

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430967 (CHEMBL2337848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430968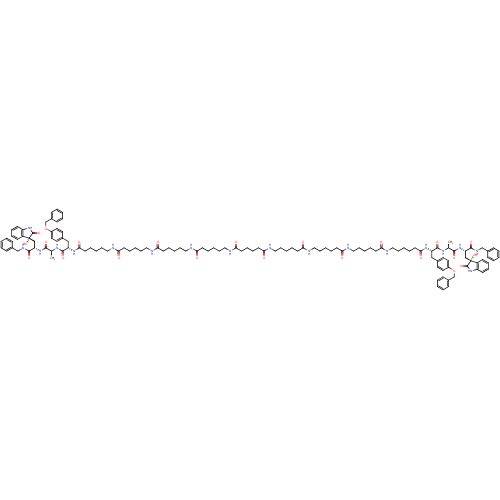 (CHEMBL2337847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430962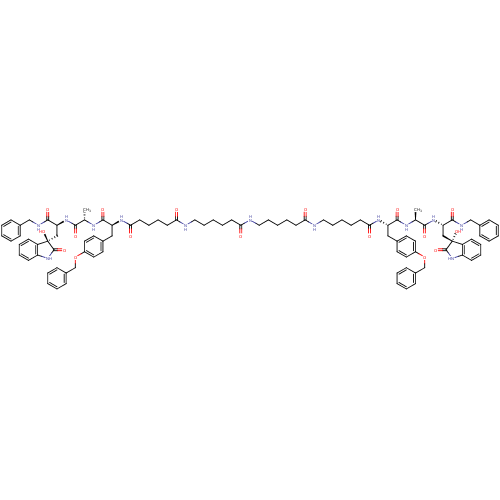 (CHEMBL2337843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430969 (CHEMBL2337846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430961 (CHEMBL2337844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430970 (CHEMBL2337845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430963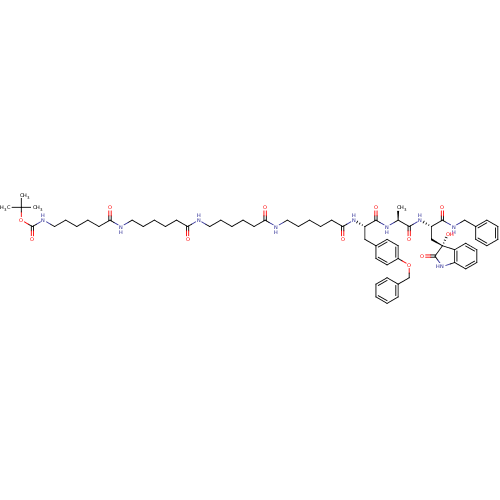 (CHEMBL2337842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430964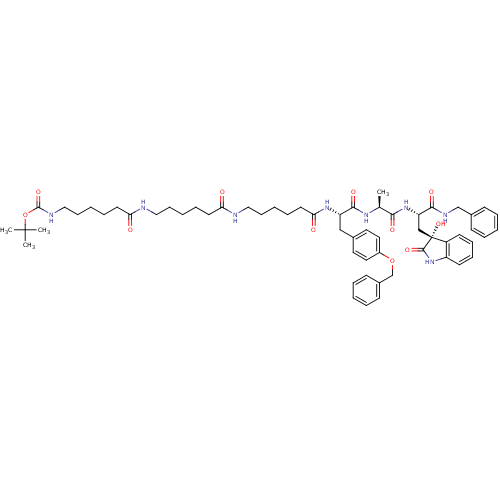 (CHEMBL2337841) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430963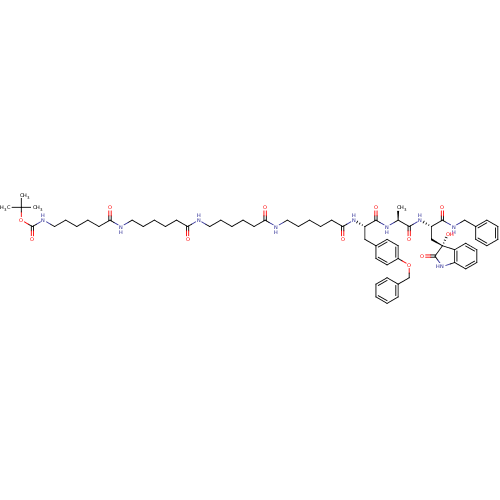 (CHEMBL2337842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430964 (CHEMBL2337841) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430966 (CHEMBL2337849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430966 (CHEMBL2337849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430965 (CHEMBL2337850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Noncompetitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430965 (CHEMBL2337850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Noncompetitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099832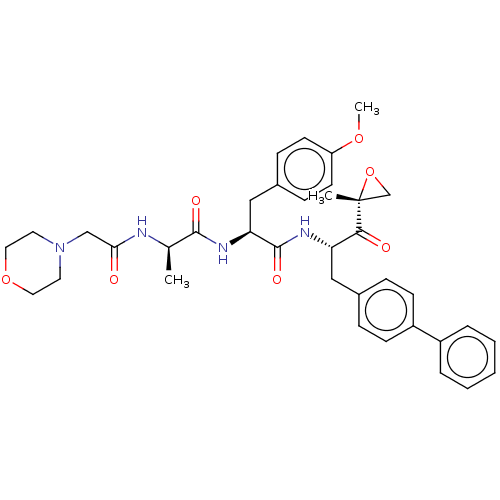 (CHEMBL3319587) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit alpha type-2 (Saccharomyces cerevisiae s288c (Yeast)) | BDBM154579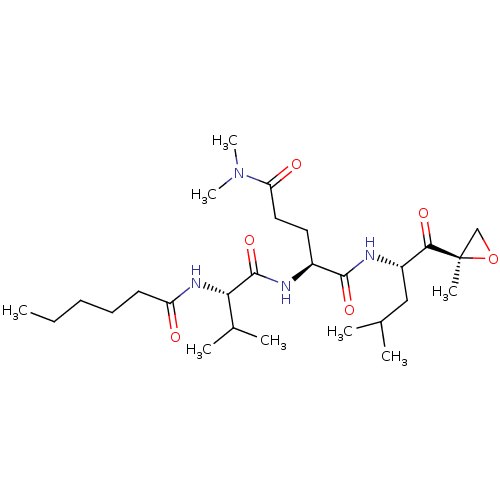 ((2S)-2-[(2S)-2-hexanamido-3-methylbutanamido]-N,N-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California at San Diego; University of Campinas | Assay Description One nanomolar of proteasome was incubated with different inhibitor concentrations in Tris 25 mM pH 7.5; SDS 0.03%; EDTA 0.5 mM for 15 min at 37 °C in... | Chem Biol 21: 782-91 (2014) Article DOI: 10.1016/j.chembiol.2014.04.010 BindingDB Entry DOI: 10.7270/Q2QR4VVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099830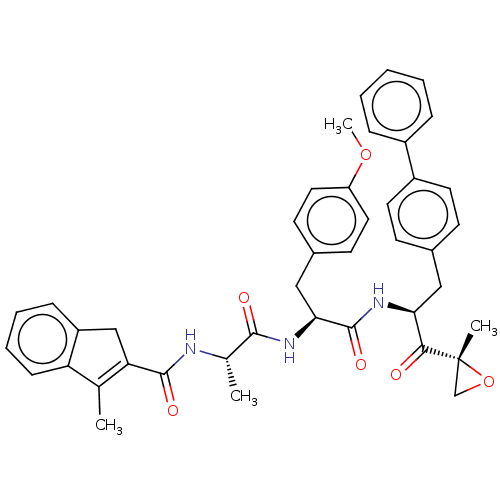 (CHEMBL3319585) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit alpha type-2 (Saccharomyces cerevisiae s288c (Yeast)) | BDBM154578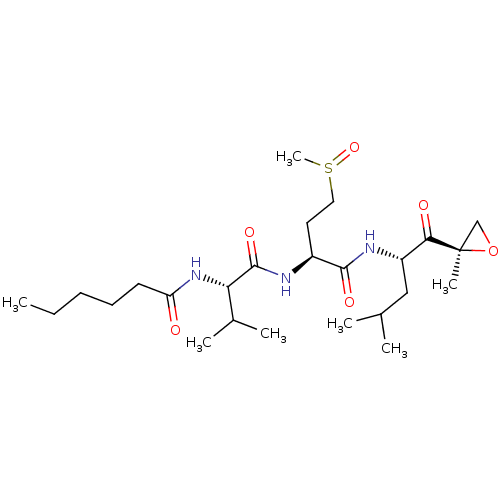 (Carmaphycin A (1)) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of California at San Diego; University of Campinas | Assay Description One nanomolar of proteasome was incubated with different inhibitor concentrations in Tris 25 mM pH 7.5; SDS 0.03%; EDTA 0.5 mM for 15 min at 37 °C in... | Chem Biol 21: 782-91 (2014) Article DOI: 10.1016/j.chembiol.2014.04.010 BindingDB Entry DOI: 10.7270/Q2QR4VVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099659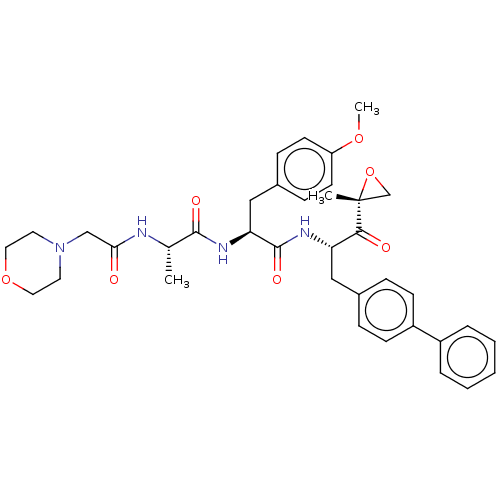 (CHEMBL3319478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425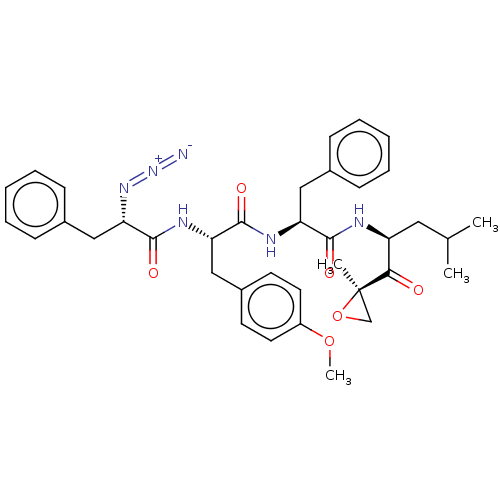 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099831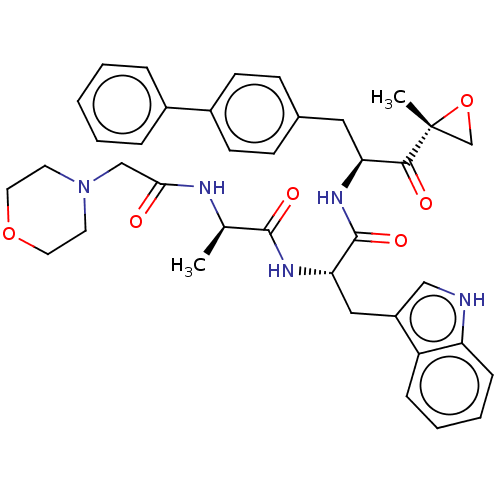 (CHEMBL3319586) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099663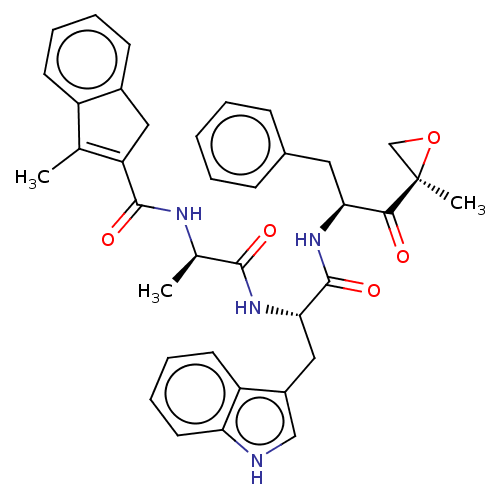 (CHEMBL3319482) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50470540 (CHEMBL4282642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
New Mexico Institute of Mining and Technology Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human Jurkat cell lysate using Suc-LLVY-AMC as substrate pretreated for 30 mins followed by substrate a... | Eur J Med Chem 157: 962-977 (2018) Article DOI: 10.1016/j.ejmech.2018.08.052 BindingDB Entry DOI: 10.7270/Q2028V7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099829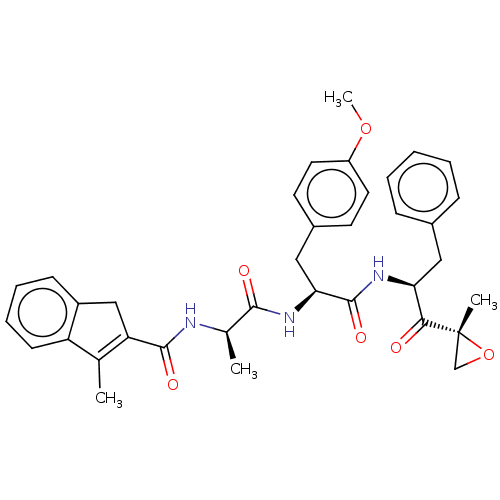 (CHEMBL3319584) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099685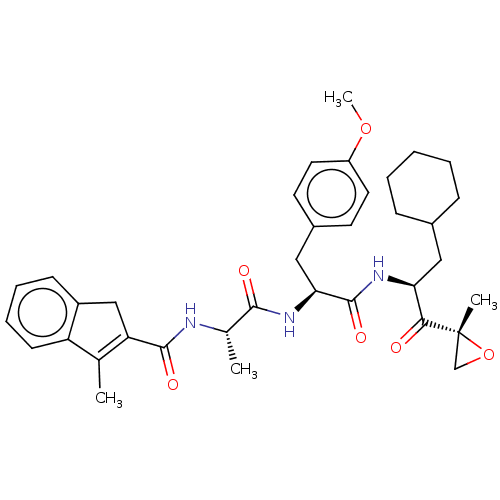 (CHEMBL3319578) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50470557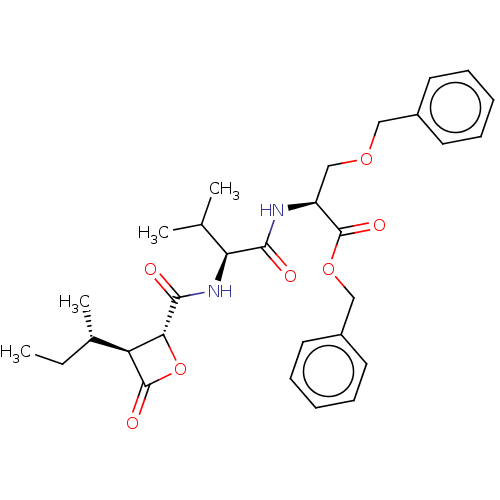 (CHEMBL4290778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
New Mexico Institute of Mining and Technology Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human Jurkat cell lysate using Suc-LLVY-AMC as substrate pretreated for 30 mins followed by substrate a... | Eur J Med Chem 157: 962-977 (2018) Article DOI: 10.1016/j.ejmech.2018.08.052 BindingDB Entry DOI: 10.7270/Q2028V7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099660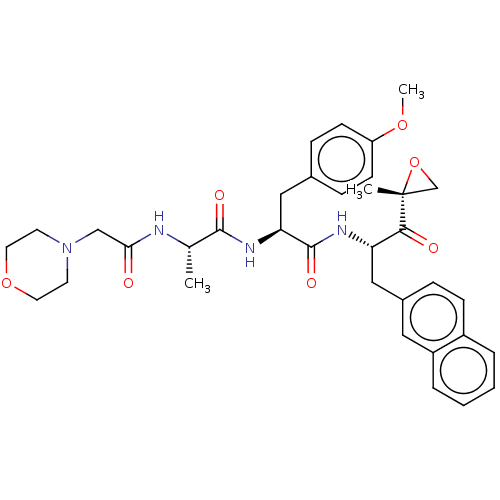 (CHEMBL3319479) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099833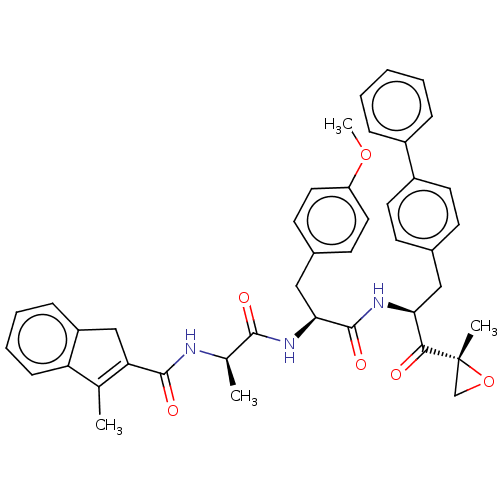 (CHEMBL3319588) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50470536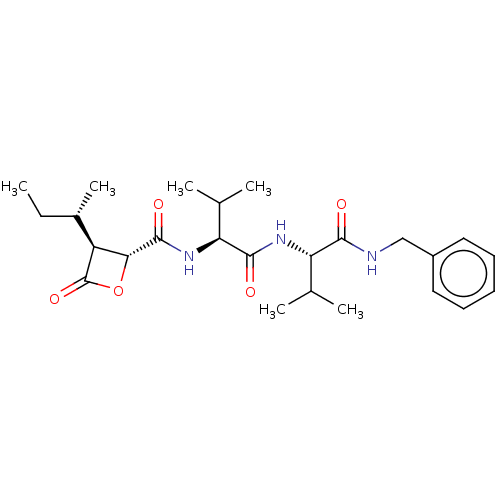 (CHEMBL4292798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
New Mexico Institute of Mining and Technology Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human Jurkat cell lysate using Suc-LLVY-AMC as substrate pretreated for 30 mins followed by substrate a... | Eur J Med Chem 157: 962-977 (2018) Article DOI: 10.1016/j.ejmech.2018.08.052 BindingDB Entry DOI: 10.7270/Q2028V7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536442 (CHEMBL4570216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich Heine University D£sseldorf Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human SEM cells using Suc-LLVY aminoluciferin as substrate after 2 hrs by proteasome-Gl... | J Med Chem 61: 10299-10309 (2018) Article DOI: 10.1021/acs.jmedchem.8b01487 BindingDB Entry DOI: 10.7270/Q26W9DMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 20S proteasome beta 5 subunit using Suc-LLVY-AMC as substrate after 60 mins by fluorescence assay | J Med Chem 56: 3689-700 (2013) Article DOI: 10.1021/jm4002296 BindingDB Entry DOI: 10.7270/Q25M673W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536439 (CHEMBL4581001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517643 (CHEMBL4451682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity in human Raji cell lysates after 1 hr by competitive ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50470555 (CHEMBL4293394) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
New Mexico Institute of Mining and Technology Curated by ChEMBL | Assay Description Inhibition of 20S proteasome beta5 subunit in human Jurkat cell lysate using Suc-LLVY-AMC as substrate pretreated for 30 mins followed by substrate a... | Eur J Med Chem 157: 962-977 (2018) Article DOI: 10.1016/j.ejmech.2018.08.052 BindingDB Entry DOI: 10.7270/Q2028V7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099683 (CHEMBL3319484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50099687 (CHEMBL3319580) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of proteasome subunit beta-5i in human Raji cells using BODIPY-NC005 by fluorescent densitometry | J Med Chem 57: 6197-209 (2014) Article DOI: 10.1021/jm500716s BindingDB Entry DOI: 10.7270/Q2BR8TZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430967 (CHEMBL2337848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as remaining ac... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 804 total ) | Next | Last >> |