 Found 826 hits with Last Name = 'knight' and Initial = 'l'
Found 826 hits with Last Name = 'knight' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM82217
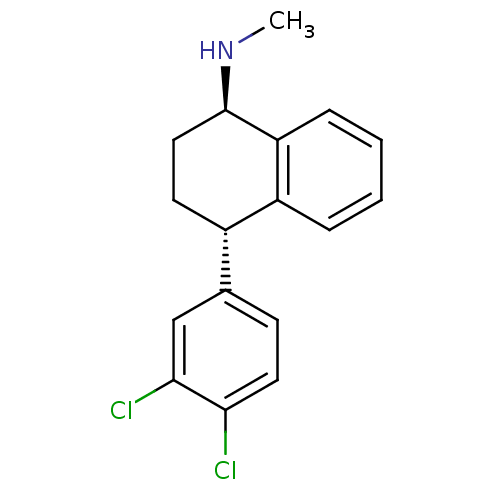
(CHEMBL284994 | CP-52003 | SERTRALINE | [4-(3,4-Dic...)Show SMILES CN[C@@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870
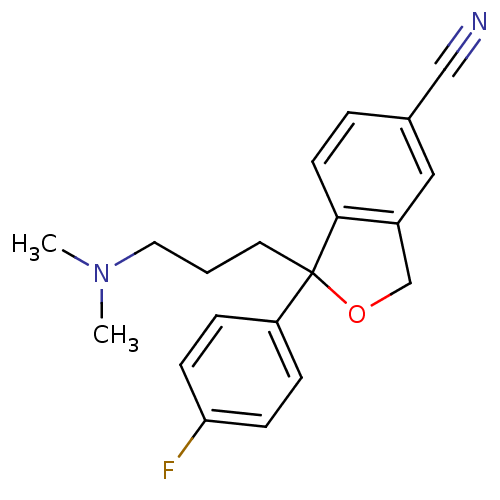
(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM81875
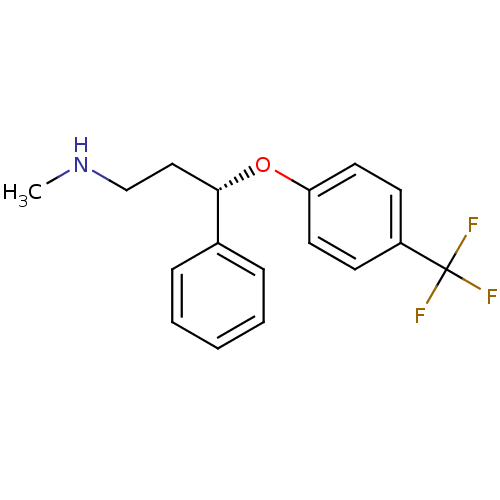
(Fluoxetine-R-(-))Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870

(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50028091
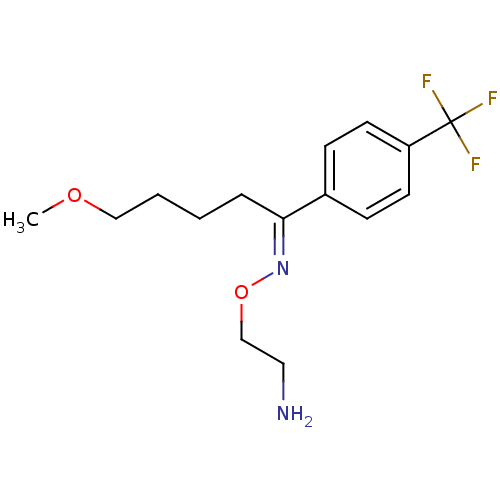
((1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan...)Show InChI InChI=1S/C15H21F3N2O2/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18/h5-8H,2-4,9-11,19H2,1H3/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870

(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM82217

(CHEMBL284994 | CP-52003 | SERTRALINE | [4-(3,4-Dic...)Show SMILES CN[C@@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372007
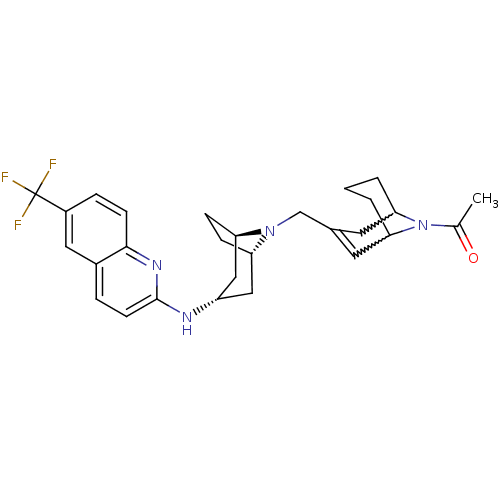
(CHEMBL257017)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3cc(ccc3n1)C(F)(F)F)C2 |w:4.40,8.9,t:10,TLB:1:3:10.35.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O/c1-17(36)35-24-3-2-4-25(35)12-18(11-24)16-34-22-7-8-23(34)15-21(14-22)32-27-10-5-19-13-20(28(29,30)31)6-9-26(19)33-27/h5-6,9-11,13,21-25H,2-4,7-8,12,14-16H2,1H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227864
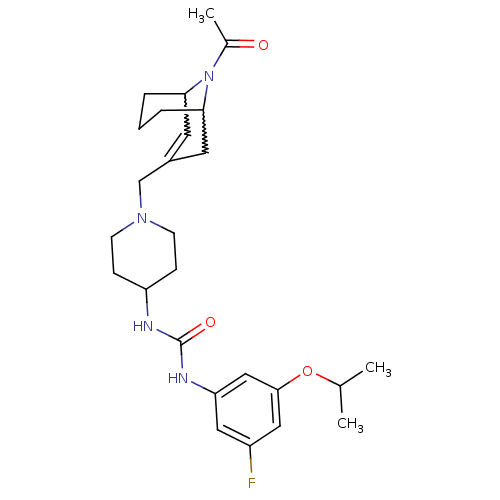
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cc(F)cc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:25.25,21.20,t:19,TLB:28:27:19.26.20:22.24.23| Show InChI InChI=1S/C26H37FN4O3/c1-17(2)34-25-14-20(27)13-22(15-25)29-26(33)28-21-7-9-30(10-8-21)16-19-11-23-5-4-6-24(12-19)31(23)18(3)32/h11,13-15,17,21,23-24H,4-10,12,16H2,1-3H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372000
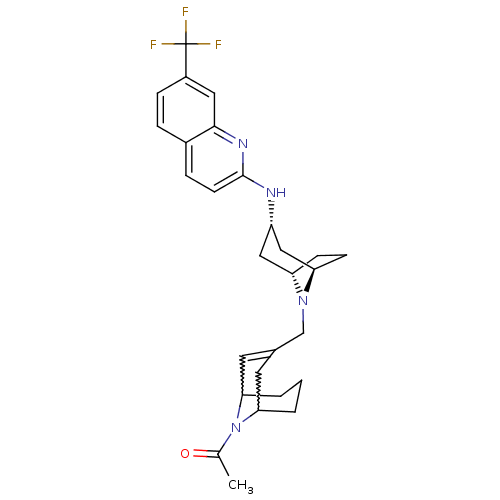
(CHEMBL256851)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3ccc(cc3n1)C(F)(F)F)C2 |w:4.40,8.9,t:10,TLB:1:3:10.35.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O/c1-17(36)35-24-3-2-4-25(35)12-18(11-24)16-34-22-8-9-23(34)15-21(14-22)32-27-10-6-19-5-7-20(28(29,30)31)13-26(19)33-27/h5-7,10-11,13,21-25H,2-4,8-9,12,14-16H2,1H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372006

(CHEMBL256850)Show SMILES CC(C)Oc1ccc2ccc(N[C@H]3C[C@@H]4CC[C@H](C3)N4CC3=CC4CCCC(C3)N4C(C)=O)nc2c1 |w:27.29,23.24,t:23,TLB:30:29:21.28.22:24.26.25,THB:20:19:13.12.18:15.16| Show InChI InChI=1S/C30H40N4O2/c1-19(2)36-28-11-7-22-8-12-30(32-29(22)17-28)31-23-15-24-9-10-25(16-23)33(24)18-21-13-26-5-4-6-27(14-21)34(26)20(3)35/h7-8,11-13,17,19,23-27H,4-6,9-10,14-16,18H2,1-3H3,(H,31,32)/t23-,24-,25+,26?,27? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM30130

(CHEMBL1201082 | CHEMBL41 | Fluoxetin | Fluoxetine ...)Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372005

(CHEMBL429598)Show SMILES CC(C)Oc1cc(F)c2ccc(N[C@H]3C[C@@H]4CC[C@H](C3)N4CC3=CC4CCCC(C3)N4C(C)=O)nc2c1 |w:28.30,24.25,t:24,TLB:31:30:22.29.23:25.27.26,THB:21:20:14.13.19:16.17| Show InChI InChI=1S/C30H39FN4O2/c1-18(2)37-26-15-28(31)27-9-10-30(33-29(27)16-26)32-21-13-22-7-8-23(14-21)34(22)17-20-11-24-5-4-6-25(12-20)35(24)19(3)36/h9-11,15-16,18,21-25H,4-8,12-14,17H2,1-3H3,(H,32,33)/t21-,22-,23+,24?,25? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227866
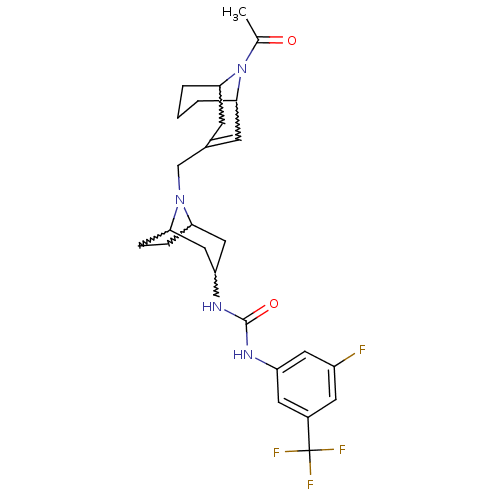
(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50227864

(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cc(F)cc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:25.25,21.20,t:19,TLB:28:27:19.26.20:22.24.23| Show InChI InChI=1S/C26H37FN4O3/c1-17(2)34-25-14-20(27)13-22(15-25)29-26(33)28-21-7-9-30(10-8-21)16-19-11-23-5-4-6-24(12-19)31(23)18(3)32/h11,13-15,17,21,23-24H,4-10,12,16H2,1-3H3,(H2,28,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse CXCR3 |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372008
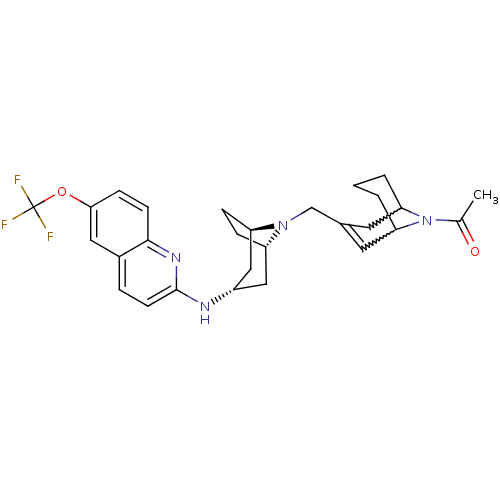
(CHEMBL444542)Show SMILES CC(=O)N1C2CCCC1C=C(CN1[C@H]3CC[C@@H]1C[C@H](C3)Nc1ccc3cc(OC(F)(F)F)ccc3n1)C2 |w:4.41,8.9,t:10,TLB:1:3:10.36.9:7.5.6,THB:11:12:19.18.17:14.15| Show InChI InChI=1S/C28H33F3N4O2/c1-17(36)35-23-3-2-4-24(35)12-18(11-23)16-34-21-6-7-22(34)15-20(14-21)32-27-10-5-19-13-25(37-28(29,30)31)8-9-26(19)33-27/h5,8-11,13,20-24H,2-4,6-7,12,14-16H2,1H3,(H,32,33)/t20-,21-,22+,23?,24? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372011
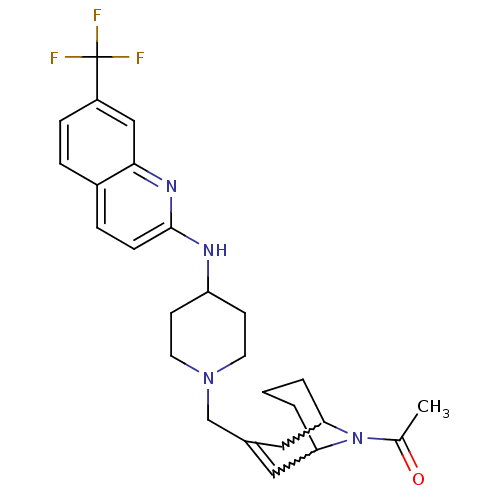
(CHEMBL271847)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)Nc1ccc3ccc(cc3n1)C(F)(F)F)C2 |w:4.37,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C26H31F3N4O/c1-17(34)33-22-3-2-4-23(33)14-18(13-22)16-32-11-9-21(10-12-32)30-25-8-6-19-5-7-20(26(27,28)29)15-24(19)31-25/h5-8,13,15,21-23H,2-4,9-12,14,16H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227866

(1-(8-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1C3CCC1CC(C3)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.39,8.9,18.22,13.14,16.16,t:10,TLB:1:3:10.35.9:7.5.6,TEB:20:18:12:14.15| Show InChI InChI=1S/C26H32F4N4O2/c1-15(35)34-23-3-2-4-24(34)8-16(7-23)14-33-21-5-6-22(33)13-20(12-21)32-25(36)31-19-10-17(26(28,29)30)9-18(27)11-19/h7,9-11,20-24H,2-6,8,12-14H2,1H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50494112
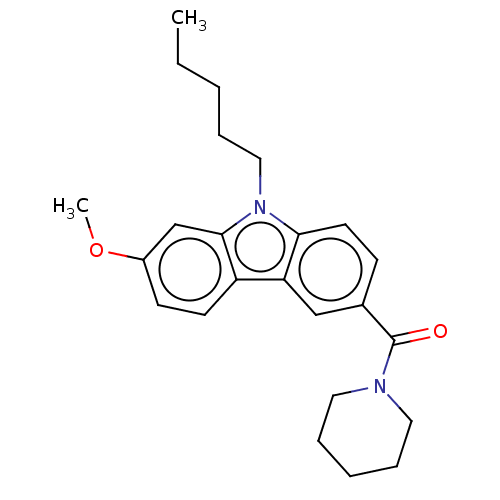
(CHEMBL2441475)Show SMILES CCCCCn1c2ccc(cc2c2ccc(OC)cc12)C(=O)N1CCCCC1 Show InChI InChI=1S/C24H30N2O2/c1-3-4-6-15-26-22-12-9-18(24(27)25-13-7-5-8-14-25)16-21(22)20-11-10-19(28-2)17-23(20)26/h9-12,16-17H,3-8,13-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227863
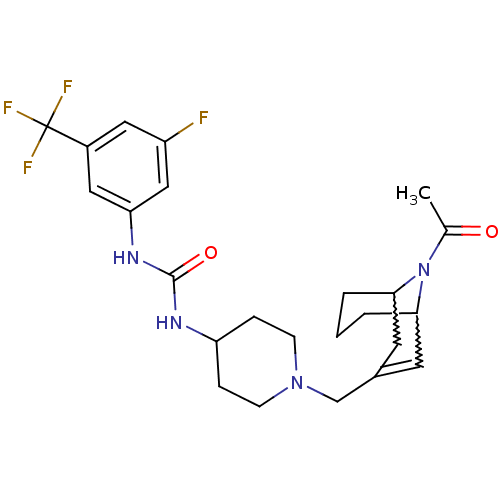
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.36,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C24H30F4N4O2/c1-15(33)32-21-3-2-4-22(32)10-16(9-21)14-31-7-5-19(6-8-31)29-23(34)30-20-12-17(24(26,27)28)11-18(25)13-20/h9,11-13,19,21-22H,2-8,10,14H2,1H3,(H2,29,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227863

(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.36,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C24H30F4N4O2/c1-15(33)32-21-3-2-4-22(32)10-16(9-21)14-31-7-5-19(6-8-31)29-23(34)30-20-12-17(24(26,27)28)11-18(25)13-20/h9,11-13,19,21-22H,2-8,10,14H2,1H3,(H2,29,30,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227861

(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((9-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.39,11.12,t:13,TLB:4:6:13.36.12:10.8.9| Show InChI InChI=1S/C27H36F4N4O2/c1-26(2,3)24(36)35-22-5-4-6-23(35)12-17(11-22)16-34-9-7-20(8-10-34)32-25(37)33-21-14-18(27(29,30)31)13-19(28)15-21/h11,13-15,20,22-23H,4-10,12,16H2,1-3H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50028091

((1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan...)Show InChI InChI=1S/C15H21F3N2O2/c1-21-10-3-2-4-14(20-22-11-9-19)12-5-7-13(8-6-12)15(16,17)18/h5-8H,2-4,9-11,19H2,1H3/b20-14+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227867
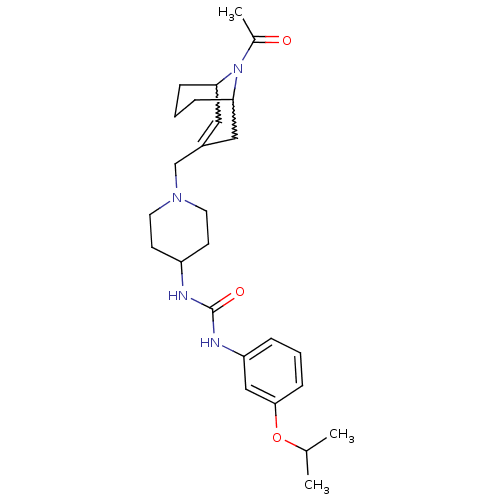
(1-(1-((9-acetyl-9-aza-bicyclo[3.3.1]non-2-en-3-yl)...)Show SMILES CC(C)Oc1cccc(NC(=O)NC2CCN(CC3=CC4CCCC(C3)N4C(C)=O)CC2)c1 |w:24.24,20.19,t:18,TLB:27:26:18.25.19:21.23.22| Show InChI InChI=1S/C26H38N4O3/c1-18(2)33-25-9-4-6-22(16-25)28-26(32)27-21-10-12-29(13-11-21)17-20-14-23-7-5-8-24(15-20)30(23)19(3)31/h4,6,9,14,16,18,21,23-24H,5,7-8,10-13,15,17H2,1-3H3,(H2,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM81875

(Fluoxetine-R-(-))Show InChI InChI=1S/C17H18F3NO/c1-21-12-11-16(13-5-3-2-4-6-13)22-15-9-7-14(8-10-15)17(18,19)20/h2-10,16,21H,11-12H2,1H3/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50494112

(CHEMBL2441475)Show SMILES CCCCCn1c2ccc(cc2c2ccc(OC)cc12)C(=O)N1CCCCC1 Show InChI InChI=1S/C24H30N2O2/c1-3-4-6-15-26-22-12-9-18(24(27)25-13-7-5-8-14-25)16-21(22)20-11-10-19(28-2)17-23(20)26/h9-12,16-17H,3-8,13-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from CB1 receptor in rat brain homogenate after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372015
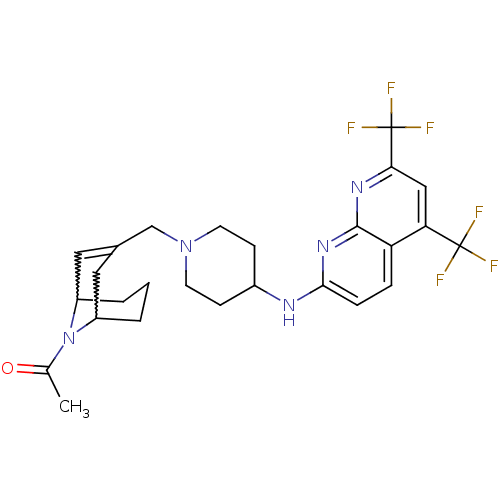
(CHEMBL402514)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)Nc1ccc3c(cc(nc3n1)C(F)(F)F)C(F)(F)F)C2 |w:4.41,8.9,t:10,TLB:1:3:10.37.9:7.5.6| Show InChI InChI=1S/C26H29F6N5O/c1-15(38)37-18-3-2-4-19(37)12-16(11-18)14-36-9-7-17(8-10-36)33-23-6-5-20-21(25(27,28)29)13-22(26(30,31)32)34-24(20)35-23/h5-6,11,13,17-19H,2-4,7-10,12,14H2,1H3,(H,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198419
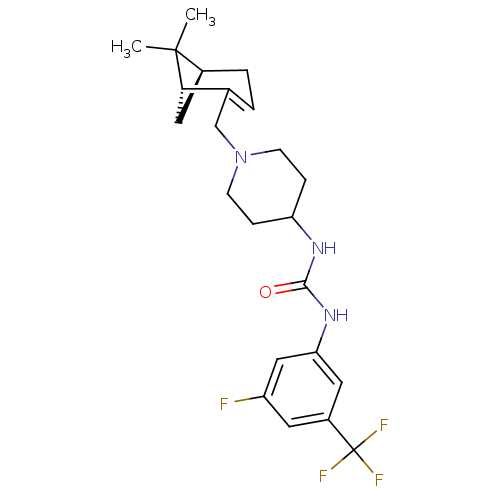
(1-(1-(((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)=CC2 |c:31| Show InChI InChI=1S/C23H29F4N3O/c1-22(2)15-4-3-14(20(22)11-15)13-30-7-5-18(6-8-30)28-21(31)29-19-10-16(23(25,26)27)9-17(24)12-19/h3,9-10,12,15,18,20H,4-8,11,13H2,1-2H3,(H2,28,29,31)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 697-701 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.088
BindingDB Entry DOI: 10.7270/Q2833RPB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198419

(1-(1-(((1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)=CC2 |c:31| Show InChI InChI=1S/C23H29F4N3O/c1-22(2)15-4-3-14(20(22)11-15)13-30-7-5-18(6-8-30)28-21(31)29-19-10-16(23(25,26)27)9-17(24)12-19/h3,9-10,12,15,18,20H,4-8,11,13H2,1-2H3,(H2,28,29,31)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor expressed in CHO membrane by ITAC-stimulated GTP gammaS assay |
Bioorg Med Chem Lett 17: 6806-10 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.029
BindingDB Entry DOI: 10.7270/Q24F1QGP |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227865
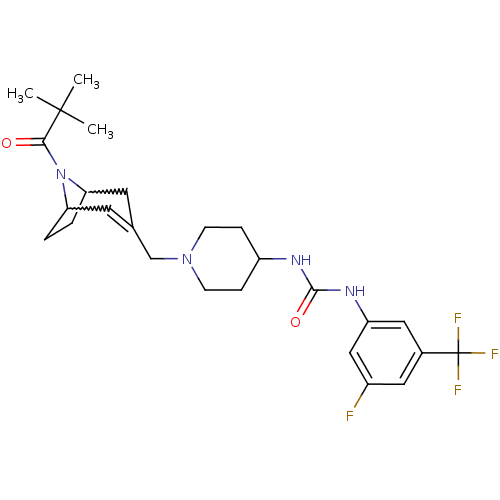
(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-pi...)Show SMILES CC(C)(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:7.38,10.11,t:12| Show InChI InChI=1S/C26H34F4N4O2/c1-25(2,3)23(35)34-21-4-5-22(34)11-16(10-21)15-33-8-6-19(7-9-33)31-24(36)32-20-13-17(26(28,29)30)12-18(27)14-20/h10,12-14,19,21-22H,4-9,11,15H2,1-3H3,(H2,31,32,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372012
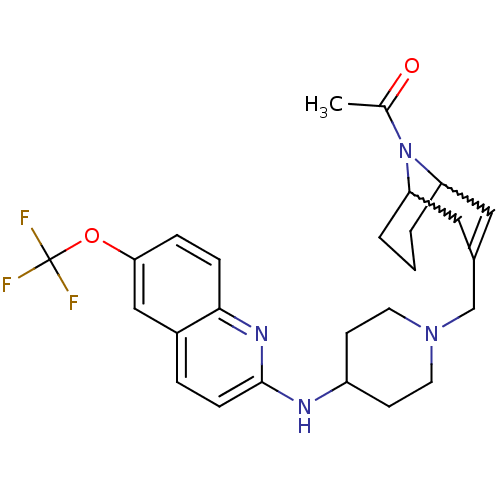
(CHEMBL404028)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)Nc1ccc3cc(OC(F)(F)F)ccc3n1)C2 |w:4.38,8.9,t:10,TLB:1:3:10.34.9:7.5.6| Show InChI InChI=1S/C26H31F3N4O2/c1-17(34)33-21-3-2-4-22(33)14-18(13-21)16-32-11-9-20(10-12-32)30-25-8-5-19-15-23(35-26(27,28)29)6-7-24(19)31-25/h5-8,13,15,20-22H,2-4,9-12,14,16H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227862
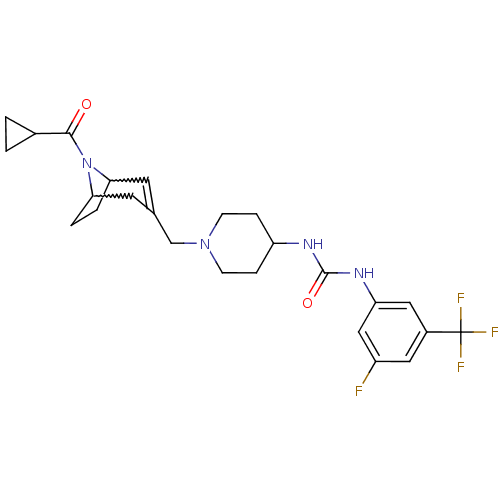
(1-(1-((8-(cyclopropanecarbonyl)-8-aza-bicyclo[3.2....)Show SMILES Fc1cc(NC(=O)NC2CCN(CC3=CC4CCC(C3)N4C(=O)C3CC3)CC2)cc(c1)C(F)(F)F |w:18.18,15.14,t:13| Show InChI InChI=1S/C25H30F4N4O2/c26-18-11-17(25(27,28)29)12-20(13-18)31-24(35)30-19-5-7-32(8-6-19)14-15-9-21-3-4-22(10-15)33(21)23(34)16-1-2-16/h9,11-13,16,19,21-22H,1-8,10,14H2,(H2,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50494086
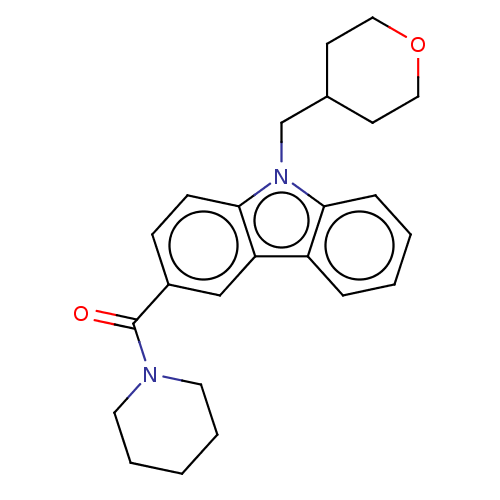
(CHEMBL2441467)Show SMILES O=C(N1CCCCC1)c1ccc2n(CC3CCOCC3)c3ccccc3c2c1 Show InChI InChI=1S/C24H28N2O2/c27-24(25-12-4-1-5-13-25)19-8-9-23-21(16-19)20-6-2-3-7-22(20)26(23)17-18-10-14-28-15-11-18/h2-3,6-9,16,18H,1,4-5,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from CB1 receptor in rat brain homogenate after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372009
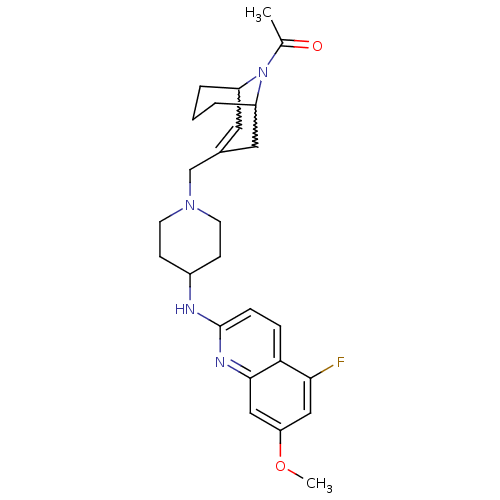
(CHEMBL271636)Show SMILES COc1cc(F)c2ccc(NC3CCN(CC4=CC5CCCC(C4)N5C(C)=O)CC3)nc2c1 |w:22.22,18.17,t:16,TLB:25:24:16.23.17:19.21.20| Show InChI InChI=1S/C26H33FN4O2/c1-17(32)31-20-4-3-5-21(31)13-18(12-20)16-30-10-8-19(9-11-30)28-26-7-6-23-24(27)14-22(33-2)15-25(23)29-26/h6-7,12,14-15,19-21H,3-5,8-11,13,16H2,1-2H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50494086

(CHEMBL2441467)Show SMILES O=C(N1CCCCC1)c1ccc2n(CC3CCOCC3)c3ccccc3c2c1 Show InChI InChI=1S/C24H28N2O2/c27-24(25-12-4-1-5-13-25)19-8-9-23-21(16-19)20-6-2-3-7-22(20)26(23)17-18-10-14-28-15-11-18/h2-3,6-9,16,18H,1,4-5,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372002
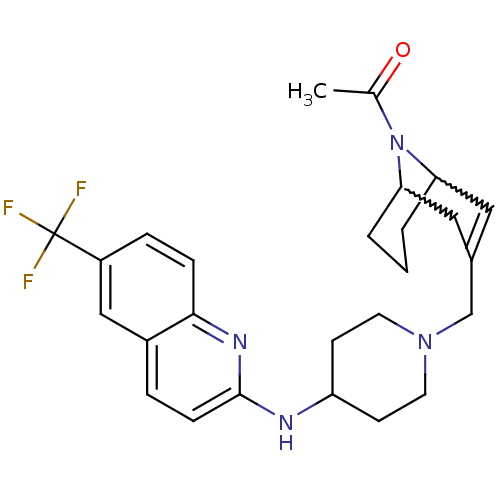
(CHEMBL256188)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)Nc1ccc3cc(ccc3n1)C(F)(F)F)C2 |w:4.37,8.9,t:10,TLB:1:3:10.33.9:7.5.6| Show InChI InChI=1S/C26H31F3N4O/c1-17(34)33-22-3-2-4-23(33)14-18(13-22)16-32-11-9-21(10-12-32)30-25-8-5-19-15-20(26(27,28)29)6-7-24(19)31-25/h5-8,13,15,21-23H,2-4,9-12,14,16H2,1H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM82217

(CHEMBL284994 | CP-52003 | SERTRALINE | [4-(3,4-Dic...)Show SMILES CN[C@@H]1CC[C@@H](c2ccc(Cl)c(Cl)c2)c2ccccc12 Show InChI InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
Encephale 28: 350-5 (2002)
BindingDB Entry DOI: 10.7270/Q24J0CPW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372001
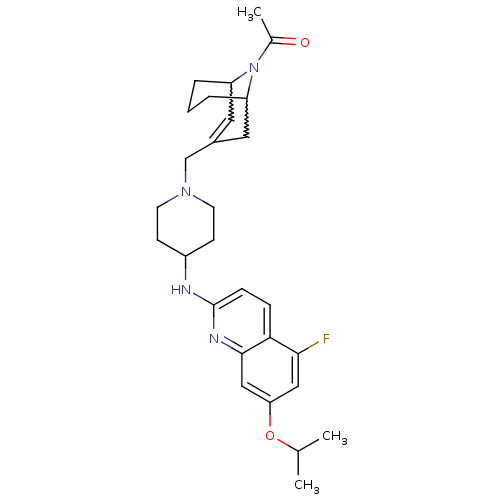
(CHEMBL257018)Show SMILES CC(C)Oc1cc(F)c2ccc(NC3CCN(CC4=CC5CCCC(C4)N5C(C)=O)CC3)nc2c1 |w:24.24,20.19,t:18,TLB:27:26:18.25.19:21.23.22| Show InChI InChI=1S/C28H37FN4O2/c1-18(2)35-24-15-26(29)25-7-8-28(31-27(25)16-24)30-21-9-11-32(12-10-21)17-20-13-22-5-4-6-23(14-20)33(22)19(3)34/h7-8,13,15-16,18,21-23H,4-6,9-12,14,17H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372010
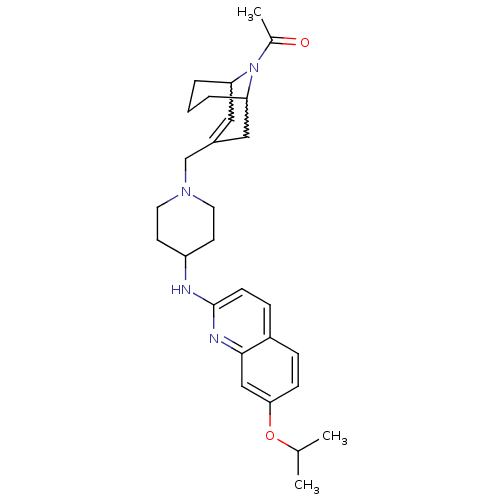
(CHEMBL402245)Show SMILES CC(C)Oc1ccc2ccc(NC3CCN(CC4=CC5CCCC(C4)N5C(C)=O)CC3)nc2c1 |w:23.23,19.18,t:17,TLB:26:25:17.24.18:20.22.21| Show InChI InChI=1S/C28H38N4O2/c1-19(2)34-26-9-7-22-8-10-28(30-27(22)17-26)29-23-11-13-31(14-12-23)18-21-15-24-5-4-6-25(16-21)32(24)20(3)33/h7-10,15,17,19,23-25H,4-6,11-14,16,18H2,1-3H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198392
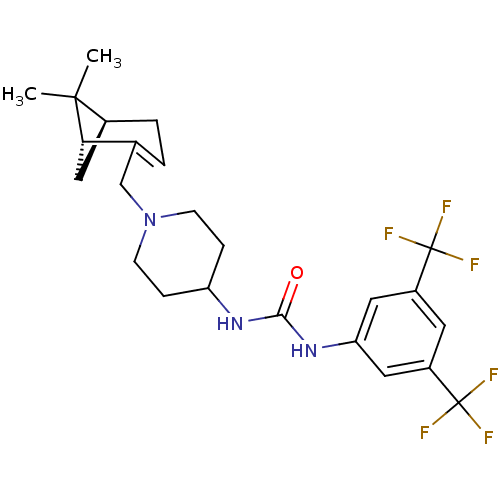
(1-(3,5-bis(trifluoromethyl)phenyl)-3-(1-(((1R,5S)-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)NC(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F)=CC2 |c:34| Show InChI InChI=1S/C24H29F6N3O/c1-22(2)15-4-3-14(20(22)12-15)13-33-7-5-18(6-8-33)31-21(34)32-19-10-16(23(25,26)27)9-17(11-19)24(28,29)30/h3,9-11,15,18,20H,4-8,12-13H2,1-2H3,(H2,31,32,34)/t15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50372013
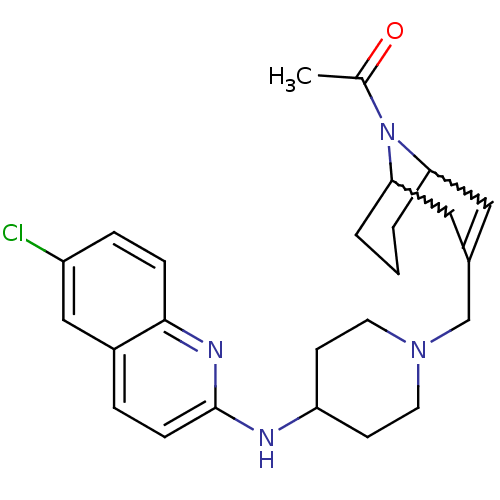
(CHEMBL256190)Show SMILES CC(=O)N1C2CCCC1C=C(CN1CCC(CC1)Nc1ccc3cc(Cl)ccc3n1)C2 |w:4.34,8.9,t:10,TLB:1:3:10.30.9:7.5.6| Show InChI InChI=1S/C25H31ClN4O/c1-17(31)30-22-3-2-4-23(30)14-18(13-22)16-29-11-9-21(10-12-29)27-25-8-5-19-15-20(26)6-7-24(19)28-25/h5-8,13,15,21-23H,2-4,9-12,14,16H2,1H3,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to CXCR3 receptor expressed in CHO cells assessed as ITAC-induced [35]GTPgammaS binding |
Bioorg Med Chem Lett 18: 629-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.075
BindingDB Entry DOI: 10.7270/Q29S1RWG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50225715
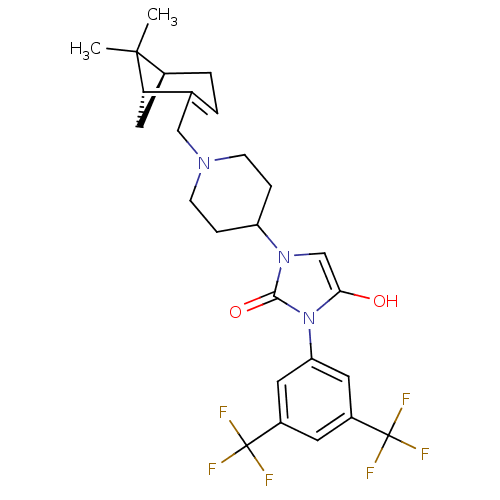
(3-(3,5-bis(trifluoromethyl)phenyl)-1-(1-(((1R,5S)-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(CN1CCC(CC1)n1cc(O)n(-c3cc(cc(c3)C(F)(F)F)C(F)(F)F)c1=O)=CC2 |c:38| Show InChI InChI=1S/C26H29F6N3O2/c1-24(2)16-4-3-15(21(24)12-16)13-33-7-5-19(6-8-33)34-14-22(36)35(23(34)37)20-10-17(25(27,28)29)9-18(11-20)26(30,31)32/h3,9-11,14,16,19,21,36H,4-8,12-13H2,1-2H3/t16-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor expressed in CHO membrane by ITAC-stimulated GTP gammaS assay |
Bioorg Med Chem Lett 17: 6806-10 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.029
BindingDB Entry DOI: 10.7270/Q24F1QGP |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50494092
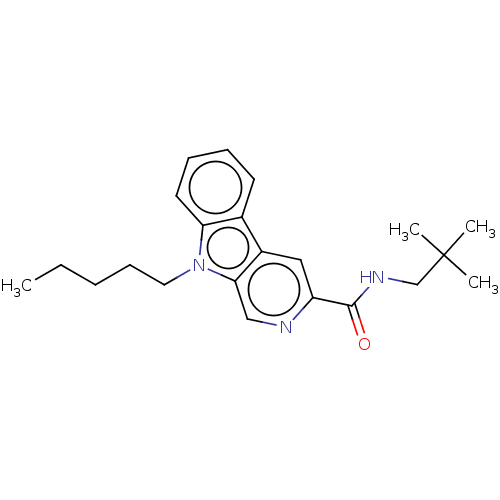
(CHEMBL2441452)Show InChI InChI=1S/C22H29N3O/c1-5-6-9-12-25-19-11-8-7-10-16(19)17-13-18(23-14-20(17)25)21(26)24-15-22(2,3)4/h7-8,10-11,13-14H,5-6,9,12,15H2,1-4H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor expressed in HEK293 cells after 1.5 hrs by microbeta liquid scintillation counting analysis |
Eur J Med Chem 69: 881-907 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.038
BindingDB Entry DOI: 10.7270/Q20P12Z8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227869
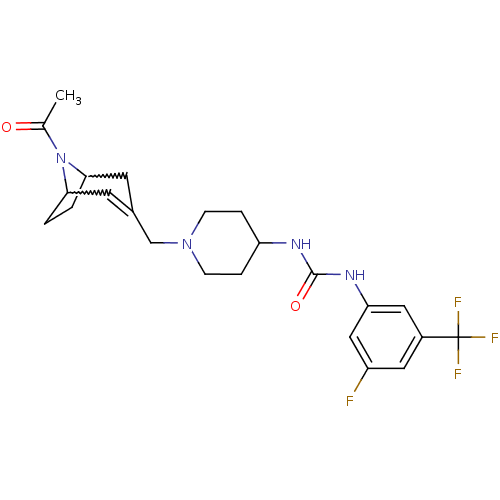
(1-[1-(8-acetyl-8-aza-bicyclo[3.2.1]oct-2-en-3-ylme...)Show SMILES CC(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:4.35,7.8,t:9| Show InChI InChI=1S/C23H28F4N4O2/c1-14(32)31-20-2-3-21(31)9-15(8-20)13-30-6-4-18(5-7-30)28-22(33)29-19-11-16(23(25,26)27)10-17(24)12-19/h8,10-12,18,20-21H,2-7,9,13H2,1H3,(H2,28,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50198395
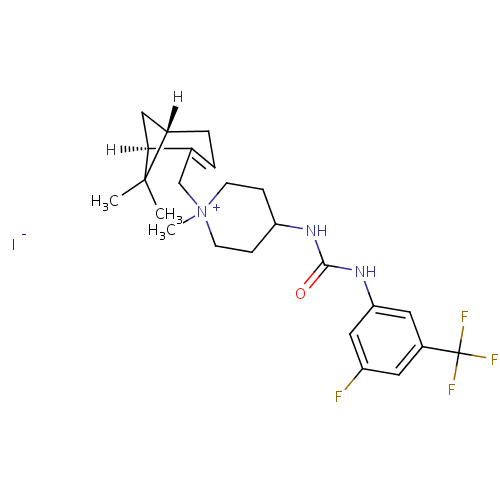
(1-{[(1R,5S)-6,6-dimethylbicyclo[3.1.1]hept-2-en-2-...)Show SMILES CC1(C)[C@@H]2C[C@H]1C(C[N+]1(C)CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)=CC2 |c:32| Show InChI InChI=1S/C24H31F4N3O.HI/c1-23(2)16-5-4-15(21(23)12-16)14-31(3)8-6-19(7-9-31)29-22(32)30-20-11-17(24(26,27)28)10-18(25)13-20;/h4,10-11,13,16,19,21H,5-9,12,14H2,1-3H3,(H-,29,30,32);1H/t16-,19?,21-,31?;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 17: 697-701 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.088
BindingDB Entry DOI: 10.7270/Q2833RPB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50227873
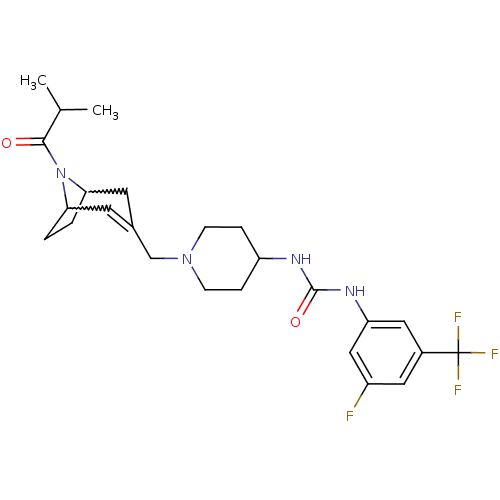
(1-(3-fluoro-5-(trifluoromethyl)phenyl)-3-(1-((8-is...)Show SMILES CC(C)C(=O)N1C2CCC1C=C(CN1CCC(CC1)NC(=O)Nc1cc(F)cc(c1)C(F)(F)F)C2 |w:6.37,9.10,t:11| Show InChI InChI=1S/C25H32F4N4O2/c1-15(2)23(34)33-21-3-4-22(33)10-16(9-21)14-32-7-5-19(6-8-32)30-24(35)31-20-12-17(25(27,28)29)11-18(26)13-20/h9,11-13,15,19,21-22H,3-8,10,14H2,1-2H3,(H2,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UCB Inflammation Discovery
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 18: 147-51 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.109
BindingDB Entry DOI: 10.7270/Q2MC8ZRG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data