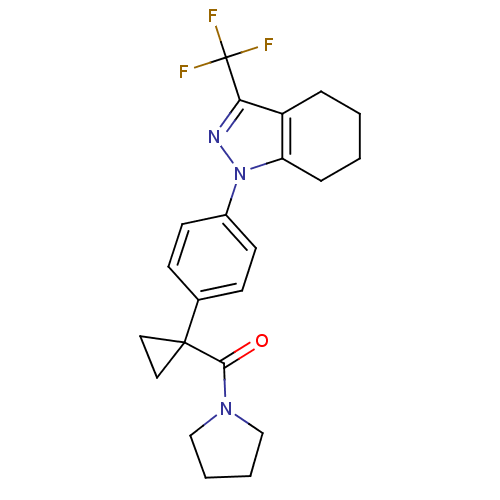
(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331371
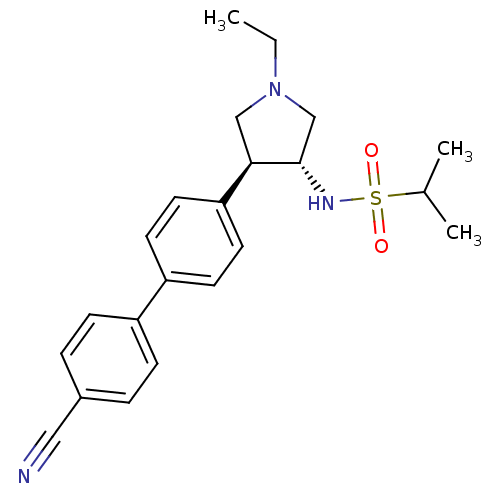
(CHEMBL1290275 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H27N3O2S/c1-4-25-14-21(22(15-25)24-28(26,27)16(2)3)20-11-9-19(10-12-20)18-7-5-17(13-23)6-8-18/h5-12,16,21-22,24H,4,14-15H2,1-3H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50323798
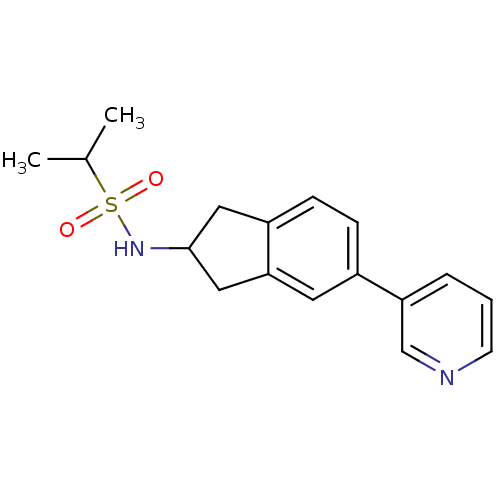
(CHEMBL1214336 | N-[5-(3-pyridinyl)-2,3-dihydro-1H-...)Show InChI InChI=1S/C17H20N2O2S/c1-12(2)22(20,21)19-17-9-14-6-5-13(8-16(14)10-17)15-4-3-7-18-11-15/h3-8,11-12,17,19H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 5801-12 (2010)
Article DOI: 10.1021/jm1005429
BindingDB Entry DOI: 10.7270/Q2FT8N0T |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 5
(Homo sapiens (Human)) | BDBM50602136

(CHEMBL5183139) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117084
BindingDB Entry DOI: 10.7270/Q2XW4PWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 5
(Homo sapiens (Human)) | BDBM50602137
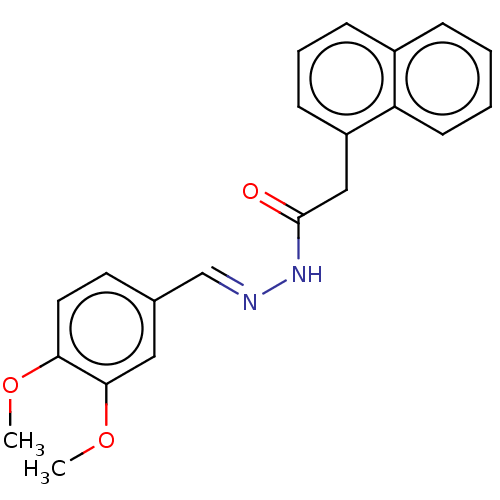
(CHEMBL5196998) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117084
BindingDB Entry DOI: 10.7270/Q2XW4PWD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334936

(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331373
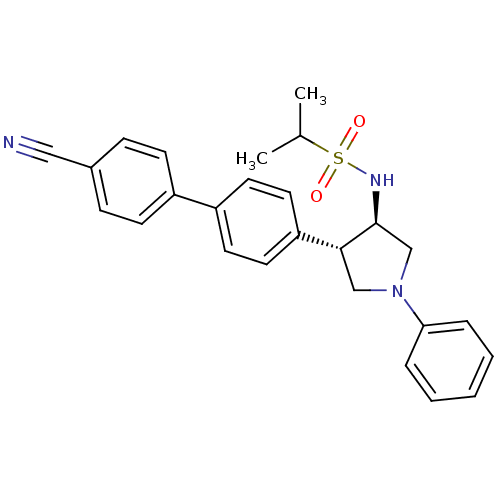
(CHEMBL1290391 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2S/c1-19(2)32(30,31)28-26-18-29(24-6-4-3-5-7-24)17-25(26)23-14-12-22(13-15-23)21-10-8-20(16-27)9-11-21/h3-15,19,25-26,28H,17-18H2,1-2H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334939

(1-({4-[3-(Trifluoromethyl)-4,5,6,7-tetrahydro-1H-i...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)18-15-4-1-2-5-16(15)25(23-18)14-9-7-13(8-10-14)12-24-11-3-6-17(24)26/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334940
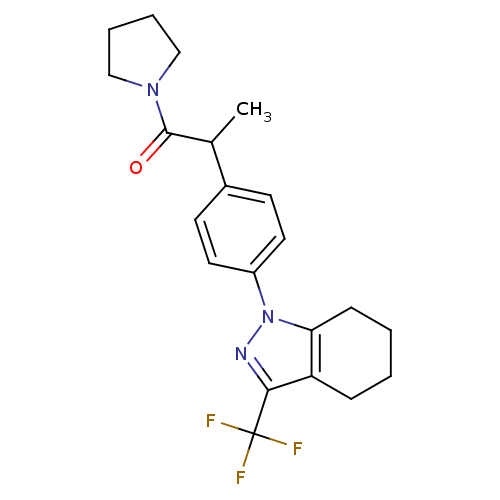
(1-{4-[1-methyl-2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES CC(C(=O)N1CCCC1)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N3O/c1-14(20(28)26-12-4-5-13-26)15-8-10-16(11-9-15)27-18-7-3-2-6-17(18)19(25-27)21(22,23)24/h8-11,14H,2-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331381
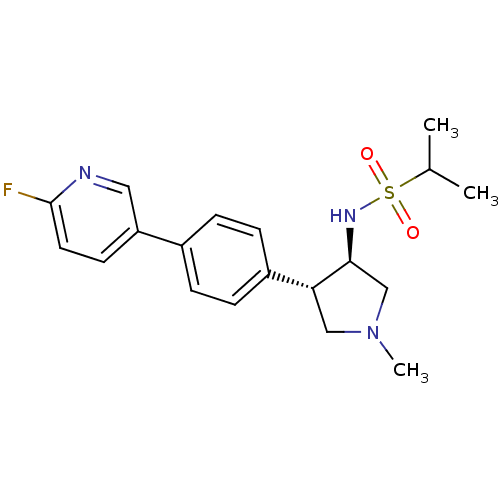
(CHEMBL1289062 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1 |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-6-4-14(5-7-15)16-8-9-19(20)21-10-16/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334948

(CHEMBL1649654 | N-methyl-N-(2-phenylethyl)-4-[3-(t...)Show SMILES CN(CCc1ccccc1)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-29(16-15-17-7-3-2-4-8-17)23(31)18-11-13-19(14-12-18)30-21-10-6-5-9-20(21)22(28-30)24(25,26)27/h2-4,7-8,11-14H,5-6,9-10,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331369
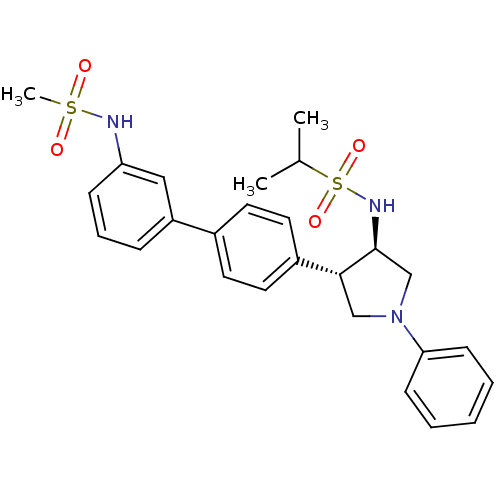
(CHEMBL1290165 | N-((3R,4R)-4-(3'-(methylsulfonamid...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1)c1ccccc1 |r| Show InChI InChI=1S/C26H31N3O4S2/c1-19(2)35(32,33)28-26-18-29(24-10-5-4-6-11-24)17-25(26)21-14-12-20(13-15-21)22-8-7-9-23(16-22)27-34(3,30)31/h4-16,19,25-28H,17-18H2,1-3H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331370
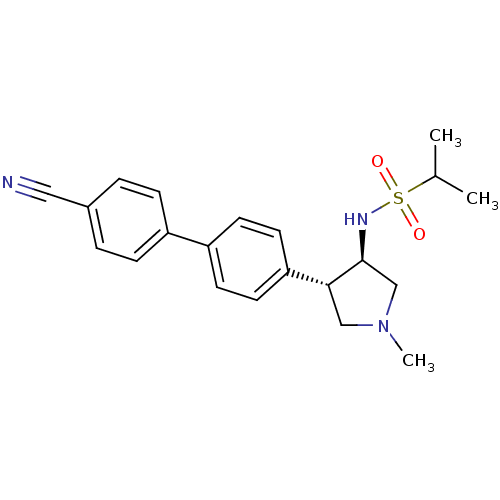
(CHEMBL1290166 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H25N3O2S/c1-15(2)27(25,26)23-21-14-24(3)13-20(21)19-10-8-18(9-11-19)17-6-4-16(12-22)5-7-17/h4-11,15,20-21,23H,13-14H2,1-3H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334937
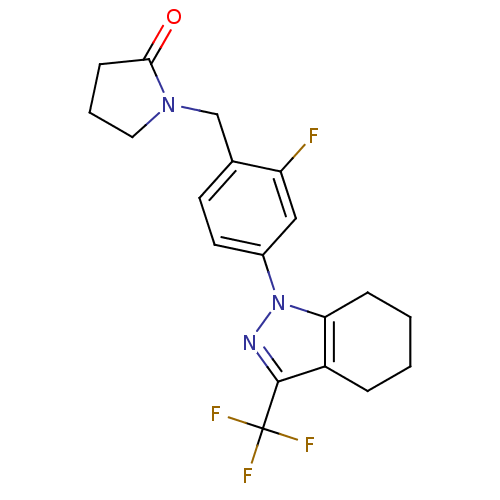
(1-({2-Fluoro-4-[3-(trifluoromethyl)-4,5,6,7-tetrah...)Show SMILES Fc1cc(ccc1CN1CCCC1=O)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C19H19F4N3O/c20-15-10-13(8-7-12(15)11-25-9-3-6-17(25)27)26-16-5-2-1-4-14(16)18(24-26)19(21,22)23/h7-8,10H,1-6,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334949
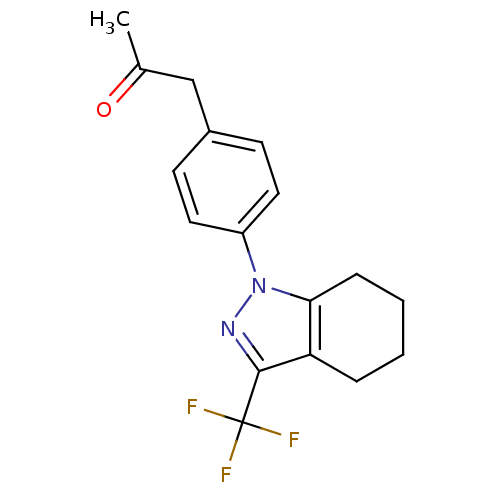
(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C17H17F3N2O/c1-11(23)10-12-6-8-13(9-7-12)22-15-5-3-2-4-14(15)16(21-22)17(18,19)20/h6-9H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334947
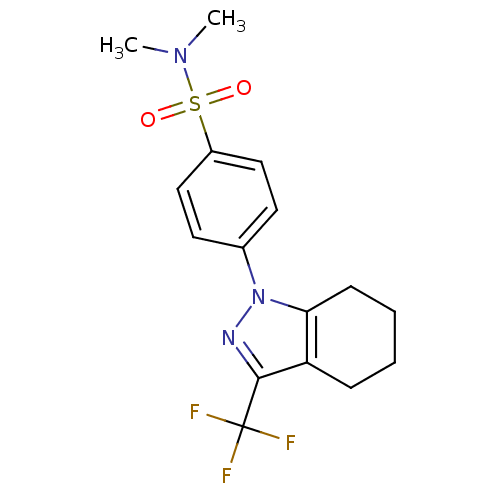
(CHEMBL1649655 | N,N-dimethyl-4-[3-(trifluoromethyl...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C16H18F3N3O2S/c1-21(2)25(23,24)12-9-7-11(8-10-12)22-14-6-4-3-5-13(14)15(20-22)16(17,18)19/h7-10H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331367
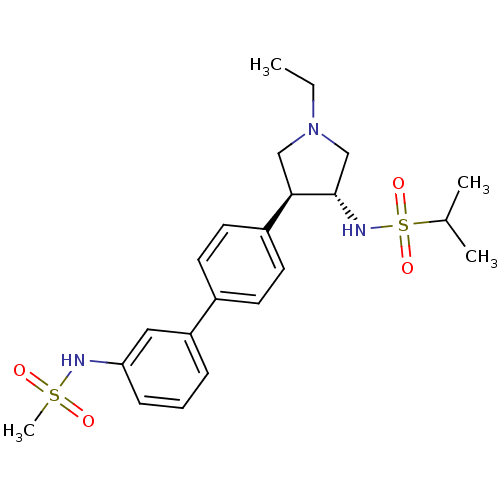
(CHEMBL1290058 | N-((3R,4R)-1-ethyl-4-(3'-(methylsu...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C22H31N3O4S2/c1-5-25-14-21(22(15-25)24-31(28,29)16(2)3)18-11-9-17(10-12-18)19-7-6-8-20(13-19)23-30(4,26)27/h6-13,16,21-24H,5,14-15H2,1-4H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334946

(1-{4-[2-OXO-2-(1-PYRROLIDINYL)ETHYL]PHENYL}-3-( TR...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)N2CCCC2)cc1 Show InChI InChI=1S/C20H22F3N3O/c21-20(22,23)19-16-5-1-2-6-17(16)26(24-19)15-9-7-14(8-10-15)13-18(27)25-11-3-4-12-25/h7-10H,1-6,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334948

(CHEMBL1649654 | N-methyl-N-(2-phenylethyl)-4-[3-(t...)Show SMILES CN(CCc1ccccc1)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-29(16-15-17-7-3-2-4-8-17)23(31)18-11-13-19(14-12-18)30-21-10-6-5-9-20(21)22(28-30)24(25,26)27/h2-4,7-8,11-14H,5-6,9-10,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334938

(CHEMBL1649672 | N-({4-[3-(trifluoromethyl)-4,5,6,7...)Show SMILES CCC(=O)NCc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-2-16(25)22-11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(23-24)18(19,20)21/h7-10H,2-6,11H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334945
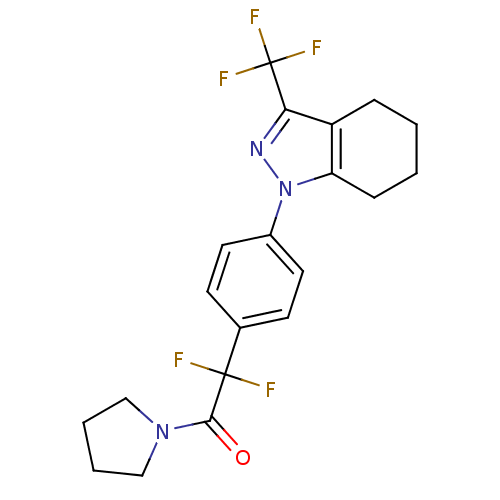
(1-{4-[1,1-difluoro-2-oxo-2-(1-pyrrolidinyl)ethyl]p...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C(F)(F)C(=O)N1CCCC1 Show InChI InChI=1S/C20H20F5N3O/c21-19(22,18(29)27-11-3-4-12-27)13-7-9-14(10-8-13)28-16-6-2-1-5-15(16)17(26-28)20(23,24)25/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334942

(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C16H15F3N2O/c1-10(22)11-6-8-12(9-7-11)21-14-5-3-2-4-13(14)15(20-21)16(17,18)19/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331384
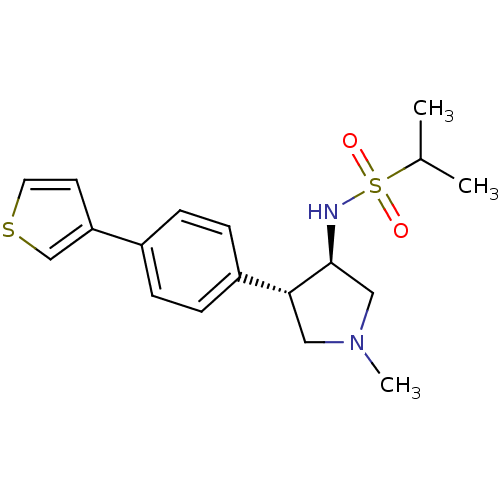
(CHEMBL1289180 | N-((3R,4R)-1-methyl-4-(4-(thiophen...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccsc1 |r| Show InChI InChI=1S/C18H24N2O2S2/c1-13(2)24(21,22)19-18-11-20(3)10-17(18)15-6-4-14(5-7-15)16-8-9-23-12-16/h4-9,12-13,17-19H,10-11H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331377
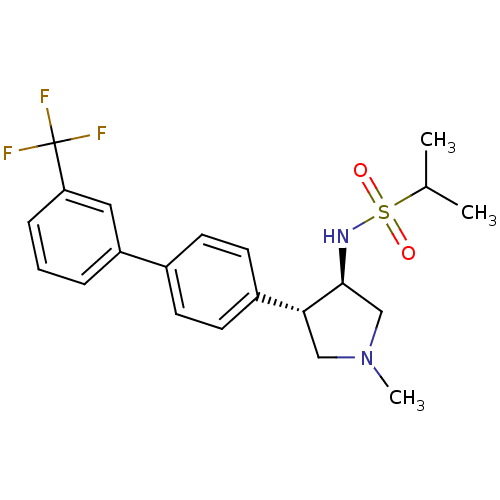
(CHEMBL1290612 | N-((3R,4R)-1-methyl-4-(3'-(trifluo...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H25F3N2O2S/c1-14(2)29(27,28)25-20-13-26(3)12-19(20)16-9-7-15(8-10-16)17-5-4-6-18(11-17)21(22,23)24/h4-11,14,19-20,25H,12-13H2,1-3H3/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334941
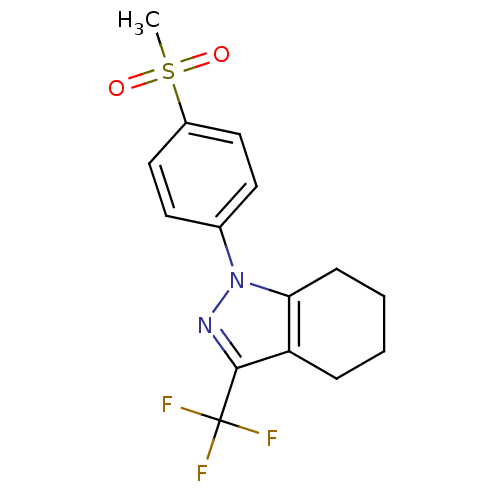
(1-(4-(methylsulfonyl)phenyl)-3-(trifluoromethyl)-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C15H15F3N2O2S/c1-23(21,22)11-8-6-10(7-9-11)20-13-5-3-2-4-12(13)14(19-20)15(16,17)18/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334939

(1-({4-[3-(Trifluoromethyl)-4,5,6,7-tetrahydro-1H-i...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)18-15-4-1-2-5-16(15)25(23-18)14-9-7-13(8-10-14)12-24-11-3-6-17(24)26/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334932

(CHEMBL1649660 | N-butyl-N-methyl-4-[3-(trifluorome...)Show SMILES CCCCN(C)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C20H24F3N3O/c1-3-4-13-25(2)19(27)14-9-11-15(12-10-14)26-17-8-6-5-7-16(17)18(24-26)20(21,22)23/h9-12H,3-8,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334950
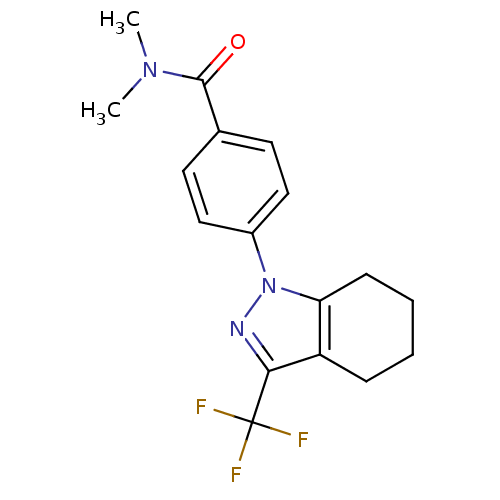
(CHEMBL1594422 | N,N-Dimethyl-4-[3-(trifluoromethyl...)Show SMILES CN(C)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C17H18F3N3O/c1-22(2)16(24)11-7-9-12(10-8-11)23-14-6-4-3-5-13(14)15(21-23)17(18,19)20/h7-10H,3-6H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334941

(1-(4-(methylsulfonyl)phenyl)-3-(trifluoromethyl)-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C15H15F3N2O2S/c1-23(21,22)11-8-6-10(7-9-11)20-13-5-3-2-4-12(13)14(19-20)15(16,17)18/h6-9H,2-5H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334940

(1-{4-[1-methyl-2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES CC(C(=O)N1CCCC1)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N3O/c1-14(20(28)26-12-4-5-13-26)15-8-10-16(11-9-15)27-18-7-3-2-6-17(18)19(25-27)21(22,23)24/h8-11,14H,2-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334943
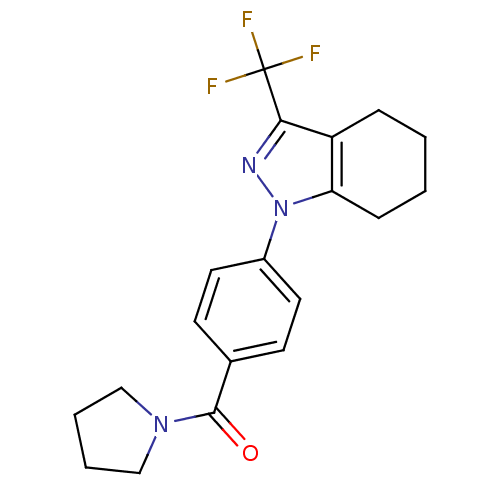
(1-[4-(1-Pyrrolidinylcarbonyl)phenyl]-3-(trifluorom...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C(=O)N1CCCC1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)17-15-5-1-2-6-16(15)25(23-17)14-9-7-13(8-10-14)18(26)24-11-3-4-12-24/h7-10H,1-6,11-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334935

(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331380
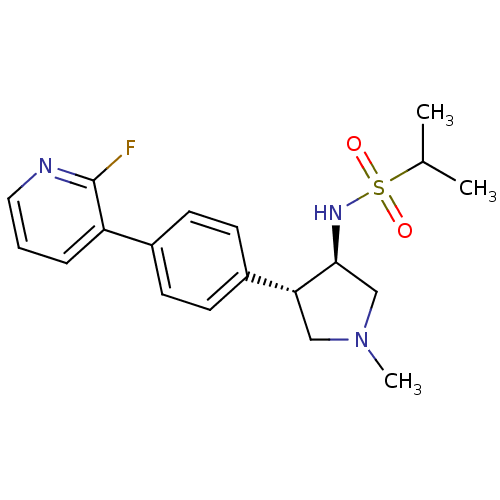
(CHEMBL1290732 | N-((3R,4R)-4-(4-(2-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccnc1F |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-8-6-14(7-9-15)16-5-4-10-21-19(16)20/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331368
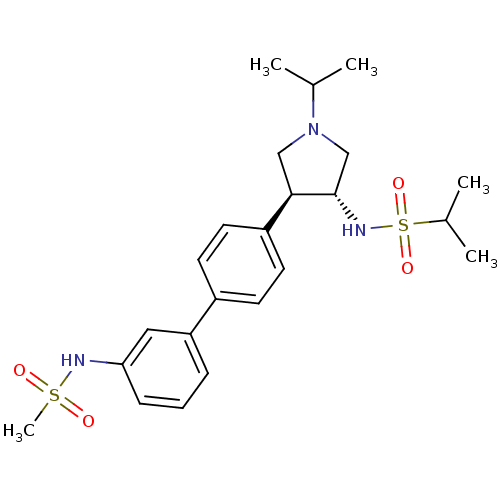
(CHEMBL1290059 | N-((3R,4R)-1-isopropyl-4-(3'-(meth...)Show SMILES CC(C)N1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C23H33N3O4S2/c1-16(2)26-14-22(23(15-26)25-32(29,30)17(3)4)19-11-9-18(10-12-19)20-7-6-8-21(13-20)24-31(5,27)28/h6-13,16-17,22-25H,14-15H2,1-5H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334931
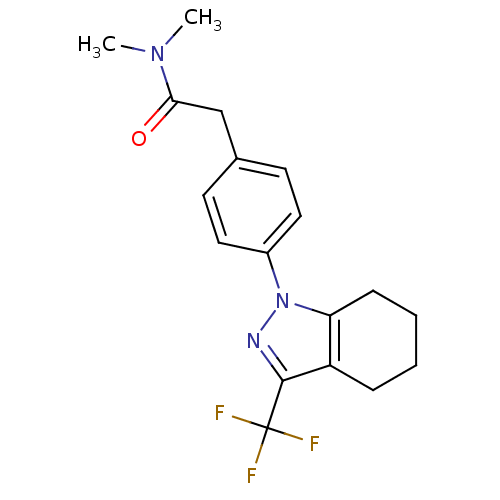
(CHEMBL1649663 | N,N-Dimethyl-2-{4-[3-(trifluoromet...)Show SMILES CN(C)C(=O)Cc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-23(2)16(25)11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(22-24)18(19,20)21/h7-10H,3-6,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334944

(1-{3-Fluoro-4-[2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES Fc1cc(ccc1CC(=O)N1CCCC1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C20H21F4N3O/c21-16-12-14(8-7-13(16)11-18(28)26-9-3-4-10-26)27-17-6-2-1-5-15(17)19(25-27)20(22,23)24/h7-8,12H,1-6,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334935

(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331376
(CHEMBL1290504 | N-((3R,4R)-4-(3'-acetylbiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(C)=O |r| Show InChI InChI=1S/C22H28N2O3S/c1-15(2)28(26,27)23-22-14-24(4)13-21(22)18-10-8-17(9-11-18)20-7-5-6-19(12-20)16(3)25/h5-12,15,21-23H,13-14H2,1-4H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334936

(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334929
(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334932

(CHEMBL1649660 | N-butyl-N-methyl-4-[3-(trifluorome...)Show SMILES CCCCN(C)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C20H24F3N3O/c1-3-4-13-25(2)19(27)14-9-11-15(12-10-14)26-17-8-6-5-7-16(17)18(24-26)20(21,22)23/h9-12H,3-8,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334938

(CHEMBL1649672 | N-({4-[3-(trifluoromethyl)-4,5,6,7...)Show SMILES CCC(=O)NCc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-2-16(25)22-11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(23-24)18(19,20)21/h7-10H,2-6,11H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50323794
(CHEMBL1214396 | N-[5-(4-methyl-3-pyridinyl)-2,3-di...)Show InChI InChI=1S/C18H22N2O2S/c1-12(2)23(21,22)20-17-9-14-4-5-15(8-16(14)10-17)18-11-19-7-6-13(18)3/h4-8,11-12,17,20H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 after 10 mins |
J Med Chem 53: 5801-12 (2010)
Article DOI: 10.1021/jm1005429
BindingDB Entry DOI: 10.7270/Q2FT8N0T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data