 Found 31852 hits with Last Name = 'hocker' and Initial = 'm'
Found 31852 hits with Last Name = 'hocker' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043760
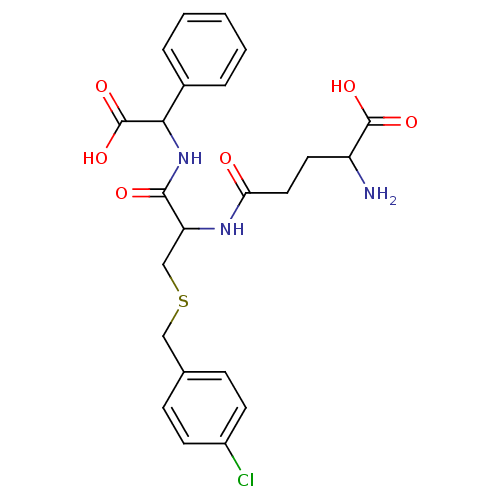
(2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES NC(CCC(=O)NC(CSCc1ccc(Cl)cc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26ClN3O6S/c24-16-8-6-14(7-9-16)12-34-13-18(26-19(28)11-10-17(25)22(30)31)21(29)27-20(23(32)33)15-4-2-1-3-5-15/h1-9,17-18,20H,10-13,25H2,(H,26,28)(H,27,29)(H,30,31)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043762
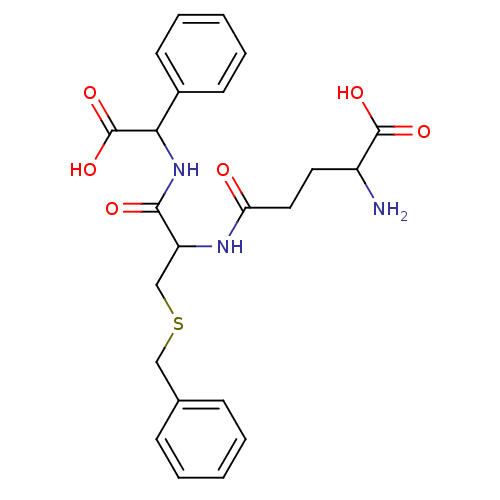
(2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H27N3O6S/c24-17(22(29)30)11-12-19(27)25-18(14-33-13-15-7-3-1-4-8-15)21(28)26-20(23(31)32)16-9-5-2-6-10-16/h1-10,17-18,20H,11-14,24H2,(H,25,27)(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043758

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...)Show InChI InChI=1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043764
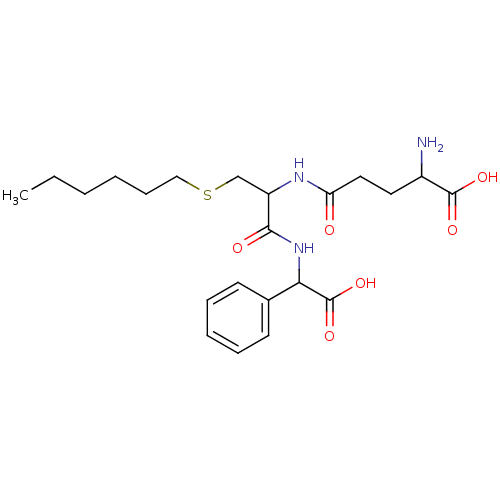
(2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C22H33N3O6S/c1-2-3-4-8-13-32-14-17(24-18(26)12-11-16(23)21(28)29)20(27)25-19(22(30)31)15-9-6-5-7-10-15/h5-7,9-10,16-17,19H,2-4,8,11-14,23H2,1H3,(H,24,26)(H,25,27)(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043758

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...)Show InChI InChI=1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043763
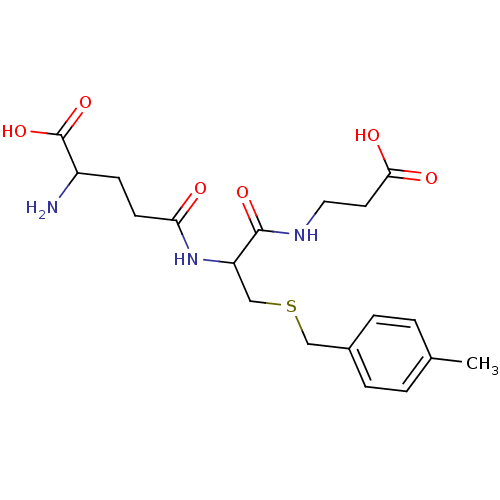
(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...)Show SMILES Cc1ccc(CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O)cc1 Show InChI InChI=1S/C19H27N3O6S/c1-12-2-4-13(5-3-12)10-29-11-15(18(26)21-9-8-17(24)25)22-16(23)7-6-14(20)19(27)28/h2-5,14-15H,6-11,20H2,1H3,(H,21,26)(H,22,23)(H,24,25)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043764

(2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C22H33N3O6S/c1-2-3-4-8-13-32-14-17(24-18(26)12-11-16(23)21(28)29)20(27)25-19(22(30)31)15-9-6-5-7-10-15/h5-7,9-10,16-17,19H,2-4,8,11-14,23H2,1H3,(H,24,26)(H,25,27)(H,28,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043758

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...)Show InChI InChI=1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043761

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C17H31N3O6S/c1-2-3-4-5-10-27-11-13(16(24)19-9-8-15(22)23)20-14(21)7-6-12(18)17(25)26/h12-13H,2-11,18H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043760

(2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES NC(CCC(=O)NC(CSCc1ccc(Cl)cc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26ClN3O6S/c24-16-8-6-14(7-9-16)12-34-13-18(26-19(28)11-10-17(25)22(30)31)21(29)27-20(23(32)33)15-4-2-1-3-5-15/h1-9,17-18,20H,10-13,25H2,(H,26,28)(H,27,29)(H,30,31)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043760

(2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES NC(CCC(=O)NC(CSCc1ccc(Cl)cc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26ClN3O6S/c24-16-8-6-14(7-9-16)12-34-13-18(26-19(28)11-10-17(25)22(30)31)21(29)27-20(23(32)33)15-4-2-1-3-5-15/h1-9,17-18,20H,10-13,25H2,(H,26,28)(H,27,29)(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase M1a enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043763

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...)Show SMILES Cc1ccc(CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O)cc1 Show InChI InChI=1S/C19H27N3O6S/c1-12-2-4-13(5-3-12)10-29-11-15(18(26)21-9-8-17(24)25)22-16(23)7-6-14(20)19(27)28/h2-5,14-15H,6-11,20H2,1H3,(H,21,26)(H,22,23)(H,24,25)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043759
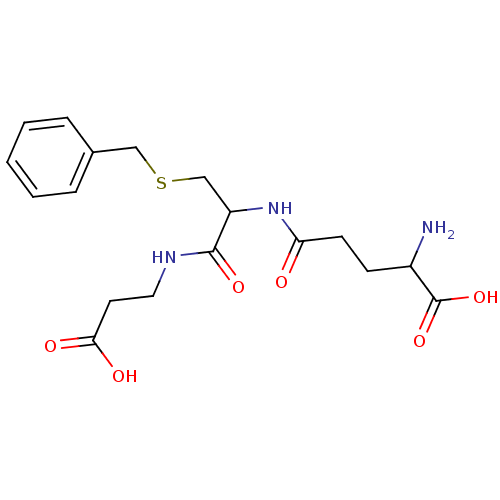
(2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NCCC(O)=O)C(O)=O Show InChI InChI=1S/C18H25N3O6S/c19-13(18(26)27)6-7-15(22)21-14(17(25)20-9-8-16(23)24)11-28-10-12-4-2-1-3-5-12/h1-5,13-14H,6-11,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043762

(2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H27N3O6S/c24-17(22(29)30)11-12-19(27)25-18(14-33-13-15-7-3-1-4-8-15)21(28)26-20(23(31)32)16-9-5-2-6-10-16/h1-10,17-18,20H,11-14,24H2,(H,25,27)(H,26,28)(H,29,30)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043759

(2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NCCC(O)=O)C(O)=O Show InChI InChI=1S/C18H25N3O6S/c19-13(18(26)27)6-7-15(22)21-14(17(25)20-9-8-16(23)24)11-28-10-12-4-2-1-3-5-12/h1-5,13-14H,6-11,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043758

(2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...)Show InChI InChI=1S/C16H29N3O6S/c1-2-3-4-5-8-26-10-12(15(23)18-9-14(21)22)19-13(20)7-6-11(17)16(24)25/h11-12H,2-10,17H2,1H3,(H,18,23)(H,19,20)(H,21,22)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043763

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...)Show SMILES Cc1ccc(CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O)cc1 Show InChI InChI=1S/C19H27N3O6S/c1-12-2-4-13(5-3-12)10-29-11-15(18(26)21-9-8-17(24)25)22-16(23)7-6-14(20)19(27)28/h2-5,14-15H,6-11,20H2,1H3,(H,21,26)(H,22,23)(H,24,25)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043764

(2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C22H33N3O6S/c1-2-3-4-8-13-32-14-17(24-18(26)12-11-16(23)21(28)29)20(27)25-19(22(30)31)15-9-6-5-7-10-15/h5-7,9-10,16-17,19H,2-4,8,11-14,23H2,1H3,(H,24,26)(H,25,27)(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043761

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C17H31N3O6S/c1-2-3-4-5-10-27-11-13(16(24)19-9-8-15(22)23)20-14(21)7-6-12(18)17(25)26/h12-13H,2-11,18H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043761

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C17H31N3O6S/c1-2-3-4-5-10-27-11-13(16(24)19-9-8-15(22)23)20-14(21)7-6-12(18)17(25)26/h12-13H,2-11,18H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043763

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...)Show SMILES Cc1ccc(CSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O)cc1 Show InChI InChI=1S/C19H27N3O6S/c1-12-2-4-13(5-3-12)10-29-11-15(18(26)21-9-8-17(24)25)22-16(23)7-6-14(20)19(27)28/h2-5,14-15H,6-11,20H2,1H3,(H,21,26)(H,22,23)(H,24,25)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043760

(2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES NC(CCC(=O)NC(CSCc1ccc(Cl)cc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26ClN3O6S/c24-16-8-6-14(7-9-16)12-34-13-18(26-19(28)11-10-17(25)22(30)31)21(29)27-20(23(32)33)15-4-2-1-3-5-15/h1-9,17-18,20H,10-13,25H2,(H,26,28)(H,27,29)(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 1
(Homo sapiens (Human)) | BDBM50043762

(2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H27N3O6S/c24-17(22(29)30)11-12-19(27)25-18(14-33-13-15-7-3-1-4-8-15)21(28)26-20(23(31)32)16-9-5-2-6-10-16/h1-10,17-18,20H,11-14,24H2,(H,25,27)(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043764

(2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C22H33N3O6S/c1-2-3-4-8-13-32-14-17(24-18(26)12-11-16(23)21(28)29)20(27)25-19(22(30)31)15-9-6-5-7-10-15/h5-7,9-10,16-17,19H,2-4,8,11-14,23H2,1H3,(H,24,26)(H,25,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase Mu 2
(Homo sapiens (Human)) | BDBM50043762

(2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NC(C(O)=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C23H27N3O6S/c24-17(22(29)30)11-12-19(27)25-18(14-33-13-15-7-3-1-4-8-15)21(28)26-20(23(31)32)16-9-5-2-6-10-16/h1-10,17-18,20H,11-14,24H2,(H,25,27)(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase A1
(Homo sapiens (Human)) | BDBM50043759

(2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NCCC(O)=O)C(O)=O Show InChI InChI=1S/C18H25N3O6S/c19-13(18(26)27)6-7-15(22)21-14(17(25)20-9-8-16(23)24)11-28-10-12-4-2-1-3-5-12/h1-5,13-14H,6-11,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzyme |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043761

(2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...)Show SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C17H31N3O6S/c1-2-3-4-5-10-27-11-13(16(24)19-9-8-15(22)23)20-14(21)7-6-12(18)17(25)26/h12-13H,2-11,18H2,1H3,(H,19,24)(H,20,21)(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Glutathione S-transferase P
(Homo sapiens (Human)) | BDBM50043759

(2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...)Show SMILES NC(CCC(=O)NC(CSCc1ccccc1)C(=O)NCCC(O)=O)C(O)=O Show InChI InChI=1S/C18H25N3O6S/c19-13(18(26)27)6-7-15(22)21-14(17(25)20-9-8-16(23)24)11-28-10-12-4-2-1-3-5-12/h1-5,13-14H,6-11,19H2,(H,20,25)(H,21,22)(H,23,24)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P |
J Med Chem 37: 189-94 (1994)
BindingDB Entry DOI: 10.7270/Q21V5D1V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 11A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for CDC2L2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CA kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50309910
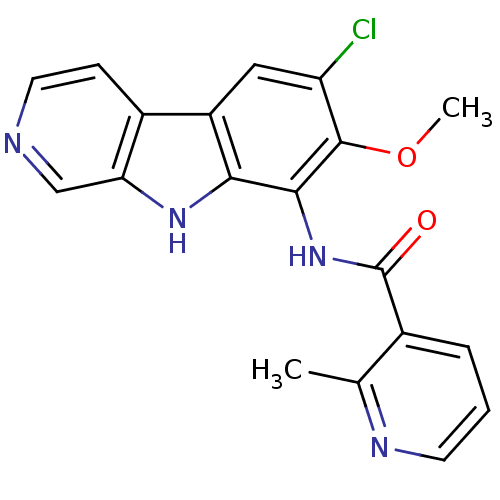
(CHEMBL608154 | ML-120B | N-(6-chloro-7-methoxy-9H-...)Show SMILES COc1c(Cl)cc2c3ccncc3[nH]c2c1NC(=O)c1cccnc1C Show InChI InChI=1S/C19H15ClN4O2/c1-10-11(4-3-6-22-10)19(25)24-17-16-13(8-14(20)18(17)26-2)12-5-7-21-9-15(12)23-16/h3-9,23H,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PRKG1 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
NUAK family SNF1-like kinase 1
(Homo sapiens (Human)) | BDBM50326053
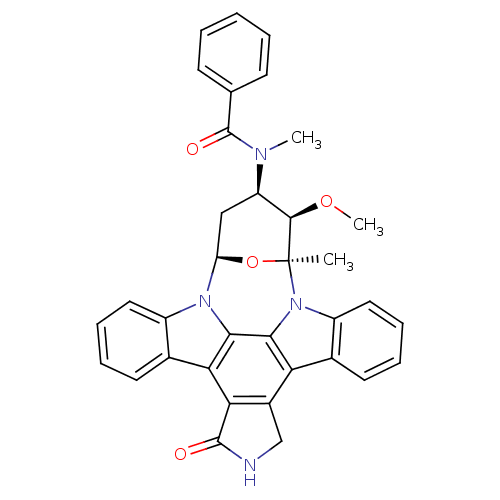
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for ARK5 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM5931
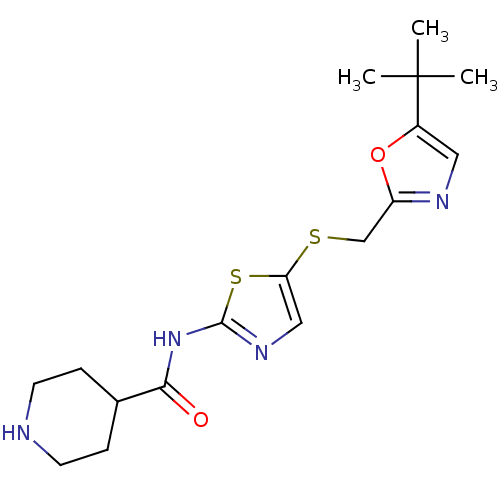
(BMS-387072 | CHEMBL296468 | N-(5-{[(5-tert-butyl-1...)Show InChI InChI=1S/C17H24N4O2S2/c1-17(2,3)12-8-19-13(23-12)10-24-14-9-20-16(25-14)21-15(22)11-4-6-18-7-5-11/h8-9,11,18H,4-7,10H2,1-3H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MINK kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM25118
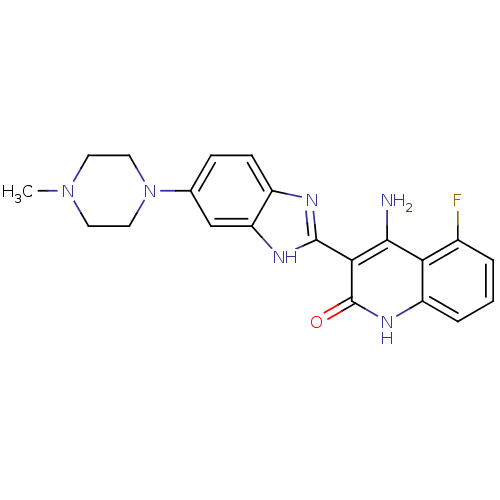
((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(F)cccc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EGFR(G719S) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 1
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA1 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for INSR kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50026612

(BIBF-1120 | Nintedanib | US10981896, Compound Nint...)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for TYK2(JH2domain-pseudokinase) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for INSR kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for HPK1 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 3
(Homo sapiens (Human)) | BDBM50308060
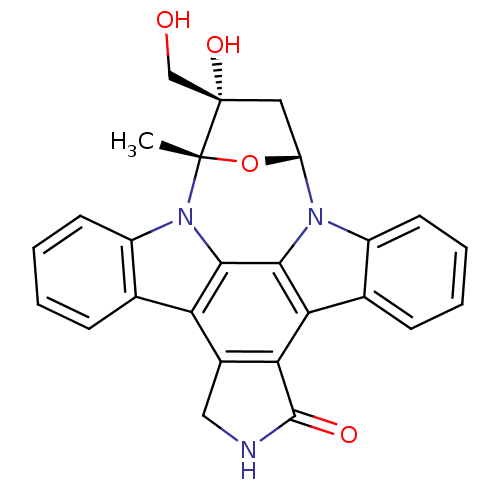
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB3 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for FLT3(D835H) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 6
(Homo sapiens (Human)) | BDBM13336
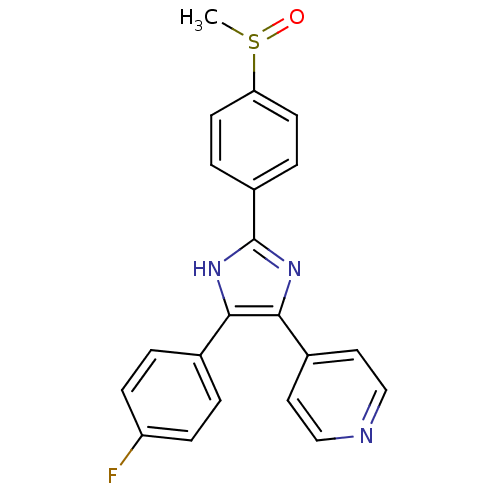
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHA6 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM4851
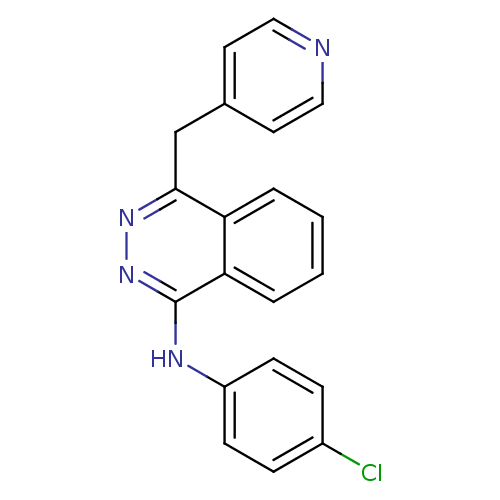
((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...)Show InChI InChI=1S/C20H15ClN4/c21-15-5-7-16(8-6-15)23-20-18-4-2-1-3-17(18)19(24-25-20)13-14-9-11-22-12-10-14/h1-12H,13H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CA(E542K) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for ABL1(F317I)-phosphorylated kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK4
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for CLK4 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for RSK4(Kin.Dom.2-C-terminal) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MET kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM26474
(5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...)Show SMILES CN(c1ccc2c(C)n(C)nc2c1)c1ccnc(Nc2ccc(C)c(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C21H23N7O2S/c1-13-5-6-15(11-19(13)31(22,29)30)24-21-23-10-9-20(25-21)27(3)16-7-8-17-14(2)28(4)26-18(17)12-16/h5-12H,1-4H3,(H2,22,29,30)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for CAMK2B kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for RSK4(Kin.Dom.2-C-terminal) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25617
(N-[3-({5-chloro-1H-pyrrolo[2,3-b]pyridin-3-yl}carb...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(Cl)cc23)c1F Show InChI InChI=1S/C17H14ClF2N3O3S/c1-2-5-27(25,26)23-13-4-3-12(19)14(15(13)20)16(24)11-8-22-17-10(11)6-9(18)7-21-17/h3-4,6-8,23H,2,5H2,1H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CA(H1047Y) kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data