 Found 725 hits with Last Name = 'borriello' and Initial = 'm'
Found 725 hits with Last Name = 'borriello' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271367
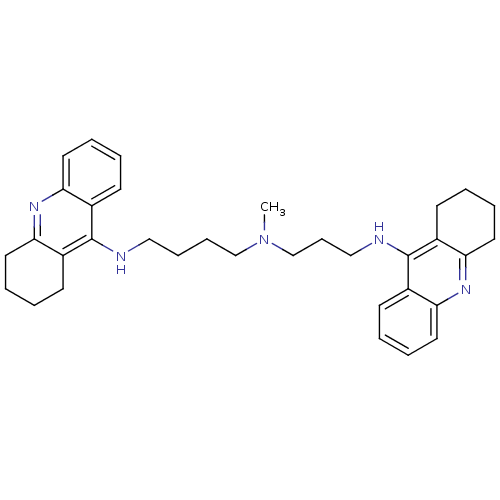
(CHEMBL489454 | N-Methyl-N-(1,2,3,4-tetrahydroacrid...)Show SMILES CN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C34H43N5/c1-39(24-12-22-36-34-27-15-4-8-19-31(27)38-32-20-9-5-16-28(32)34)23-11-10-21-35-33-25-13-2-6-17-29(25)37-30-18-7-3-14-26(30)33/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,35,37)(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50271556
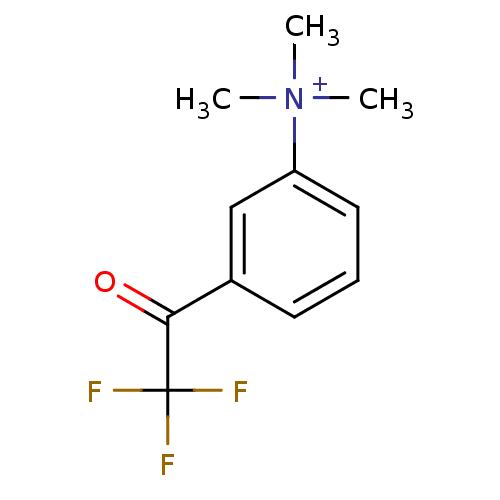
(CHEMBL525622 | N,N,N-trimethyl-3-(2,2,2-trifluoroa...)Show InChI InChI=1S/C11H13F3NO/c1-15(2,3)9-6-4-5-8(7-9)10(16)11(12,13)14/h4-7H,1-3H3/q+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10597
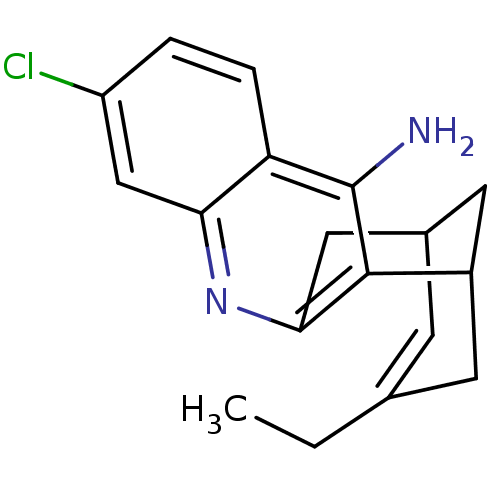
((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universita di Siena
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by E llman. Inhibition of enzyme activity was measured over a substrate c... |
J Med Chem 49: 3421-5 (2006)
Article DOI: 10.1021/jm060257t
BindingDB Entry DOI: 10.7270/Q2WW7FWB |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549
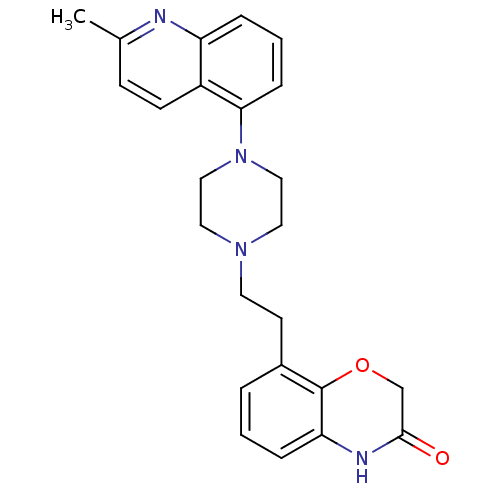
(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413549

(CHEMBL513715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)COc23)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-8-9-19-20(25-17)5-3-7-22(19)28-14-12-27(13-15-28)11-10-18-4-2-6-21-24(18)30-16-23(29)26-21/h2-9H,10-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441
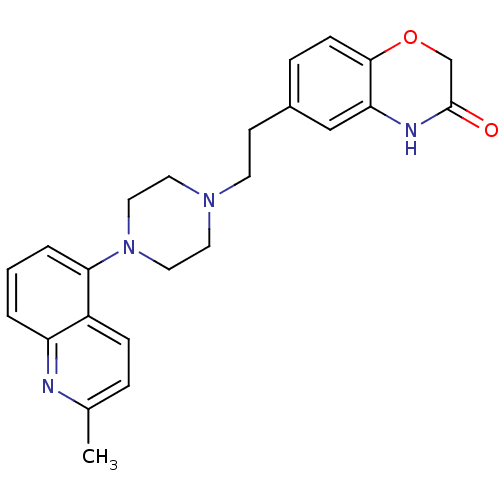
(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50149201
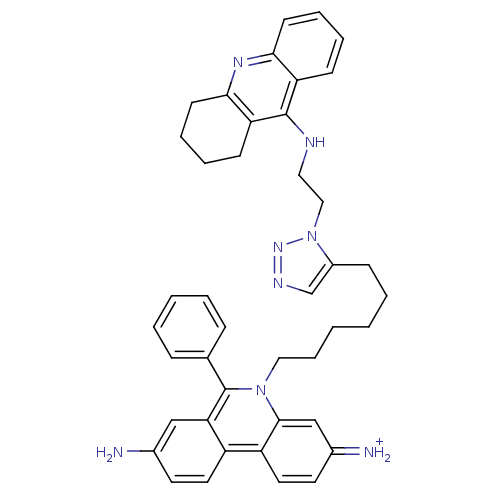
(3,8-DIAMINO-6-PHENYL-5-[6-[1-[2-[(1,2,3,4-TETRAHYD...)Show SMILES Nc1ccc2c(c1)c(-c1ccccc1)n(CCCCCCc1cnnn1CCNc1c3CCCCc3nc3ccccc13)c1cc(=[NH2+])ccc21 Show InChI InChI=1S/C42H44N8/c43-30-19-21-33-34-22-20-31(44)27-40(34)49(42(37(33)26-30)29-12-4-3-5-13-29)24-11-2-1-6-14-32-28-46-48-50(32)25-23-45-41-35-15-7-9-17-38(35)47-39-18-10-8-16-36(39)41/h3-5,7,9,12-13,15,17,19-22,26-28,44H,1-2,6,8,10-11,14,16,18,23-25,43H2,(H,45,47)/p+1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413077
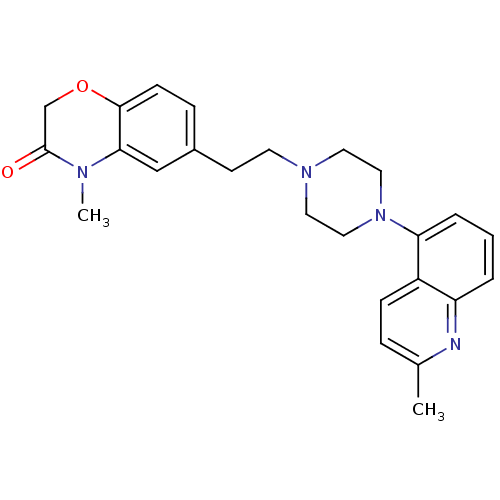
(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413560
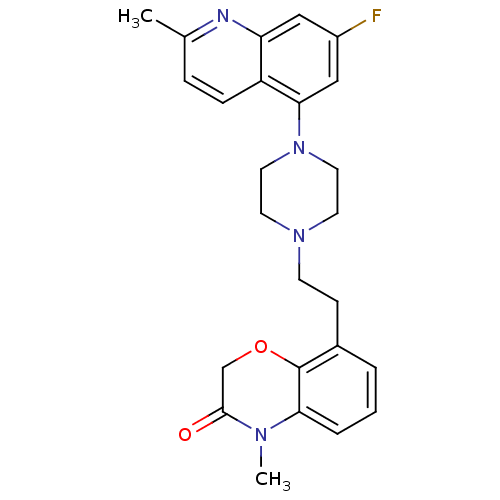
(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417409

(CHEMBL1290487)Show SMILES CN1C(=O)CCc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O/c1-19-9-10-22-23(27-19)6-4-8-25(22)30-17-15-29(16-18-30)14-13-20-5-3-7-24-21(20)11-12-26(31)28(24)2/h3-10H,11-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271469
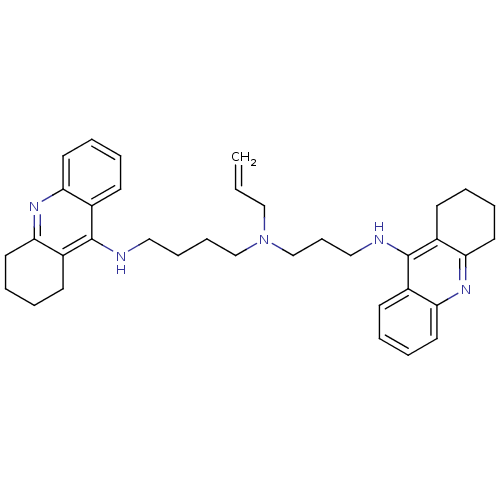
(CHEMBL507174 | N-Allyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES C=CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C36H45N5/c1-2-24-41(26-13-23-38-36-29-16-5-9-20-33(29)40-34-21-10-6-17-30(34)36)25-12-11-22-37-35-27-14-3-7-18-31(27)39-32-19-8-4-15-28(32)35/h2-3,5,7,9,14,16,18,20H,1,4,6,8,10-13,15,17,19,21-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50265775
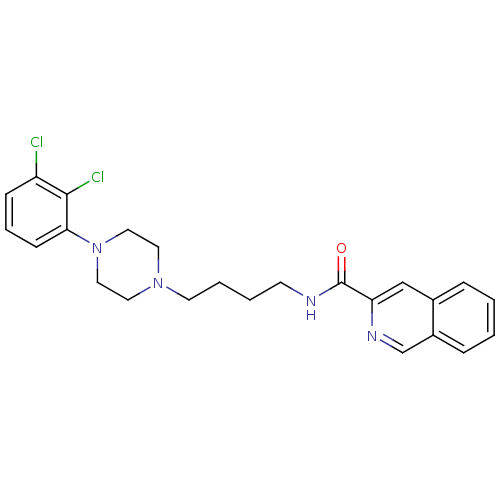
(CHEMBL462508 | N-[4-[4-(2,3-Dichlorophenyl)piperaz...)Show SMILES Clc1cccc(N2CCN(CCCCNC(=O)c3cc4ccccc4cn3)CC2)c1Cl Show InChI InChI=1S/C24H26Cl2N4O/c25-20-8-5-9-22(23(20)26)30-14-12-29(13-15-30)11-4-3-10-27-24(31)21-16-18-6-1-2-7-19(18)17-28-21/h1-2,5-9,16-17H,3-4,10-15H2,(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413550
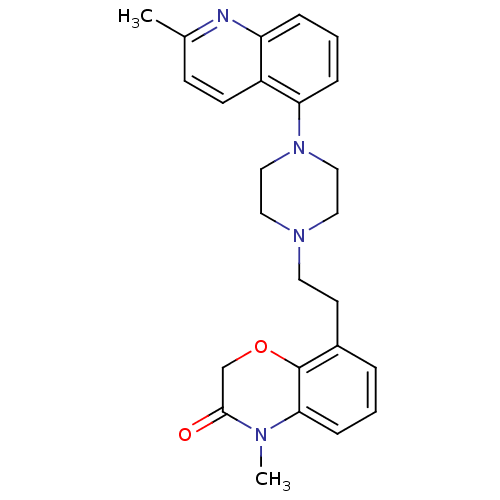
(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417420
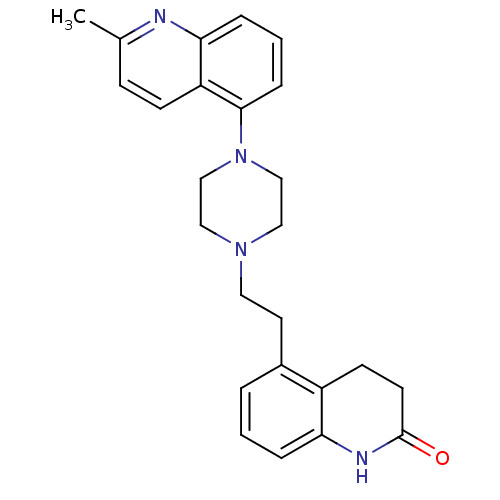
(CHEMBL1290486)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3NC(=O)CCc23)CC1 Show InChI InChI=1S/C25H28N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-9H,10-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413555
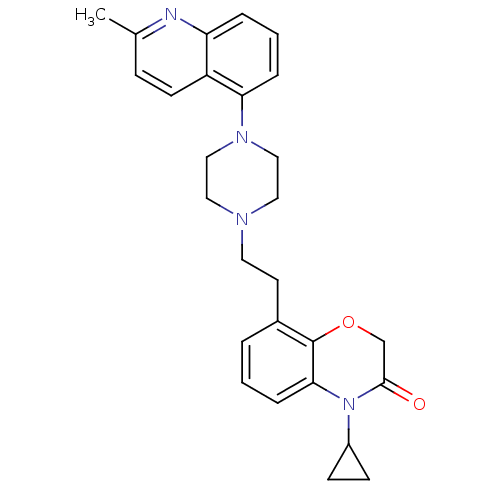
(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413077

(CHEMBL522257)Show SMILES CN1C(=O)COc2ccc(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cc12 Show InChI InChI=1S/C25H28N4O2/c1-18-6-8-20-21(26-18)4-3-5-22(20)29-14-12-28(13-15-29)11-10-19-7-9-24-23(16-19)27(2)25(30)17-31-24/h3-9,16H,10-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50265375
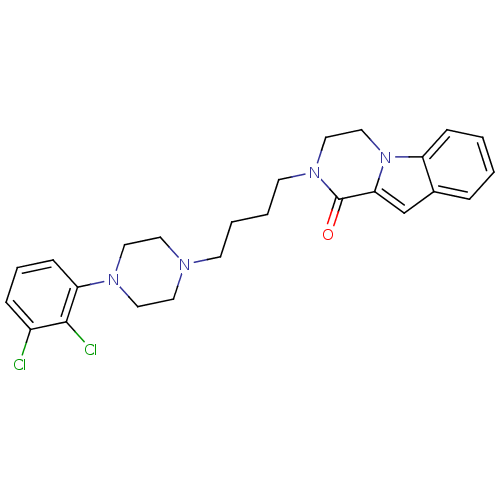
(CHEMBL496531 | N-[4-[4-(2,3-Dichlorophenyl)piperaz...)Show SMILES Clc1cccc(N2CCN(CCCCN3CCn4c(cc5ccccc45)C3=O)CC2)c1Cl Show InChI InChI=1S/C25H28Cl2N4O/c26-20-7-5-9-22(24(20)27)29-14-12-28(13-15-29)10-3-4-11-30-16-17-31-21-8-2-1-6-19(21)18-23(31)25(30)32/h1-2,5-9,18H,3-4,10-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271470
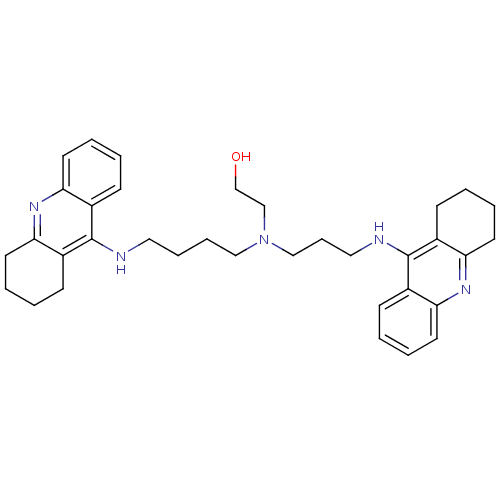
(CHEMBL499224 | N-(2-Hydroxyethyl)-N-(1,2,3,4-tetra...)Show SMILES OCCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5O/c41-25-24-40(23-11-21-37-35-28-14-3-7-18-32(28)39-33-19-8-4-15-29(33)35)22-10-9-20-36-34-26-12-1-5-16-30(26)38-31-17-6-2-13-27(31)34/h1,3,5,7,12,14,16,18,41H,2,4,6,8-11,13,15,17,19-25H2,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417411
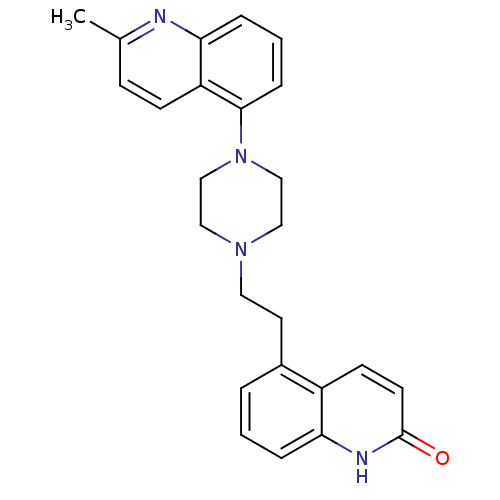
(CHEMBL1290715)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3[nH]c(=O)ccc23)CC1 Show InChI InChI=1S/C25H26N4O/c1-18-8-9-21-23(26-18)6-3-7-24(21)29-16-14-28(15-17-29)13-12-19-4-2-5-22-20(19)10-11-25(30)27-22/h2-11H,12-17H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413553
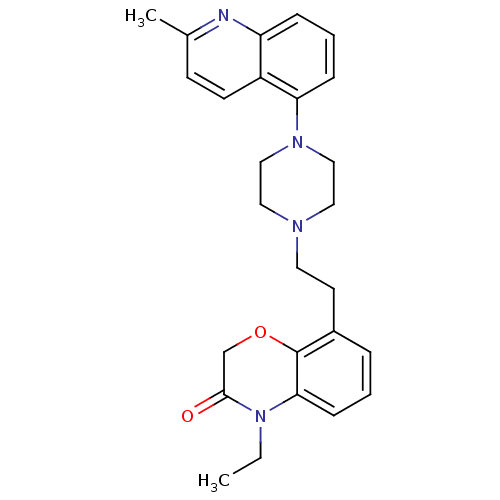
(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413086
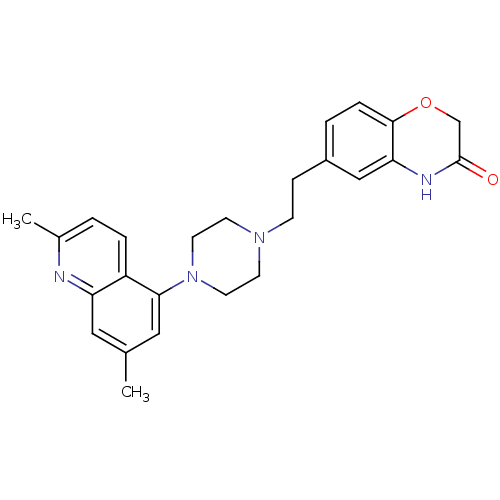
(CHEMBL484260)Show SMILES Cc1cc(N2CCN(CCc3ccc4OCC(=O)Nc4c3)CC2)c2ccc(C)nc2c1 Show InChI InChI=1S/C25H28N4O2/c1-17-13-21-20(5-3-18(2)26-21)23(14-17)29-11-9-28(10-12-29)8-7-19-4-6-24-22(15-19)27-25(30)16-31-24/h3-6,13-15H,7-12,16H2,1-2H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50417424
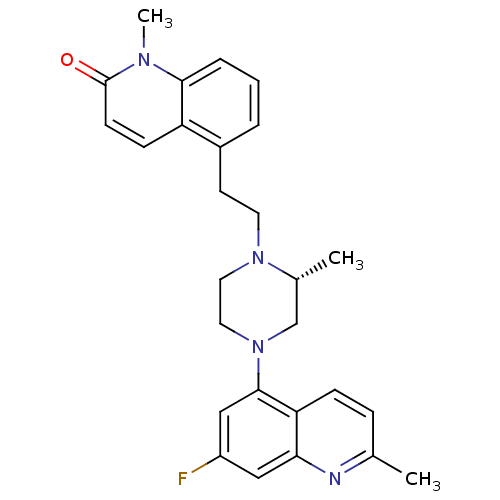
(CHEMBL1289394)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2n(C)c(=O)ccc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C27H29FN4O/c1-18-7-8-23-24(29-18)15-21(28)16-26(23)32-14-13-31(19(2)17-32)12-11-20-5-4-6-25-22(20)9-10-27(33)30(25)3/h4-10,15-16,19H,11-14,17H2,1-3H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50369748
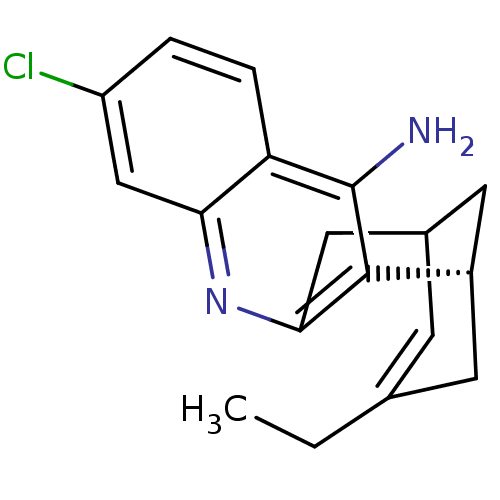
(CHEMBL208599)Show SMILES CCC1=CC2C[C@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:19:8:5:3.2.7,THB:11:9:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21)/t11?,12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of Torpedo californica AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271471
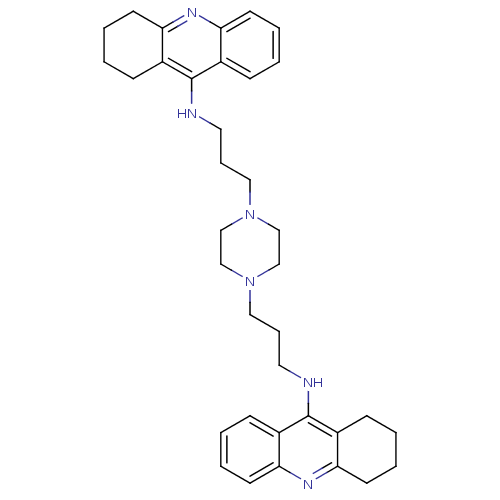
(1,4-bis[3-(1,2,3,4-Tetrahydroacridin-9-yl)aminopro...)Show SMILES C(CNc1c2CCCCc2nc2ccccc12)CN1CCN(CCCNc2c3CCCCc3nc3ccccc23)CC1 Show InChI InChI=1S/C36H46N6/c1-5-15-31-27(11-1)35(28-12-2-6-16-32(28)39-31)37-19-9-21-41-23-25-42(26-24-41)22-10-20-38-36-29-13-3-7-17-33(29)40-34-18-8-4-14-30(34)36/h1,3,5,7,11,13,15,17H,2,4,6,8-10,12,14,16,18-26H2,(H,37,39)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50265445
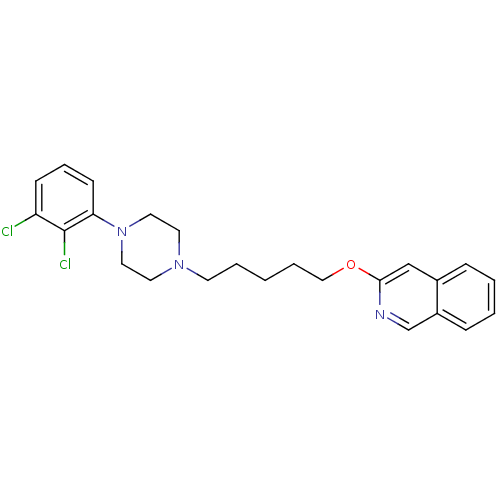
(3-[5-[4-(2,3-Dichlorophenyl)piperazin-1-yl]pentylo...)Show SMILES Clc1cccc(N2CCN(CCCCCOc3cc4ccccc4cn3)CC2)c1Cl Show InChI InChI=1S/C24H27Cl2N3O/c25-21-9-6-10-22(24(21)26)29-14-12-28(13-15-29)11-4-1-5-16-30-23-17-19-7-2-3-8-20(19)18-27-23/h2-3,6-10,17-18H,1,4-5,11-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from 5HT2A receptor in CRL:CD(SD)BR-COBS rat cortex by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50413083
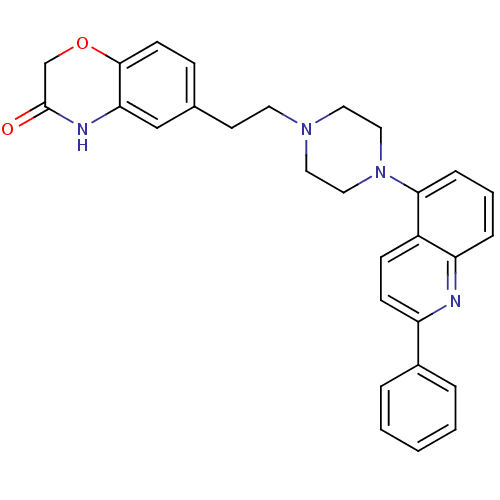
(CHEMBL484059)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4nc(ccc34)-c3ccccc3)cc2N1 Show InChI InChI=1S/C29H28N4O2/c34-29-20-35-28-12-9-21(19-26(28)31-29)13-14-32-15-17-33(18-16-32)27-8-4-7-25-23(27)10-11-24(30-25)22-5-2-1-3-6-22/h1-12,19H,13-18,20H2,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from human SerT expressed pig LLCPK cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413078
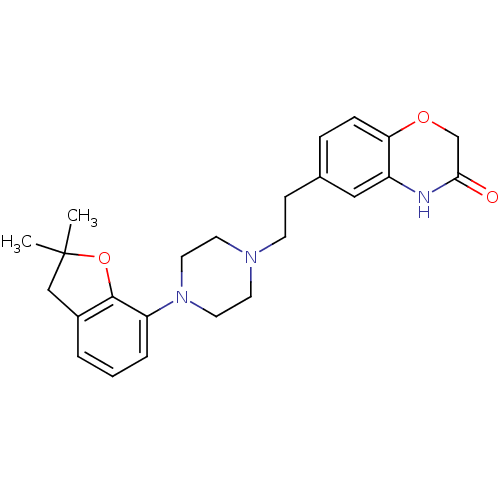
(CHEMBL491839)Show SMILES CC1(C)Cc2cccc(N3CCN(CCc4ccc5OCC(=O)Nc5c4)CC3)c2O1 Show InChI InChI=1S/C24H29N3O3/c1-24(2)15-18-4-3-5-20(23(18)30-24)27-12-10-26(11-13-27)9-8-17-6-7-21-19(14-17)25-22(28)16-29-21/h3-7,14H,8-13,15-16H2,1-2H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413550

(CHEMBL469345)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H28N4O2/c1-18-9-10-20-21(26-18)6-4-7-22(20)29-15-13-28(14-16-29)12-11-19-5-3-8-23-25(19)31-17-24(30)27(23)2/h3-10H,11-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5-HT1A receptor expressed in HEK293 cells assessed as inhibition of GTPgammaS binding by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7092-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.085
BindingDB Entry DOI: 10.7270/Q2MC919V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413555

(CHEMBL469568)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cccc3N(C4CC4)C(=O)COc23)CC1 Show InChI InChI=1S/C27H30N4O2/c1-19-8-11-22-23(28-19)5-3-6-24(22)30-16-14-29(15-17-30)13-12-20-4-2-7-25-27(20)33-18-26(32)31(25)21-9-10-21/h2-8,11,21H,9-10,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413084
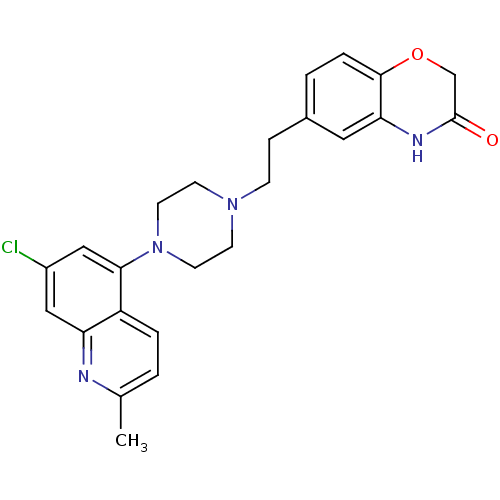
(CHEMBL521506)Show SMILES Cc1ccc2c(cc(Cl)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271468
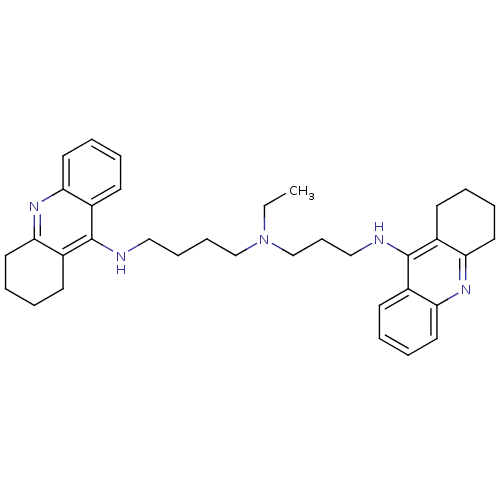
(CHEMBL490060 | N-Ethyl-N-(1,2,3,4-tetrahydroacridi...)Show SMILES CCN(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H45N5/c1-2-40(25-13-23-37-35-28-16-5-9-20-32(28)39-33-21-10-6-17-29(33)35)24-12-11-22-36-34-26-14-3-7-18-30(26)38-31-19-8-4-15-27(31)34/h3,5,7,9,14,16,18,20H,2,4,6,8,10-13,15,17,19,21-25H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50131925
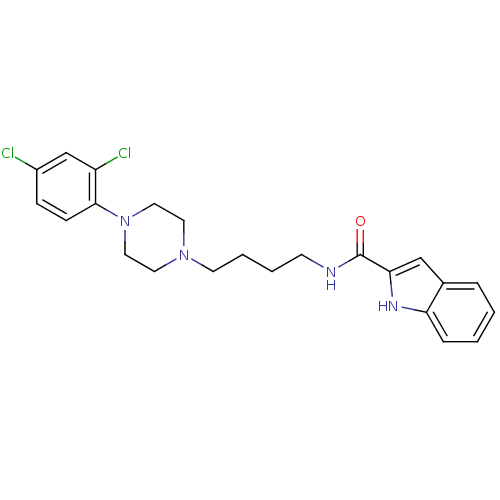
(1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4ccccc4[nH]3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H26Cl2N4O/c24-18-7-8-22(19(25)16-18)29-13-11-28(12-14-29)10-4-3-9-26-23(30)21-15-17-5-1-2-6-20(17)27-21/h1-2,5-8,15-16,27H,3-4,9-14H2,(H,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]7OH-DPAT from dopamine D3 receptor (unknown origin) expressed in Sf9 cells by scintillation spectrometry |
J Med Chem 52: 151-69 (2009)
Article DOI: 10.1021/jm800689g
BindingDB Entry DOI: 10.7270/Q2J67GSD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413075
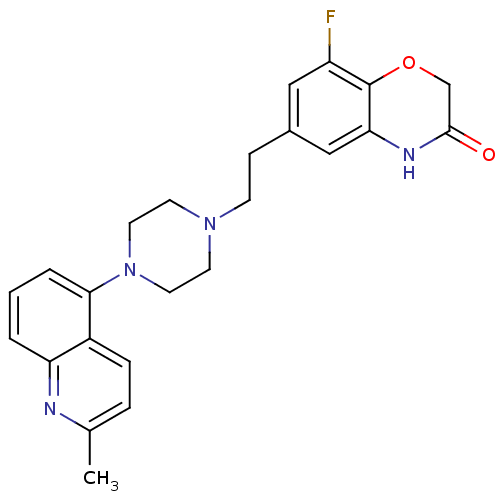
(CHEMBL489394)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cc(F)c3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-6-18-20(26-16)3-2-4-22(18)29-11-9-28(10-12-29)8-7-17-13-19(25)24-21(14-17)27-23(30)15-31-24/h2-6,13-14H,7-12,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413079
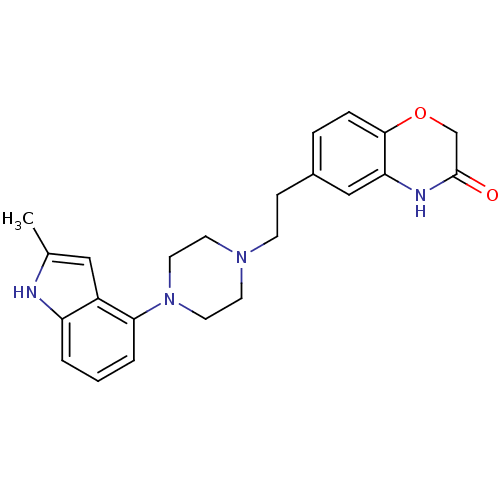
(CHEMBL444398)Show SMILES Cc1cc2c(cccc2[nH]1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C23H26N4O2/c1-16-13-18-19(24-16)3-2-4-21(18)27-11-9-26(10-12-27)8-7-17-5-6-22-20(14-17)25-23(28)15-29-22/h2-6,13-14,24H,7-12,15H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413085
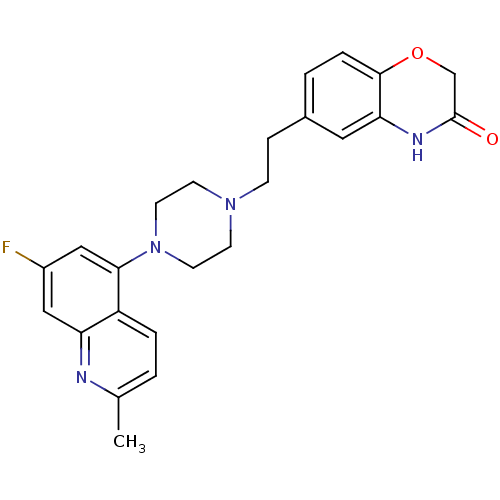
(CHEMBL485491)Show SMILES Cc1ccc2c(cc(F)cc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-2-4-19-20(26-16)13-18(25)14-22(19)29-10-8-28(9-11-29)7-6-17-3-5-23-21(12-17)27-24(30)15-31-23/h2-5,12-14H,6-11,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413561
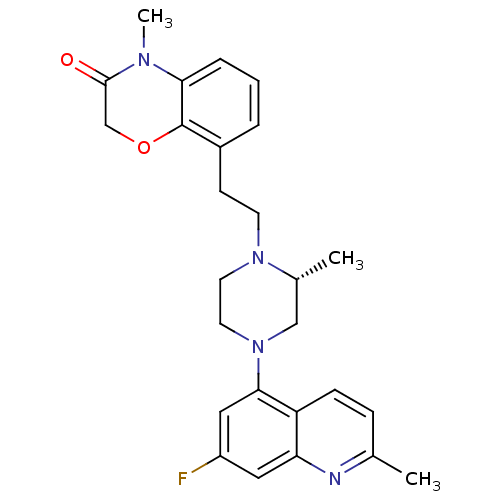
(CHEMBL513875)Show SMILES C[C@@H]1CN(CCN1CCc1cccc2N(C)C(=O)COc12)c1cc(F)cc2nc(C)ccc12 |r| Show InChI InChI=1S/C26H29FN4O2/c1-17-7-8-21-22(28-17)13-20(27)14-24(21)31-12-11-30(18(2)15-31)10-9-19-5-4-6-23-26(19)33-16-25(32)29(23)3/h4-8,13-14,18H,9-12,15-16H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413553

(CHEMBL472290)Show SMILES CCN1C(=O)COc2c(CCN3CCN(CC3)c3cccc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C26H30N4O2/c1-3-30-24-9-4-6-20(26(24)32-18-25(30)31)12-13-28-14-16-29(17-15-28)23-8-5-7-22-21(23)11-10-19(2)27-22/h4-11H,3,12-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413560

(CHEMBL469374)Show SMILES CN1C(=O)COc2c(CCN3CCN(CC3)c3cc(F)cc4nc(C)ccc34)cccc12 Show InChI InChI=1S/C25H27FN4O2/c1-17-6-7-20-21(27-17)14-19(26)15-23(20)30-12-10-29(11-13-30)9-8-18-4-3-5-22-25(18)32-16-24(31)28(22)2/h3-7,14-15H,8-13,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413087
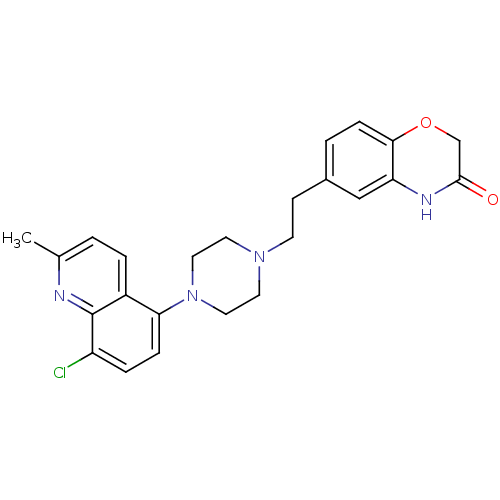
(CHEMBL497980)Show SMILES Cc1ccc2c(ccc(Cl)c2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O2/c1-16-2-4-18-21(6-5-19(25)24(18)26-16)29-12-10-28(11-13-29)9-8-17-3-7-22-20(14-17)27-23(30)15-31-22/h2-7,14H,8-13,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50271325
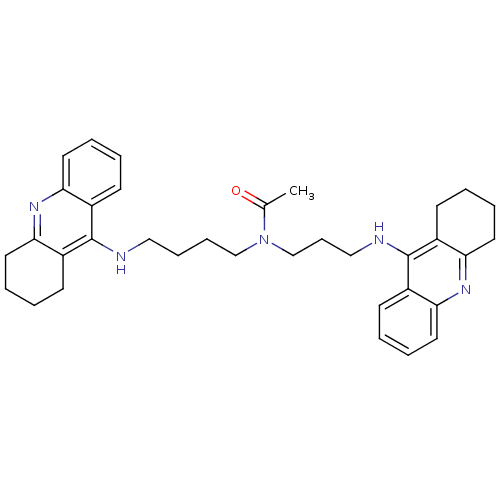
(CHEMBL451277 | N-{4-[(1,2,3,4-Tetrahydroacridin-9-...)Show SMILES CC(=O)N(CCCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C35H43N5O/c1-25(41)40(24-12-22-37-35-28-15-4-8-19-32(28)39-33-20-9-5-16-29(33)35)23-11-10-21-36-34-26-13-2-6-17-30(26)38-31-18-7-3-14-27(31)34/h2,4,6,8,13,15,17,19H,3,5,7,9-12,14,16,18,20-24H2,1H3,(H,36,38)(H,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE |
J Med Chem 51: 3154-70 (2008)
Article DOI: 10.1021/jm701253t
BindingDB Entry DOI: 10.7270/Q22Z16D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413074
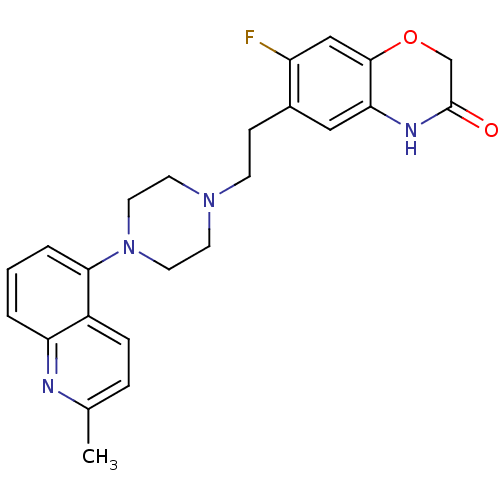
(CHEMBL496317)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cc3NC(=O)COc3cc2F)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-6-18-20(26-16)3-2-4-22(18)29-11-9-28(10-12-29)8-7-17-13-21-23(14-19(17)25)31-15-24(30)27-21/h2-6,13-14H,7-12,15H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413074

(CHEMBL496317)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2cc3NC(=O)COc3cc2F)CC1 Show InChI InChI=1S/C24H25FN4O2/c1-16-5-6-18-20(26-16)3-2-4-22(18)29-11-9-28(10-12-29)8-7-17-13-21-23(14-19(17)25)31-15-24(30)27-21/h2-6,13-14H,7-12,15H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412441

(CHEMBL490417 | SB-744185)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3OCC(=O)Nc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-23-21(15-18)26-24(29)16-30-23/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY-100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413081
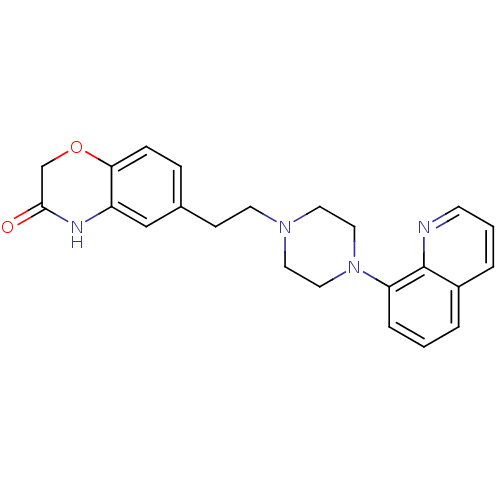
(CHEMBL523095)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3cccc4cccnc34)cc2N1 Show InChI InChI=1S/C23H24N4O2/c28-22-16-29-21-7-6-17(15-19(21)25-22)8-10-26-11-13-27(14-12-26)20-5-1-3-18-4-2-9-24-23(18)20/h1-7,9,15H,8,10-14,16H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413082
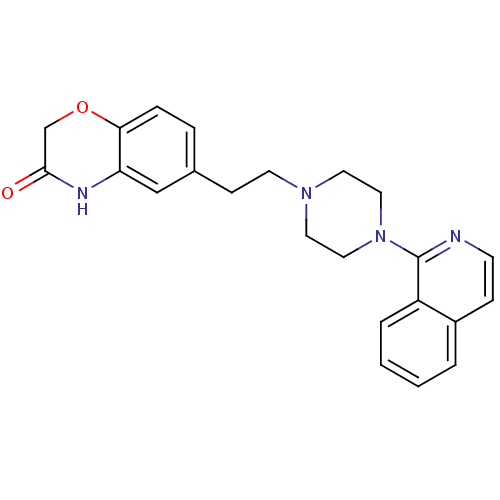
(CHEMBL490211)Show SMILES O=C1COc2ccc(CCN3CCN(CC3)c3nccc4ccccc34)cc2N1 Show InChI InChI=1S/C23H24N4O2/c28-22-16-29-21-6-5-17(15-20(21)25-22)8-10-26-11-13-27(14-12-26)23-19-4-2-1-3-18(19)7-9-24-23/h1-7,9,15H,8,10-14,16H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 5653-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.084
BindingDB Entry DOI: 10.7270/Q2BR8TC6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413551
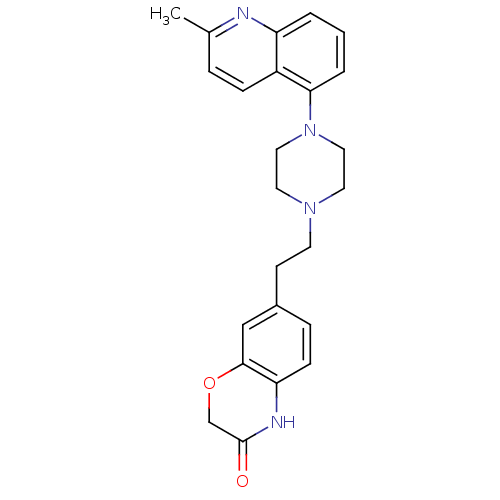
(CHEMBL512724)Show SMILES Cc1ccc2c(cccc2n1)N1CCN(CCc2ccc3NC(=O)COc3c2)CC1 Show InChI InChI=1S/C24H26N4O2/c1-17-5-7-19-20(25-17)3-2-4-22(19)28-13-11-27(12-14-28)10-9-18-6-8-21-23(15-18)30-16-24(29)26-21/h2-8,15H,9-14,16H2,1H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT1A assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HT |
Bioorg Med Chem Lett 19: 2338-42 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.056
BindingDB Entry DOI: 10.7270/Q2J967MX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data